Prev Page--Waters of Kansas--Chlor-Sulfate || Next Page--Waters of Kansas--Chlor-Carbonate
Part II--The Mineral Waters of Kansas, Arranged and Classified, with Analyses, continued
Chapter XIII--The Carbonate Group
Carbonated waters are perhaps the most numerous of any class. These include some very heavily charged with mineral matter which has been dissolved from the rocks or soil by the carbon dioxid dissolved in the water, and others that contain only small quantities of the bicarbonates, as they are called. Most of the ordinary spring waters which have a local reputation for great therapeutic virtues are of this class; those having an excess of sodium carbonate are also included in this group. These are called by many authors the" alkaline" class, because they include the alkaline carbonates, potassium, sodium, and lithium, as well as carbonates of the alkaline earths, calcium, magnesium, and strontium. These waters usually contain an excess of carbon-dioxid (carbonic-acid) gas, more than enough to keep the bases in solution. Lime and magnesia, it should be said, are dissolved by water surcharged with this gas, in accordance with the well-known reaction:
CaCO3 + H2O + CO2 = CaH2(CO3)2.
When a water of this composition evaporates spontaneously, as in the roof of a cave, or when it is heated, the water and carbon dioxid are expelled, and the calcium carbonate is precipitated or separates out in accordance with the reaction:
CaH2(CO3)2 = CaCO3 + H2O + CO2
This accounts for the formation of stalactites and many similar deposits.
These waters usually have an alkaline or neutral reaction. If sodium carbonate is present, the reaction is strongly alkaline, since carbonic acid is a very weak acid.
The amount of carbon-dioxid gas dissolved in the Kansas waters is small; in fact, there are none of this class that correspond with the waters of many localities that yield a sparkling and effervescent product. Some have attributed this excess of carbonic acid to the volcanic origin of a water.
This group is represented by the following waters:- Atchison, Dixon's spring.
- Baxter Springs, Cherokee county, Nos. 2, 3, and 4.
- Bonner Springs, Leavenworth county, Nos. 1, 2, and 3.
- Chico spring, Cherokee county.
- Chautauqua springs, Chautauqua county.
- Coffeyville, Montgomery county.
- Eagle springs, Doniphan county, Nos. 1 and 2.
- Eudora, Douglas county.
- Kickapoo springs, Leavenworth county.
- Moodyville, Pottawatomie county.
- Murphy's springs, Geary county.
- Onaga, Hoover's spring, Pottawatomie county.
- Ottawa, Sylvan springs, Johnson county.
- Stanley spring, Johnson county.
Dixon's Spring
In the city of Atchison, on South Sixth street, between Park and Spring streets, is a strongly flowing spring, which has had considerable local reputation for medicinal properties. From the time of the earliest settlers it has never been known to become dry, though it has a stronger flow after rains, but the water is never turbid. It flows from beneath the Oread limestone, with a flow of at least seven gallons per minute.
This water was formerly sold throughout the city, but on account of the fact that the spring is in a thickly populated locality it was thought probable that the water might be impure. The sanitary analysis seems to confirm this suspicion, and, furthermore, the determination of chlorin, made at different times, shows that the quantity is quite variable, thus indicating surface contamination.
| The Dixon Sprtng Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .1110 | Sodium oxid (Na2O) | .1496 | |
| Potassium (K) | .0060 | Potassium oxid (K2O) | .0072 | |
| Ammonium (NH4) | trace | Ammonia | trace | |
| Calcium (Ca) | .1560 | Calcium oxid (CaO) | .2184 | |
| Magnesium (Mg) | .0250 | Magnesium oxid (MgO) | .0417 | |
| Iron (Fe) | .0060 | Iron oxid (FeO) | .0077 | |
| Aluminum (Al) | trace | Aluminum oxid (Al2O3) | trace | |
| Chlorin (Cl) | .0580 | Chlorin (Cl) | .0580 | |
| Sulfuric acid ion (SO4) | .1164 | Sulfuric anhydrid (SO3) | .0970 | |
| Phosphate ion (PO4) | .0054 | Phosphoric anhydrid (P2O5) | .0040 | |
| Nitrate ion (NO3) | .1065 | Nitric anhydrid (N2O5) | .0927 | |
| Nitrite ion (NO2) | trace | Nitrous anhydrid (N2O3) | trace | |
| Silicic acid ion (SiO3) | .0376 | Silica (SiO2) | .0300 | |
| Water (H2O) | .0831 | |||
| Carbonic anhydrid (CO2) | .4044 | |||
| Oxygen equivalent | .0130 | |||
| Total | 1.1808 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium sulfate (Na2SO4) | .1076 | 6.2760 |
| Potassium nitrate (KNO3) | .0155 | .9041 |
| Sodium phosphate (NaHPO4) | .0080 | .4666 |
| Sodium chlorid (NaCl) | .0956 | 5.5760 |
| Sodium nitrate (NaNO3) | .1329 | 7.7520 |
| Calcium sulfate (CaSO4) | .0618 | 3.6050 |
| Calcium bicarbonate (CaH2(CO3)2) | .5582 | 32.5600 |
| Magnesium bicarbonate (MgH2(CO3)2) | .1522 | 8.8775 |
| Iron bicarbonate (FeH2(CO3)2) | .0190 | 1.1080 |
| Silica (SiO2) | .0300 | 1.7500 |
| Total mineral matter | 1.1808 | 68.8752 |
| Temperature, 13.3° C. (56° F.) Specific gravity, 1.00078 Analysis by E. B. Knerr. |
||
Baxter Springs
The Baxter chalybeate springs, in Cherokee county, on the St. Louis & San Francisco railroad, were for many years among the most noted of any in the state. The place is of great interest geologically on account of the fact that it is just in the edge of the Mississippian formation that extends from Missouri across the extreme southeastern corner of Kansas. The region is well watered by abundant springs, and drained by Spring river, that flows about eighteen miles through the state and thence southward into the territory. The city of Baxter Springs is six miles from the Missouri line and a mile and a half north of Indian Territory. On account of its peculiar situation, Baxter Springs has attained considerable commercial importance and carries on trade with the Indians in the vicinity.
The springs were developed in 1883, but the hotel is of an earlier date, belonging to the time when the city was known only as an important trading point. The streams of this section of the state are very clear, and the banks are well wooded, so that, since the surface of the country is very much broken by hills and valleys, it presents a marked difference in appearance from most other parts of the state. On both sides of the small branch that flows easterly through the city, as many as thirty springs have been discovered, many of which have an abundant flow of clear, sparkling water. Within a few hundred yards no less than ten excellent springs are to be seen.
A list of these springs, with the temperature as shown on September 4, 1898, is as follows:
- West pavilion, Iron spring, 16° C. (60.8° F.)
- East pavilion, Medical spring, 17° C. (62.6° F.)
- Spring in high way east of latter.
- Mann spring, on right bank of creek, 17° C. (62.6° F.)
- Doty spring, near residence of Mr. Doty, 19° C. (66.2°F.)
- Spring in pier of bridge.
- Spring in highway, north of No. 1.
- Spring in rock near residence of Mr. Newhouse, 19° C. (66.2° F.)
- Sulfur spring near the Scott property, 19° C. (66.2° F.)
- Spring beside bridge pier northwest of schoolhouse, 20° C. (68° F.)
Plate 21--West Pavilion, Baxter Springs.

Plate 21--Bath-house, Baxter Springs.

Improvements
Springs Nos. 1 and 2, as above noted, are situated in a small park. They are about 100 feet apart, and each is covered by a pavilion (Plate XXI). The springs are walled up and cemented, and furnish an abundance of water, which is utilized very extensively by the people of the city. The flow of spring No. 1 is estimated at about 480 gallons per hour, and that of No. 2 about 160 gallons per hour. At the northeast corner of the park is a bath-house with several bath-rooms, arranged for the use of hot and cold water. The water is pumped from either of the springs, and stored in a tank in the upper part of the bath-house.
On account of a change of proprietors, this resort was not in operation during the summer of 1898, nor has it been since that time. At some seasons of the year, especially during the summer, these springs are used by the people of the vicinity, and in fact spring No. 1 may be regarded as the source of city supply for good, wholesome drinking water. Spring No. 2 furnishes water that has been shipped quite extensively, but the water of No. 1 is said to contain so much iron that it deposits after standing a short time, so that, without special arrangements for carbonating, it cannot be conveniently shipped. The following analyses are from samples taken personally in June, 1901:
| Baxter Springs No. 2, "Medical Spring" Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0120 | Sodium oxid (Na2O) | .0161 | |
| Potassium (K) | .0042 | Potassium oxid (K2O) | .0050 | |
| Lithium (Li) | trace | Lithium oxid (Li2O) | trace | |
| Calcium (Ca) | .1260 | Calcium oxid (CaO) | .1763 | |
| Magnesium (Mg) | .0054 | Magnesium oxid (MgO) | .0090 | |
| Iron (Fe) | .0036 | Iron oxid (FeO) | .0046 | |
| Chlorin (Cl) | .0162 | Chlorin (Cl) | .0162 | |
| Sulfuric acid ion (SO4) | .1416 | Sulfuric anhydrid (SO3) | .1180 | |
| Silicic acid ion (SiO3) | .0149 | Silica (SiO2) | .0132 | |
| Water (H2O) | .0868 | |||
| Carbonic anhydrid (CO2) | .1803 | |||
| Oxygen equivalent | .0036 | |||
| Total | .5719 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0203 | 1.1857 |
| Sodium sulfate (Na2SO4) | .0122 | .7126 |
| Potassium chlorid (KCl) | .0080 | .4673 |
| Lithium bicarbonate (LiHCO3) | trace | trace |
| Calcium sulfate (CaSO4) | .1584 | 9.2522 |
| Calcium bicarbonate (CaH2(CO3)2) | .3214 | 18.7730 |
| Magnesium sulfate (MgSO4) | .0270 | 1.5770 |
| Iron bicarbonate (FeH2(CO3)2) | .0115 | .6717 |
| Silica (SiO2) | .0132 | .7710 |
| Totals | .5720 | 33.4105 |
| Analysis by E. B. Knerr. | ||
| Baxter Springs, No. 3, Doty's Spring Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0078 | Sodium oxid (Na2O) | .0105 | |
| Potassium (K) | .0063 | Potassium oxid (K2O) | .0076 | |
| Lithium (Li) | .0002 | Lithium oxid (Li2O) | .0003 | |
| Calcium (Ca) | .1118 | Calcium oxid (CaO) | .1568 | |
| Magnesium (Mg) | .0060 | Magnesium oxid (MgO) | .0101 | |
| Iron (Fe) | .0027 | Iron oxid (FeO) | .0034 | |
| Chlorin (Cl) | .0101 | Chlorin (Cl) | .0101 | |
| Nitrous acid ion (NO2) | .0003 | Nitrous anhydrid (N2O5) | .0002 | |
| Sulfuric acid ion (SO4) | .1345 | Sulfuric anhydrid (SO3) | .1121 | |
| Silicic acid ion (SiO3) | .0147 | Silica (SiO2) | .0131 | |
| Water (H2O) | .0323 | |||
| Carbonic anhydrid(CO2) | .1590 | |||
| Oxygen equivalent | .0023 | |||
| Total | .5132 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0076 | .4422 |
| Sodium sulfate (Na2SO4) | .0147 | .8587 |
| Potassium chlorid (KCl) | .0116 | .6743 |
| Potassium nitrite (KNO2) | .0006 | .0355 |
| Lithium bicarbonate (LiHCO3) | .0015 | .0875 |
| Calcium sulfate (CaSO4) | .1422 | 8.2950 |
| Calcium bicarbonate (CaH2(CO3)2) | .2832 | 16.5100 |
| Magnesium sulfate (MgSO4) | .0302 | 1.7620 |
| Iron bicarbonate (FeH2(CO3)2) | .0084 | .4900 |
| Silica (SiO2) | .0132 | .7700 |
| Totals | .5132 | 29.9252 |
| Analysis by E. B. Knerr. | ||
| Baxter Springs, No. 4, Mann Spring Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0069 | Sodium oxid (Na2O) | .0093 | |
| Potassium (K) | .0026 | Potassium oxid (K2O) | .0032 | |
| Lithium (Li) | .0008 | Lithium oxid (Li2O) | .0018 | |
| Calcium (Ca) | .1244 | Calcium oxid (CaO) | .1741 | |
| Magnesium (Mg) | .0005 | Magnesium oxid (MgO) | .0008 | |
| Iron (Fe) | .0017 | Iron oxid (FeO) | .0022 | |
| Chlorin (Cl) | .0222 | Chlorin (Cl) | .0222 | |
| Nitric acid ion (NO3) | .0089 | Nitric anhydrid (N2O5) | .0066 | |
| Nitrous acid ion (NO2) | trace | Nitrous anhydrid (N2O3) | trace | |
| Sulfuric acid ion (SO4) | .1139 | Sulfuric anhydrid (SO3) | .0949 | |
| Silicic acid ion (SiO3) | .0192 | Silica (SiO2) | .0152 | |
| Water (H2O) | .0338 | |||
| Carbonic anhydrid (CO2) | .1608 | |||
| Oxygen equivalent | .0050 | |||
| Total | .5199 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0176 | 1.0280 |
| Potassium nitrite (KNO2) | trace | trace |
| Potassium nitrate (KNO3) | .0068 | .3972 |
| Lithium bicarbonate (LiHCO3) | .0080 | .4673 |
| Calcium nitrate (Ca(NO3)2) | .0062 | .3621 |
| Calcium chlorid (CaCl2) | .0180 | 1.0514 |
| Calcium sulfate (CaSO4) | .1587 | 9.2696 |
| Calcium bicarbonate (CaH2(CO3)2) | .2817 | 16.4541 |
| Magnesium sulfate (MgSO4) | .0024 | .1402 |
| Iron bicarbonate (FeH2(CO3)2) | .0053 | .3096 |
| Silica | .0152 | .8878 |
| Totals | .5199 | 30.3673 |
| Analysis by E. B. Knerr. | ||
Plate 22--The Springs Hotel, Baxter Springs.

Plate 22--Group of Springs, Bonner Springs.

Bonner Springs
In Wyandotte county, at the northern end of the beautiful stretch of the Kaw river running northeast, is situated the Bonner Springs resort. This has been well known for many years. As originally settled it was called Tiblow, after an Indian chief, but when the proposition was made to make of it an important suburban resort for Kansas City, the present name, from Robert Bonner, was given it. Bonner Springs is seventeen miles west of Kansas City, on the Union Pacific railroad at the crossing of a branch of the Atchison, Topeka & Santa Fe railway running from Kansas City to Leavenworth; so it is very accessible from all directions. On account of the well-kept park in which the springs are situated, Bonner Springs has become a favorite camp-meeting resort. The park is not public property, but is owned by J. W. McDanield.
In the valley of the small creek that flows into the Kaw river just above the town, there are found as many as twenty springs, and at the head of these there is a lake, also fed by springs. Four of them are picturesquely grouped in the upper park, just below the camping-ground, and there are several in the lower park.
Improvements
The improvements are of considerable importance. In addition to the lake, which furnishes abundant facilities for boating, there are a large pavilion and several permanent buildings designed for the use of camp-meeting associations, and others who may tent upon these grounds. The trees are large, and many belong to the first growth. In the lower park a building has been erected for the protection of visitors from the weather, and in connection with this there is a large stand for the sale of refreshments. Near this building is a pavilion built over two of the most valuable springs. The water of one of these is carried by a pipe-line to the pump-house in the valley below the "Lodge," where a pump run by a windmill to a gasoline-engine elevates the water to the top of the "Lodge."
The sanitarium is some distance south of the park, and, situated as it is, upon something of aa elevation, it commands a beautiful view to the south across the Kaw river. The building contains fifty rooms, with 400 feet of verandas. It has steam heat, and is furnished with all modern conveniences. No special bath-houses are provided for using the spring water. The sanitarium is owned and managed by Dr. M. P. Sexton, and is at present devoted especially to the treatment of nervous and mental diseases. On account of the proximity of the sanitarium to Kansas City, it affords a convenient suburban home 'where patients may be kept. quiet, and away from the noise and bustle of the city. No attempt has been made to ship the water from Bonner Springs, although at some seasons of the year the quantity is ample for this purpose. The analyses of seven of the springs, made in 1884, and published in bulletin of the United States Geological Survey No. 32, is as follows:
| Bonner Springs, No. 1 | |
|---|---|
| Ions | Grams per liter |
| Calcium (Ca) | .0926 |
| Magnesium (Mg) | .0268 |
| Iron (Fe) | .0112 |
| Chlorin (Cl) | .0082 |
| Sulfuric acid ion (SO4) | .0315 |
| Silicic acid ion (SiO3) | .0107 |
| Phosphorie acid ion (PO4) | trace |
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Calcium chlorid (CaCl2) | .0129 | .751 |
| Calcium sulfate (CaSO4) | .0447 | 2.612 |
| Calcium bicarbonate (CaH2(CO3)2) | .3126 | 18.261 |
| Magnesium bicarbonate (MgH2(CO3)2) | .1630 | 9.525 |
| Iron bicarbonate (FeH2(CO3)2) | .0361 | 2.107 |
| Silica (SiO2) | .0085 | .496 |
| Phosphoric acid | trace | trace |
| Organic matter | small amt. | small amt. |
| Totals | .5778 | 33.752 |
| Analysis by Wm. Jones, M. D. | ||
Plate 23--Pavilion, Bonner Springs Park.

Plate 23--Bonner Springs Lake.
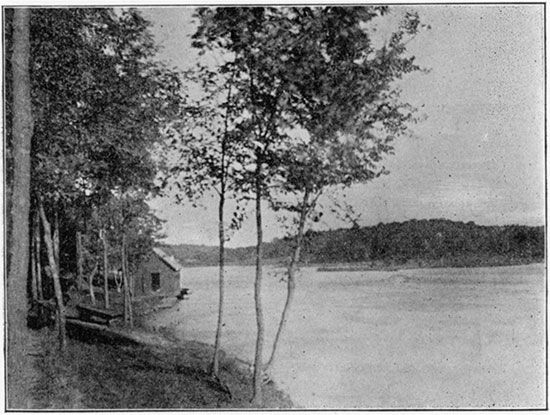
| Bonner Springs, No. 2 | |
|---|---|
| Ions | Grams per liter |
| Calcium (Ca) | .0923 |
| Magnesium (Mg) | trace |
| Iron (Fe) | .0012 |
| Chlorin (Cl) | .0114 |
| Silicic acid ion (SiO3) | .0082 |
| Phosphoric acid ion (PO4) | trace |
| Total | .1131 |
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Calcium chlorid (CaCl2) | .0179 | 1.042 |
| Calcium bicarbonate (CaH2(CO3)2) | .3729 | 21.782 |
| Magnesium bicarbonate (MgH2(CO3)2) | trace | trace |
| Iron bicarbonate (FeH2(CO3)2) | .0037 | .217 |
| Phosphoric acid | trace | trace |
| Silica (SiO2) | .0065 | .381 |
| Organic matter | small amt. | small amt. |
| Total | .4010 | 23.422 |
| Analysis by Wm. Jones. M. D. | ||
| Bonner Springs, No. 3 | |
|---|---|
| Ions | Grams per liter |
| Sodium (Na) | .5636 |
| Calcium (Ca) | .0485 |
| Magnesium (Mg) | .0374 |
| Chlorin (Cl) | .1301 |
| Sulfuric acid ion (SO4) | trace |
| Phosphoric acid ion (PO4) | trace |
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .2105 | 12.293 |
| Sodium bicarbonate (NaHCO3) | 1.7493 | 102.178 |
| Calcium chlorid (CaCl2) | trace | trace |
| Calcium sulfate (CaSO4) | trace | trace |
| Calcium bicarbonate (CaH2(CO3)2) | .1952 | 11.403 |
| Magnesium bicarbonate (MgH2(CO3)2) | .2230 | 13.024 |
| Phosphoric acid | trace | trace |
| Organic matter | small amt. | small amt. |
| Totals | 2.3780 | 138.898 |
| Analysis by Wm. Jones, M. D. | ||
Plate 24--Bonner Springs Sanitarium.

Plate 24--Chautauqua Springs Hotel.

Chautauqua Springs
In Chautauqua county there is a pure spring of water which has attracted considerable attention. It flows from beneath a sandstone rock in a little valley south of the village of Chautauqua Springs. Few improvements have been made, with the exception of erecting a spring-house. This region is much diversified by hills and valleys, and the streams are clear and sparkling. In this respect the region differs from most of the Kansas mineral-spring localities. This water belongs to that class of comparatively pure waters or "soft" waters referred to in chapter XVIII. Such waters are very useful when it is of advantage to the patient to drink copiously, and when he does not desire the effect of any mineral salts. As the water is free from large quantities of salts of lime and magnesium, which are so abundant in Kansas, it may in many cases be very beneficial to patients. Chautauqua may be reached by the A. T. & S. F. railroad.
| Chautauqua Springs Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0270 | Sodium oxid (Na2O) | .0364 | |
| Calcium (Ca ) | .0367 | Calcium oxid (CaO) | .0514 | |
| Magnesium (Mg) | .0084 | Magnesium oxid (MgO) | .0140 | |
| Iron (Fe) | .0024 | Iron oxid (FeO) | .0031 | |
| Chlorin (Cl) | .0342 | Chlorin (Cl) | .0342 | |
| Sulfuric acid ion (SO4) | .0490 | Sulfuric anhydrid (SO3) | .0409 | |
| Silicic acid ion (SiO3) | .0353 | Silicic anhydrid (SiO2) | .0279 | |
| Carbonic anhydrid (CO2) | .0799 | |||
| Water (H2O) | .0162 | |||
| Oxygen equivalent | .0077 | |||
| Total | .2963 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0565 | 3.300 |
| Sodium bicarbonate (Na4HCO3) | .0174 | 1.019 |
| Calcium bicarbonate (CaH2(CO3)2) | .0660 | 3.860 |
| Calcium sulfate (CaSO4) | .0695 | 4.051 |
| Magnesium bicarbonate (MgH2(CO3)2) | .0512 | 2.994 |
| Iron bicarbonate (FeH2(CO3)2) | .0078 | .456 |
| Silica (SiO2) | .0279 | 1.628 |
| Totals | .2963 | 17.308 |
| Analysis by E. H. S. Bailey and E. C. Franklin. | ||
Coffeyville Well
This well is situated on the property of Joseph Kloehr, one and one half-miles east of Coffeyville, in the Verdigris river bottom, and a quarter of a mile from that stream. The heavy timber of the vicinity adds much to the attractiveness of the situation. The well is dug sixteen feet deep, and from the bottom a pipe is driven sixteen feet and eight inches into the sand and gravel. Coffeyville is on the lines of the Missouri Pacific, the Atchison, Topeka & Santa Fe, and the Missouri, Kansas & Texas railroads.
Improvements
The improvements are a driveway from the main road, leading to the new two-story building arranged as a water-cure establishment for the accommodation of boarders. The water is sold to the people in the vicinity. The analysis is as follows:
| Coffeyville Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0088 | Sodium oxid (Na2O) | .0118 | |
| Calcium (Ca) | .1661 | Calcium oxid (CaO) | .2325 | |
| Magnesium (Mg) | .0357 | Magnesium oxid (MgO) | .0595 | |
| Iron (Fe) | .0095 | Iron oxid (FeO) | .0122 | |
| Chlorin (Cl) | .0135 | Chlorin (Cl) | .0135 | |
| Sulfuric acid ion (SO4) | .0276 | Sulfuric anhydrid (SO3) | .0230 | |
| Silicic acid ion (SiO3) | .0304 | Silicic anhydrid SiO2) | .0240 | |
| Carbonic anhydrid (CO2) | .4859 | |||
| Water (H2O) | .0993 | |||
| Oxygen equivalent | .0031 | |||
| Totals | .9586 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0223 | 1.304 |
| Calcium sulfate (CaSO4) | .0391 | 2.282 |
| Calcium bicarbonate (CaH2(CO3)2) | .6259 | 36.559 |
| Magnesium bicarbonate (MgH2(CO3)2) | .2172 | 12.686 |
| Iron bicarbonate (FeH2(CO3)2) | .0301 | 1.755 |
| Silica (SiO2) | .0240 | 1.405 |
| Total | .9586 | 55.991 |
| Analysis by E. H. S. Hailey | ||
Plate 25--Chautauqua Springs.

Plate 25--Eagle Springs House.

Eagle Springs (Highland Station)
In the extreme eastern part of Doniphan county, surrounded on three sides by a bend of the Missouri river, is a peculiar section of country covered with high, rounded elevations, separated by deep valleys. The hills become higher in the vicinity of the river on the east, forming the so-called bluffs. These are well wooded, and it is interesting to note that there is here a greater variety of trees than is found in other sections of the state. Much of this timber has been recently cut, however, to give place to orchards which thrive so well in this section. The apple and peach especially seem to be well adapted to this soil, which is composed largely of gravel and clay, and it is even asserted by some that apples grown upon these bluffs have a better flavor than those grown upon prairie soil. From the bluffs on the east a very extensive view of the windings of the Missouri river may be obtained; this view extends from St. Joseph, Mo., northward for perhaps forty miles, and to the south and west the high prairie uplands are visible. This section of Doniphan county is drained by Wolf river, which flows northward and empties into the Missouri near White Cloud. Among the hills east of Wolf river, a short distance from the Burlington & Missouri River railroad (Highland Station), are situated Eagle Springs, which were developed in 1882.
Improvements
There is here a hotel having a capacity for forty guests, which is owned and managed by Pryor Plank, a bath-house, two cottages, and a fine artificial lake well stocked with fish. During the summer a resident physician may be usually found at the Eagle Springs hotel. The two most important springs are the upper spring, which has a flow of not over thirty gallons per hour, and the lower spring, which is quite near the bathhouse, and has a flow of 300 gallons per hour and a temperature of 13° C. (55.4° F.)
| Eagle Springs, No. 1, Lower Spring Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0055 | Sodium oxid (Na2O) | .0084 | |
| Calcium (Ca) | .1022 | Calcium oxid (CaO) | .1431 | |
| Magnesium (Mg) | .0374 | Magnesium (MgO) | .0623 | |
| Iron (Fe) | .0051 | Ferric oxid (Fe2O3) | .0072 | |
| Chlorin (Cl) | .0096 | Chlorin (Cl) | .0096 | |
| Sulfuric acid ion (SO4) | .0122 | Sulfuric anhydrid (SO3) | .0102 | |
| Silicic acid ion (SiO3) | .0330 | Silica (SiO2) | .0261 | |
| Carbonic anhydrid (CO2) | .3583 | |||
| Water (H2O) | .0730 | |||
| Oxygen equivalent | .0022 | |||
| Total | .6960 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0158 | .9229 |
| Calcium sulfate (CaSO4) | .0173 | 1.0105 |
| Calcium bicarbonate (CaH2(CO3)2) | .3934 | 22.9784 |
| Magnesium bicarbonate (MgH2(CO3)2) | .2274 | 13.2825 |
| Iron bicarbonate (FeH2(CO3)2) | .0160 | .9346 |
| Silica (SiO2) | .0261 | 1.5247 |
| Totals | .6960 | 40.6536 |
| Temperature, 11.8° C. (53.4° F.) Analysis by E. H. B. Bailey and D. F. McFarland. |
||
| Eagle Springs, No. 2, Upper Spring | ||||
|---|---|---|---|---|
| Ions | Grams per liter |
|||
| Sodium (Na) | .0232 | |||
| Potassium (K) | .0647 | |||
| Calcium (Ca) | .0617 | |||
| Magnesium (Mg) | .0186 | |||
| Iron and aluminum (Fe and Al) | .0007 | |||
| Chlorin (Cl) | .0006 | |||
| Silicic acid ion (SiO3) | .0241 | |||
| Sulfuric acid ion (SO4) | .0101 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0010 | .0575 |
| Sodium bicarbonate (NaHCO3) | .0530 | 3.1507 |
| Potassium sulfate (K2SO4) | .0181 | 1.0527 |
| Potassium bicarbonate (KHCO3) | .1015 | 5.9357 |
| Calcium bicarbonate (CaH2(CO3)2) | .2523 | 16.0269 |
| Magnesium bicarbonate (MgH2(CO3)2) | .1135 | 6.6376 |
| Iron bicarbonate (FeH2(CO3)2) | .0022 | .1277 |
| Silica (SiO2) | .0191 | 1.1197 |
| Organic matter | .0167 | .9797 |
| Totals | .5774 | 35.0882 |
| Analysis by Walter C. Brown, Chicago. | ||
Eudora Mineral Springs, Douglas County
There were four springs developed here about ten years ago. The principal springs are situated on the right bank of the Wakarusa, a few rods above the crossing of the Atchison, Topeka & Santa Fe railroad. They are on the first terrace of the river, and are walled with brick and cement. The surrounding grounds were improved by cutting out the timber and constructing stairways, walks, and pavilions. Very little attention has recently been paid to this property, though formerly it attracted picnic and excursion parties. The analysis of spring No. 2 is given below. The water of the other springs is quite similar, although the amount of iron differs in the different waters. These might with equal propriety he classified as chalybeate springs.
| Eudora Mineral Spring No. 2 Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0201 | Sodium oxid (Na2O) | .0270 | |
| Potassium (K) | .0052 | Potassium oxid (K2O) | .0063 | |
| Calcium (Ca) | .1491 | Calcium oxid (CaO) | .2095 | |
| Magnesium (Mg) | .0155 | Magnesium oxid (MgO) | .0258 | |
| Iron (Fe) | .0140 | Iron oxid (FeO) | .0180 | |
| Manganese (Mn) | trace | Manganese oxid (MnO) | trace | |
| Chlorin (Cl) | .0049 | Chlorin (Cl) | .0049 | |
| Sulfuric acid ion (SO4) | .0302 | Sulfuric anhydrid (SO3) | .0252 | |
| Silicic acid ion (SiO3) | .0420 | Silicic anhydrid (SiO2) | .0332 | |
| Organic and volatile matter | .0261 | |||
| Carbonic anhydrid (CO2) | .4176 | |||
| Water (H2O) | .0856 | |||
| Oxygen equivalent | .0011 | |||
| Total | .8781 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0081 | .472 |
| Sodium bicarbonate (NaHCO3) | .0601 | 3.510 |
| Potassium sulfate (K2SO4) | .0117 | .683 |
| Calcium sulfate (CaSO4) | .0337 | 1.968 |
| Calcium bicarbonate (CaH2(CO3)2) | .5664 | 33.083 |
| Magnesium bicarbonate (MgH2(CO3)2) | .0943 | 5.502 |
| Iron bicarbonate (FeH2(CO3)2) | .0445 | 2.600 |
| Manganese bicarbonate (MgH2(CO3)2) | trace | trace |
| Silica (SiO2) | .0332 | 1.941 |
| Organic and volatile matter | .0261 | 1.520 |
| Totals | .8781 | 51. 279 |
| Analysis by E. H. S. Bailey. | ||
Kickapoo Springs, Leavenworth County
These springs are situated about seven miles northwest of the city of Leavenworth, on the farm of George W. Few. They are located in a valley where the land overlooks the Missouri river bluffs, and on the south is a range of hills. There are three springs in the group, all of which are more or less chalybeate. No. 1, the analysis of which is given below, has been carefully boxed, and is five and one-half feet in depth. The waters from all these springs are clear and sparkling when first drawn, but deposit the iron hydrate in the stream below for a long distance. This is of course due to the escape of carbon-dioxid gas, as has been previously noticed.
Stone implements, such as arrow-heads and spear-heads that are often found near the springs, indicate that this locality was a favorite camping-ground for the Indians, and, as the Kickapoos were the last tribe having a reservation in this vicinity, the springs have been named after them. No improvements have been made in the property and the water is not shipped.
The composition of spring No. 1 is as follows:
| Kickapoo Springs Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0096 | Sodium oxid (Na2O) | .0130 | |
| Potassium (K) | .0009 | Potassium oxid (K2O) | .0012 | |
| Calcium (Ca) | .0636 | Calcium oxid (CaO) | .0891 | |
| Magnesium (Mg) | .0132 | Magnesium oxid (MgO) | .0220 | |
| Iron (Fe) | .0007 | Iron oxid (FeO) | .0009 | |
| Aluminum (Al) | .0006. | Aluminum oxid (Al2O3) | .0011 | |
| Chlorin (Cl) | .0029 | Chlorin (Cl) | .0029 | |
| Sulfuric acid (ion) (SO4) | .0238 | Sulfuric anhydrid (SO3) | .0199 | |
| Nitric acid ion (NO3) | trace | Nitric anhydrid (N2O5) | trace | |
| Silicic acid ion (SiO3) | .0320 | Silica (SiO2) | .0252 | |
| Carbonic anhydrid (CO2) | .1845 | |||
| Water (H2O) | .0376 | |||
| Oxygen equivalent | .0004 | |||
| Total | .3970 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Potassium sulfate (K2SO4) | .0029 | .1611 |
| Sodium chlorid (NaCl) | .0049 | .286 |
| Sodium bicarbonate (NaHCO3) | trace | trace |
| Sodium nitrate (NaNO3) | trace | trace |
| Sodium sulfate (Na2SO4) | .0238 | 1.394 |
| Calcium sulfate (CaSO4) | .0088 | .513 |
| Calcium bicarbonate (CaH2(CO3)2) | .2468 | 14.415 |
| Magnesium bicarbonate (MgH2(CO3)2) | .0813 | 4.748 |
| Iron bicarbonate (FeH2(CO3)2) | .0022 | .128 |
| Aluminum oxid (Al2O3) | .0011 | .064 |
| Silica (SiO2) | .0252 | 1.471 |
| Totals | .3970 | 23.188 |
| Analysis by E. H. S, Bailey. | ||
Moodyville Springs
At Moodyville, in Pottawatomie county, about four miles northeast of Westmoreland, in Rock creek bottom, on the Westmoreland branch of a road connecting with the Leavenworth, Kansas & Western railroad at Blaine, are situated several springs that have obtained a local reputation. These springs are the property of E. M. Moody, and are situated at the base of a limestone bluff surrounded by a fine grove of natural timber.
Improvements
An avenue bordered with large trees connects the springs property with the main road. Some years ago a hotel was built on the land just west of the grove, and extensive improvements, including walks, fountains, swinging bridges, etc., were made on the property. Recently, however, no attempt has been made to keep the property in repair. On the bluff to the east of the spring is a so-called Indian mound, and a lookout has been built here which commands an extensive prospect over the surrounding territory. Some rocks tumbled together on the side of the bluff near by form an interesting cave. The water of the main spring has a temperature of 12.2° C.(54° F.) and a flow of 360 gallons per hour. It has a specific gravity of 1.00395. The qualitative analysis made by J. R. Eaton, chemist of William Jewell College, Liberty, Mo., showed calcium carbonate, magnesium carbonate, magnesium sulfate, sodium sulfate, sodium chlorid, iron carbonate, silica, alumina, and a trace of organic matter. The water contains an abundance of free carbonic-acid gas, and, when concentrated, gives an alkaline reaction.
Plate 26--Moodyville Springs.

Plate 26--Murphy's Springs.
Murphy's Springs, Junction City, Geary County
.These springs, of which there are seven in the group, are situated six miles southwest of Junction City, near Kansas Falls station, on the line of the Union Pacific railroad. No improvements except the building of a spring-house have been made. The water is forced by a windmill pump to the dwelling house of Charles Murphy, about a quarter mile distant. There is an abundance of excellent water here, but it is only utilized for domestic and stock-feeding purposes.
| Murphy's Springs (Bull. U. S. Geol. Surv. No. 32, p. 113.) |
||||
|---|---|---|---|---|
| Ions | Grams per liter | |||
| Sodium (Na) | .0163 | |||
| Calcium (Ca) | .0557 | |||
| Magnesium (Mg) | .0147 | |||
| Iron (Fe) | trace | |||
| Aluminum (Al) | trace | |||
| Silicic acid ion (SiO3) | .0213 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium bicarbonate (NaHCO3) | .0597 | 3.475 |
| Calcium bicarbonate (CaH2(CO3)2) | .2251 | 13.149 |
| Magnesium bicarbonate (MgH2(CO3)2) | .0899 | 5.243 |
| Iron and aluminum oxids (Al2O3 and Fe2O3) | trace | trace |
| Silica (SiO2) | .0168 | .982 |
| Totals | .3915 | 22.849 |
| Analysis by Barnes and Sim. | ||
Plate 27--Hoover's Spring, Onaga.

Plate 27--Interior of Spring-house, Hoover's Spring.
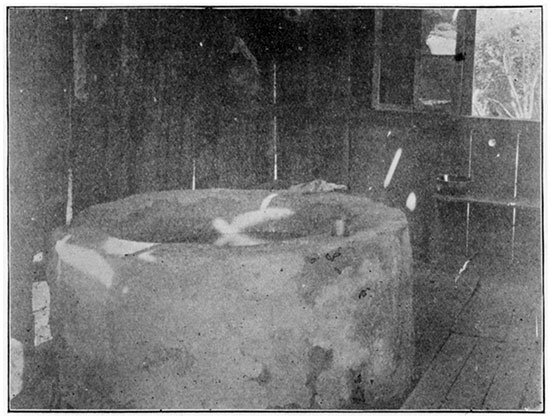
Hoover's Spring, Onaga, Pottawatomie County
In the northern part of Pottawatomie county, about one-half mile north of the village of Onaga, which is accessible by the Kansas City, Leavenworth & Western railway, is a mineral spring owned and managed by Henry Hoover. The spring is in a depression east of the owner's residence, and in the vicinity, both north and west, are hills overlooking the valley of the Vermillion on the east. The whole region is diversified by numerous hills and valleys, and is well watered and timbered. Hoover's spring, which is 200 feet above the Vermillion, has been excavated twelve feet, to the clay. It was then walled and cemented to a point three feet above the floor of the spring-house. Clear water, which may be seen to bubble from the sand at the bottom, is conducted away to a cement reservoir about twenty feet distant. No attempt is made to utilize the water, except by local patrons and an occasional shipment of a few gallons. The flow is 180 gallons per hour. The composition of the water is as follows:
| Hoover's Spring, Onaga Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0049 | Sodium oxid (Na2O) | .0066 | |
| Calcium (Ca) | .0273 | Calcium oxid (CaO) | .0383 | |
| Magnesium (Mg) | .0199 | Magnesium oxid (MgO) | .0333 | |
| Iron (Fe) | .0029 | Iron oxid (FeO) | .0038 | |
| Chlorin (Cl) | .0076 | Chlorin (Cl) | .0076 | |
| Sulfuric acid ion (SO4) | .0141 | Sulfuric anhydrid (SO3) | .0118 | |
| Silicic acid ion (SiO3) | .0308 | Silica (SiO2) | .0244 | |
| Carbonic anhydrid (CO2) | .1258 | |||
| Water (H2O) | .0253 | |||
| Oxygen equivalent | .0017 | |||
| Total | .2752 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0125 | .7301 |
| Calcium sulfate (CaSO4) | .0200 | 1.1682 |
| Calcium bicarbonate (CaH2(CO3)2) | .0873 | 5.0992 |
| Magnesium bicarbonate (MgH2(CO3)2) | .1216 | 7.1027 |
| Iron bicarbonate (FeH2(CO3)2) | .0094 | .5490 |
| Silica (SiO2) | .02404 | 1.4252 |
| Totals | .2752 | 16.0744 |
| Temperature, 16.6° C. (61.9° F.) Analysis by E. H. S. Bailey. |
||
Plate 28--Sylvan Springs, Ottawa.

Plate 28--Mineral Well, Iola.

Sylvan Springs, Ottawa
These springs are situated about two miles northwest of Eight Mile creek, a branch of the Marais des Cygnes. There is a ford at this point over the creek, and a drive has been cut through the timber, so that the springs are accessible. The springs are the property of the estate of S. E. Allison, and perhaps $100 was spent in the improvements noted. The water of the main spring comes out of the rock on a level with the creek, but when confined it rises above that level, so that by carefully walling up and cementing about the spring an abundant supply is retained. In order to obtain the water conveniently a platform has been built above the spring, and the water is brought to this platform by means of a pump.
The flow is about 200 gallons per hour in the driest weather. Within a few rods are several other springs, but the one of which the analysis has been made has the greatest flow. Within the last five years the water has been hauled to Forest Park Ottawa, and placed in permanent tanks for use during the Chautauqua assembly, which meets for about two weeks in July of each year. The water has found quite an extensive use locally, but has not been shipped.
| Sylvan Spring, Ottawa Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .0242 | Sodium oxid (Na2O) | .0325 | |
| Calcium (Ca) | .1238 | Calcium oxid (CaO) | .1730 | |
| Magnesium (Mg) | .0260 | Magnesium oxid (MgO) | .0437 | |
| Iron (Fe) | .0008 | Iron oxid (FeO) | .0011 | |
| Chlorin (Cl) | .0371 | Chlorin (Cl) | .0371 | |
| Sulfuric acid ion (SO4) | .0371 | Sulfuric anhydrid (SO3) | .0308 | |
| Silicic acid ion (SiO3) | .0235 | Silica (SiO2) | .0186 | |
| Carbonic anhydrid (CO2) | .3362 | |||
| Water (H2O) | .0679 | |||
| Oxygen equivalent | .0083 | |||
| Total | .7326 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | .0613 | 3.575 |
| Calcium bicarbonate (CaH2(CO3)2) | .5003 | 29.221 |
| Magnesium sulfate (MgSO4) | .0461 | 2.688 |
| Magnesium bicarbonate (MgH2(CO3)2) | .1037 | 6.052 |
| Iron bicarbonate (FeH2(CO3)2) | .0026 | .155 |
| Silica (SiO2) | .0186 | 1.085 |
| Totals | .7326 | 42.776 |
| Temperature, 20° C. (68° F.) Analysis by E. H. S. Bailey. |
||
Stanley Spring, Johnson County
The water of a spring on the farm of Freeman Shreve, at Stanley, has been used with considerable success by the local physicians. It has a flow of about twelve barrels per hour. The spring has been dug out to the depth of five feet and securely boxed. This water gives upon evaporation 18.3 grains of mineral matter per gallon. This consists of calcium, magnesium, sodium and iron carbonates, with some sulfates. It belongs to the light carbonate group of waters.
Comparison of Similar Waters
Waukesha, Wis., Bethesda Spring
| Grains per gallon Analysis by C. F. Chandler |
|
|---|---|
| Sodium chlorid | 1.16 |
| Sodium bicarbonate | 1.26 |
| Sodium sulfate | .54 |
| Potassium sulfate | .46 |
| Calcium bicarbonate | 17.02 |
| Magnesium bicarbonate | 12.39 |
| Iron bicarbonate | .04 |
| Aluminum oxid | .12 |
| Silica | .74 |
| Organic matter | 1.98 |
| Total | 35.71 |
Napa Soda Springs, California, Pagoda Spring
| Grains per gallon Analysis by Winslow Anderson |
|
|---|---|
| Sodium chlorid | 7.14 |
| Sodium bicarbonate | 12.95 |
| Sodium carbonate | 1.10 |
| Sodium sulfate | 1.62 |
| Potassium bicarbonate | trace |
| Calcium bicarbonate | .78 |
| Calcium carbonate | 9.55 |
| Magnesium bicarbonate | 3.04 |
| Magnesium carbonate | 21.76 |
| Ferrous carbonate | 7.90 |
| Silica | .74 |
| Alumina | .57 |
| Organic matter | trace |
| Total | 67.15 |
| Free carbon-dioxid gas, 113.62 cubic inches. Temperature of water, 67.7° F. |
|
Manitou, Colo., Manitou Soda Springs
| Grains per gallon Analysis by Elwyu Waller |
|
|---|---|
| Sodium chlorid | 23.94 |
| Sodium carbonate | 40.66 |
| Sodium sulfate | 11.14 |
| Potassium sulfate | 10.68 |
| Lithium carbonate | .71 |
| Calcium carbonate | 69.08 |
| Magnesium carbonate | 16.68 |
| Iron oxid | .02 |
| Alumina | .07 |
| Silica | 2.49 |
| Total | 174.47 |
| Free carbon-dioxid gas, abundant | |
Highland Springs, California, Ems Spring
| Grains per gallon Analysis by Winslow Anderson |
|
|---|---|
| Sodium chlorid | 1.76 |
| Sodium bicarbonate | 17.50 |
| Sodium carbonate | 2.45 |
| Potassium bicarbonate | .78 |
| Calcium bicarbonate | 57.32 |
| Magnesium bicarbonate | 66.55 |
| Magnesium carbonate | 1.63 |
| Ferrous carbonate | 1.53 |
| Manganese bicarbonate | trace |
| Silica | 7.23 |
| Alumina | .12 |
| Organic matter | trace |
| Total solids | 156.87 |
| Free carbon-dioxid gas, 85.90 cubic inches Temperature of water 77° F. |
|
Saratoga, New York, Vichy Spring
| Grains per gallon Analysis by C. F. Chandler |
|
|---|---|
| Sodium chlorid | 128.69 |
| Sodium bicarbonate | 82.87 |
| Sodium bromid | .99 |
| Potassium chlorid | 14.11 |
| Lithium bicarbonate | 1.76 |
| Calcium bicarbonate | 95.52 |
| Magnesium bicarbonate | 41.50 |
| Barium bicarbonate | .59 |
| Iron bicarbonate | .05 |
| Alumina | .48 |
| Silica | .76 |
| Total | 367.32 |
| Carbon-dioxid gas, 383.07 cubic inches. | |
Vichy, France, Grande Grille
| Grains per gallon Analysis by Bouquet |
|
|---|---|
| Sodium chlorid | 7.827 |
| Sodium bicarbonate | 319.442 |
| Sodium sulfate | 16.997 |
| Sodium phosphate | 7.592 |
| Sodium arsenite | .175 |
| Potassium bicarbonate | 22.594 |
| Calcium bicarbonate | 28.517 |
| Magnesium bicarbonate | 20.187 |
| Ferrous bicarbonate | .260 |
| Silica | 4.089 |
| Total | 427.680 |
Apollinaris, Neuenahar, Rhenish Prussia
| Grains per gallon Analysis by Professor Bischof |
|
|---|---|
| Sodium chlorid | 28.561 |
| Sodium carbonate | 77.20 |
| Sodium sulfate | 18.40 |
| Calcium carbonate | 3.60 |
| Magnesium carbonate | 27.12 |
| Ferric oxid | 1.20 |
| Alumina | 1.20 |
| Silica | .48 |
| Total | 157.76 |
| Free carbon-dioxid gas, 376.32 cubic inches. | |
A Comparison of the Waters of the Carbonate Group
| Grains per gallon | ||||||
|---|---|---|---|---|---|---|
| Name | Total solids |
Sodium chlorid |
Sodium bicarbonate |
Calcium sulfate |
Calcium bicarbonate |
Magnesium bicarbonate |
| Dixon | 68 | 5 | 3 | 32 | 8 | |
| Baxter No. 2 | 33 | 1 | 9 | 18 | ||
| Bonner No. 1 | 33 | 2 | 18 | 9 | ||
| Bonner No. 3 | 138 | 12 | 102 | trace | 11 | 13 |
| Chautauqua | 17 | 3 | 1 | 4 | 3 | 3 |
| Coffeyville | 56 | 1 | 2 | 36 | 12 | |
| Eagle No. 1 | 40 | 1 | 1 | 23 | 13 | |
| Eagle No. 2 | 35 | 3 | 16 | 6 | ||
| Eudora No. 2 | 51 | 3 | 2 | 33 | 5 | |
| Kickapoo | 23 | trace | 14 | 5 | ||
| Murphy | 22 | 3 | 13 | 5 | ||
| Hoover's | 16 | 1 | 5 | 7 | ||
| Sylvan | 42 | 3 | 29 | 6 | ||
| Waukesha, Wis. | 35 | 1 | 1 | 17 | 12 | |
| Manitou, Colo. | 174 | 24 | 40 | * 69 | * 16 | |
| Napa Soda, Cal. | 67 | 7 | 13 | * 10 | * 24 | |
| Highland, Cal. | 156 | 1 | 20 | 57 | 67 | |
| Apollinaris, Prussia | 157 | 28 | 71 | * 3 | * 27 | |
| * Carbonate | ||||||
These waters in Kansas contain calcium and magnesium bicarbonates and little besides. Some chemists have reported sodium sulfate in some of the waters of this general class. It is apparent that the sodium bicarbonate, the ingredient that gives an alkaline quality to such waters, is usually absent, or only present in small quantities. There are waters in the state, like the Abilena, and a water from Cherryvale that has been recently analyzed, which do contain considerable sodium carbonate, but a very large quantity of some other ingredients entirely overshadows the sodium carbonate in those waters.
The total solids are usually low, and in fact so low that some of the waters mentioned might be considered in the soft-water group.
Prev Page--Waters of Kansas--Chlor-Sulfate || Next Page--Waters of Kansas--Chlor-Carbonate
Kansas Geological Survey, Geology
Placed on web April 7, 2017; originally published 1902.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/Vol7/15_carbon.html