Prev Page--Site 6 || Next Page--Site 10
Site 7
Soil profile and physical and chemical characteristics
The soil profile of site 7--an Attica soil, a coarse-grained, loamy, mixed, thermic Udic Haplustalf--consists of brown to grayish-brown loamy fine-grained sand down to 15 cm (5.9 in.). This sand forms the soil Ap horizon, which is characterized by a weak granular structure. The Ap horizon is followed by an AB horizon of grayish-brown loamy, fine-grained sand down to 32 cm (12.6 in.). This horizon is characterized by a weak, medium subangular, blocky structure with a relatively high bulk density compared with other horizons in the upper part of the profile. Underlying the AB horizon is a brown to yellowish-brown fine-grained sandy loam down to 65 cm (26 in.) (the Bt horizon). The Bt soil horizon, which can be subdivided into two units [Bt1, at 32-50 cm (1.0-1.6 ft) and Bt2, at 50-65 cm (1.6-2.1 ft)], is slightly hard and friable with a moderate, medium subangular, blocky structure. Yellowish-brown loamy fine-grained sand forms the underlying C horizon, down to 157 cm (5.15 ft), which can be subdivided into three subhorizons (C1, C2, and C3). The C horizon is massive and friable with a few fine, distinct brown to dark-brown mottles. Underlying this soil horizon is another buried soil consisting of a weak red sandy clay loam forming another C horizon down to 177 cm (5.81 ft). This soil horizon has a massive structure with many reddish-brown mottles. Underlying this is a reddish-gray B horizon, which can be subdivided into a silty clay Bw horizon [down to 215 cm (7.05 ft)] characterized by several wide [6 cm (2 in.)] vertical cracks filled with coarser soil and a silty clay loam Bk horizon [down to 240 cm (7.87 ft)] characterized by strongly effervescent plate-like soft masses of lime.
The specific soil horizons and their grain-size distributions, as determined by the SCS, are shown in fig. 29. The figure indicates that site 7 has a much sandier soil profile down to 160 cm (5.2 ft) than site 6. X-ray diffraction patterns of the clays of the lower soil horizons (2C4, 2Bw, and 2Bk) exhibit large montmorillonite peaks and medium mica and kaolinite peaks; patterns for the upper soil horizons (Bt1 and Bt2) exhibit medium mica and montmorillonite peaks. The organic carbon content (fig. 30) of the upper foot of soil is much lower than that at site 6, and the CEC of the clay content at site 7 is also much lower than that at site 6, except at depths below 160 cm (6 ft), where the silty clay to clay loam layers are encountered (fig. 29).
Figure 29--Grain-size distribution and soil horizons at site 7.
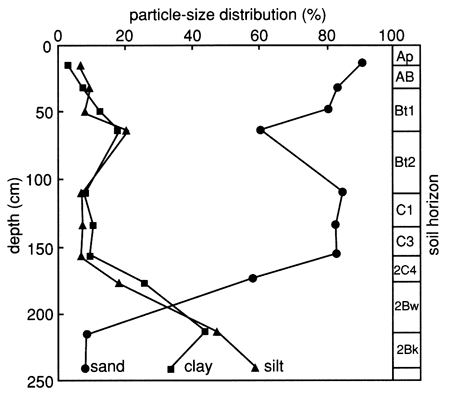
Figure 30--Organic carbon content and cation-exchange capacity profiles for site 7.
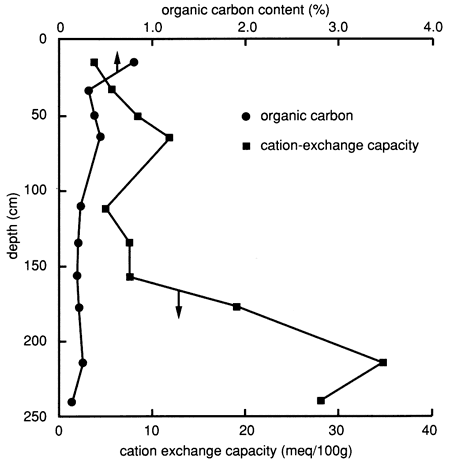
The bulk density of the soil profile, determined by the clod and core methods, shows relatively high compaction at the bottom of the upper 30 cm (1 ft) (fig. 31). The soil becomes less compact below this level until the silty clay soil is reached, at which point compaction increases again. The available water capacity of the soil profile at site 7 is shown in fig. 32; it is generally lower than that at site 6 (see fig. 8) because of the sandier nature of the soil profile, except where the silty clay soil is encountered at depth [below 180 cm (6 ft)]. The nature of the silty clay soil causes the available water capacity to increase significantly (fig. 32).
Figure 31--Soil bulk density distributions determined by the core and clod methods for site 7.
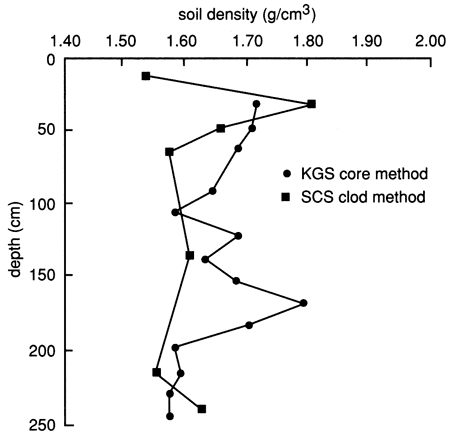
Figure 32--Available water-holding capacity of the soil profile at site 7.
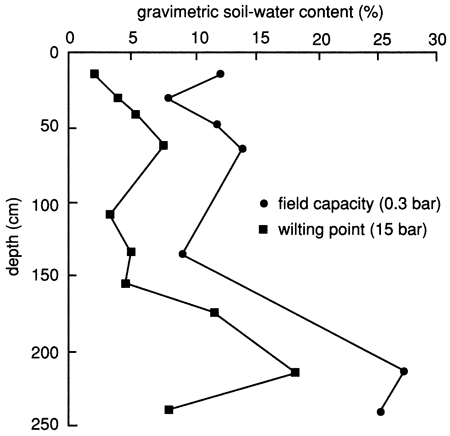
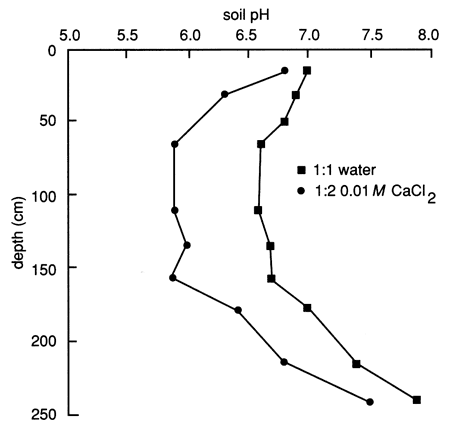
The pH distribution in the soil profile (fig. 33) has a narrower range (6.6-7.9 based on a 1:1 soil to water extract) compared with site 6 (5.9-9.0 based on a 1:1 soil to water extract). The pH decreases slightly with depth until the silty clay soil is encountered at depth, at which point the pH progressively changes from nearly neutral to slightly alkaline.
Figure 33--Soil solution pH profile for site 7.
The electrical conductivity of water extracted from saturated soil pastes and the exchangeable sodium percentage of the soil profile (fig. 34) are much less compared with site 6 (see fig. 10), indicating a much lower salt content in the soil profile of site 7. The nitrate and chloride depth distributions in the soil profile, as sampled from suction lysimeters before chemical flooding of the site, are shown in fig. 35. In general, there are high nitrate and chloride concentrations in the upper 30 cm (1 ft) of soil that progressively decrease with depth down to 90-120 cm (3-4 ft), beyond which they progressively increase with depth. The patterns of electrical conductivity and chloride concentration with depth are similar to each other at site 7, in contrast to the corresponding situation at site 6. Both sulfate and chloride concentrations in soil solutions from site 7 are appreciably lower than at site 6. As will be shown later, the higher hydraulic conductivity of the soil profile at site 7 in comparison with that at site 6 apparently does not allow as great a buildup of salts in the soil from evapotranspiration because the salts are more easily flushed by water recharge.
Figure 34--Electrical conductivity and exchangeable sodium percentage for the soil profile at site 7.
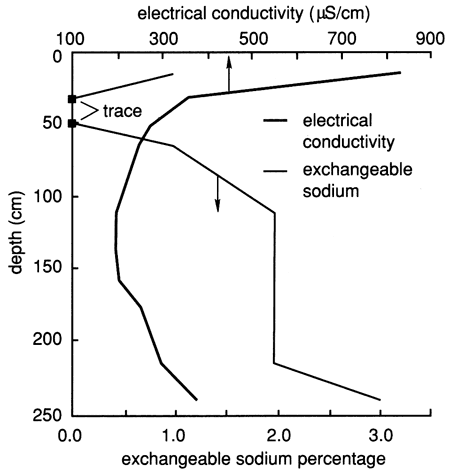
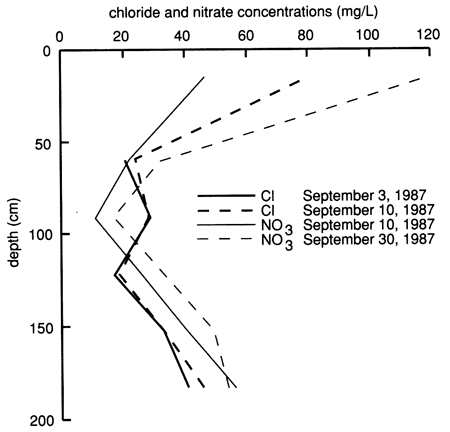
Figure 35--Profiles of chloride and nitrate concentrations from suction lysimeters before flooding at site 7.
Water chemistry
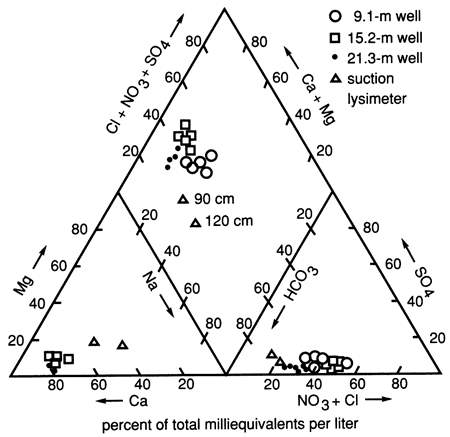
Site 7 in Pratt County is center-pivot irrigated and was used to grow com, wheat, and soybeans during this study. The soils are sandy and highly permeable and have low clay and organic carbon contents, as mentioned previously. The waters at the site are predominantly Ca-Mg-HCO3-Cl type (fig. 36), and the soil zone has less salt present than at site 6.
Figure 36--Trilinear diagram showing water chemistry data for three wells and two suction lysimeters at site 7.
The specific conductance, chloride, and nitrate time-series distributions showed an overall gradual decline during this study (figs. 37, 38, and 39). In all three figures there is a change in concentration approximately at the time of the flooding experiment. The declining trend may indicate that the shallow water table was reequilibrating to its background level.
Figure 37--Specific conductance time-series distribution of observation wells at site 7. Shallow water table is also shown.
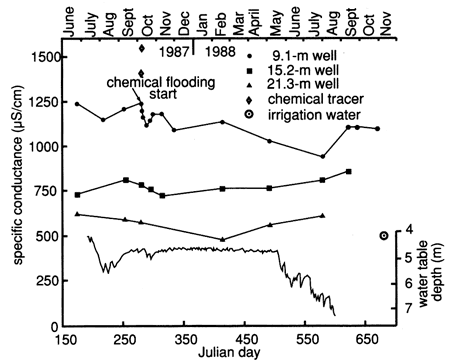
The results of the flooding test indicate that near-saturation to a depth of 400 cm (13 ft) occurred within 1 day (see next section). Application of 1000 gal (3.79 ml) of water may have caused a slight piston effect in that lower conductivity irrigation water (500 µS/cm) in the system may have been pushed down to the water table at 470 cm (15.5 ft) depth, causing the deflection seen in the 9.1-m (30-ft) well (fig. 37). This idea is substantiated by the movement of chloride through the soil zone, as will be seen in the next section. Within 5 days of flooding there was evidence that some of the higher-chloride-containing water, added during the chemical flood (specific conductance of 900 µS/cm) had moved to a depth of 100 cm (40 in.), whereas the chloride content decreased slightly at the 180-cm (6-ft) depth up to 8 days after flooding.
Figure 38--Chloride time-series distribution for observation wells at site 7. Shallow water table is also shown.
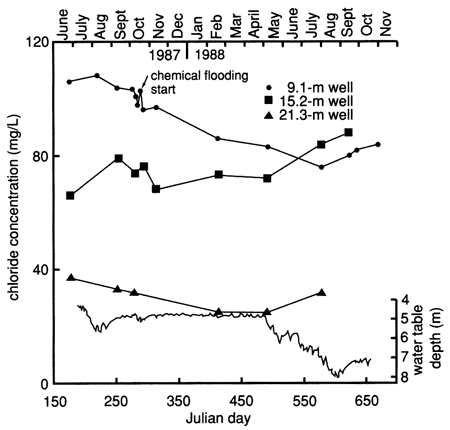
At site 7 nitrate was the inorganic constituent of most concern. The concentration of nitrate in all the observation wells reflects previous farming practices at the site and in the general vicinity of the site. There is little correlation between the irrigation at the site and the concentration found in the ground water. The increased nitrate concentration in the 9.1 m (30-ft) well at the time of the flooding experiment is probably related to a combination of macropore flow and piston-type flushing of preexisting nitrate in the deeper vadose zone. The fluctuation in the fall of 1987 may reflect application of nitrogen fertilizer in preparation for a new crop (see table C.5).
The nitrate data for the lysimeters (table C.2) show a close relationship among fertilizer application, irrigation, and movement of nitrates into the subsurface. There is a noticeable difference in concentrations between times of active irrigation and application of chemicals and times of irrigation with no chemical application (fig. 40 and table C.5). The extremely low values of nitrate noted in table C.2 for the period after September 14, 1988, are difficult to explain at present. Table C.5 shows that no fertilizer was applied after June 1988. Figure 39 shows that irrigation continued into September 1988, during a drought period. High nitrate concentrations at the 120-, 150-, and 180-cm (4-, 5-, and 6-ft) depths in August 1988 (table C.2) indicate how slowly nitrate moves through the soil profile. The low nitrate values in September 1988 may indicate utilization of the nitrate by soybeans (the crop planted at the time) or by bacteria (with a release of nitrogen gas), continued movement through the soil profile, or problems with the sampling apparatus. Additional sampling and analyses are required to elucidate these results.
Figure 39--Nitrate time-series distribution for observation wells at site 7. Shallow water table also is shown.
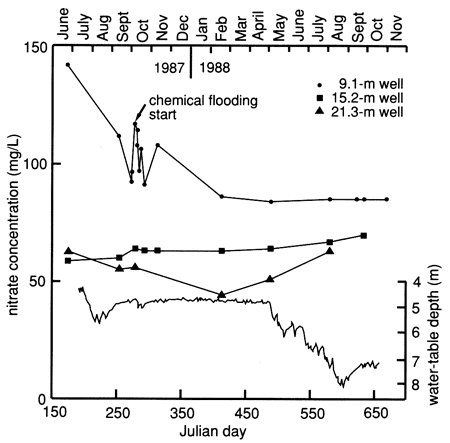
Figure 40--Nitrate concentration profiles from suction lysimeters during 1987 and 1988. Nitrogen fertilizer application dates are also indicated.
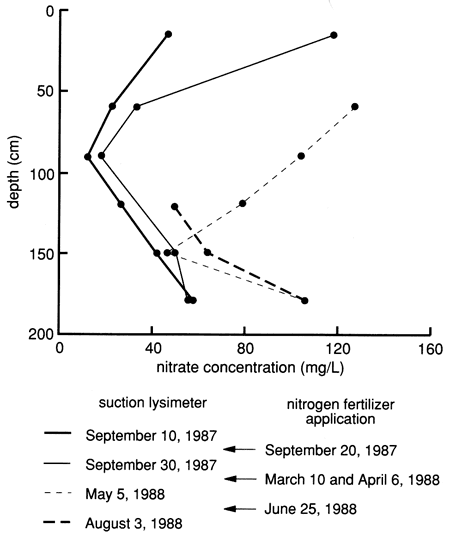
The source of most chloride and nitrate-nitrogen in an agricultural setting is usually from the concentration of salts in the soil zones resulting from evapotranspiration and from the fertilizers used on the field, respectively. Chloride is a conservative tracer because there are not many chemical processes that affect its movement through the vadose zone to the ground water. The Cl/NO3-N weight ratio is often used to estimate the role of denitrification for elimination of nitrate before the ion moves into the ground water (for references see the literature review on nitrate in appendix A). When the Cl/NO3-N ratio becomes large, denitrification processes usually explain the decrease in nitrate. A low ratio indicates that more nitrate than chloride is present and that more nitrate has moved through the vadose zone to the water table, causing an overall increase in ground-water nitrate concentration. During the study period, potassium chloride was added once (see table C.5). Other than dissolved salts in the soil zone, there was no additional source of chloride at this site.
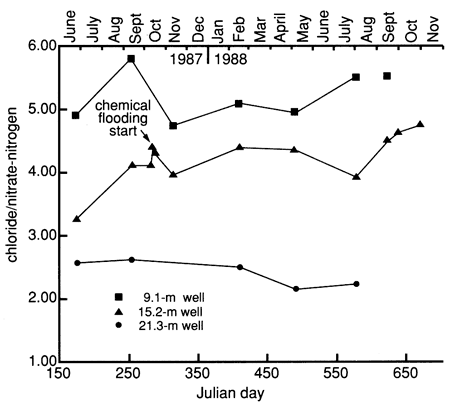
Figure 41 shows the Cl/NO3-N ratio for the wells at site 7. A comparison of the absolute concentrations of chloride and nitrate at the site 7 wells shows a general decrease in concentration with depth (table C.2). There is a clay zone that occurs between the 9.1- and 15.2-m (30- and 50-ft) wells that may slow the flow of water and hence promote stratification between these wells. The Cl/NO3-N ratio of 4.0-4.43 in the 9.1-m (30-ft) well may indicate rapid movement of chloride and nitrate into the subsurface with no possibility of denitrification occurring. The Cl/NO3-N ratio of 5.0 or greater, shown in the 15.2-m (50-ft) well (top line in fig. 41), indicates that nitrate and chloride are not moving into the lower part of the aquifer as quickly and that some of the nitrate is probably removed through denitrification. The ratio for the 21.3-m (70-ft) well is somewhat anomalous; it is lower than the ratio for the 9.1-in (30-ft) well. Examination of table C.2 shows that the 21.3-m (70-ft) well generally has lower chloride values than the other two wells and is similar to the irrigation well water [at 33.5 m (110 ft)] at the site. The Cl/NO3-N ratio for the irrigation well is 5.0 because of slightly higher chloride than nitrate concentrations (table C.2). The nitrate values in the 21.3-m (70-ft) well are similar to those of the 15.2-m (50-ft) well. The lower Cl/NO3-N ratio for the 21.3-m (70-ft) well may be due to the imposition of excess nitrate onto the background water quality at the site.
Figure 41--Chloride/nitrate-nitrogen ratio time-series distribution for observation wells at site 7.
Chemical flooding
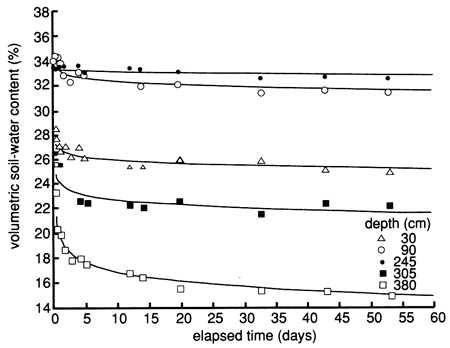
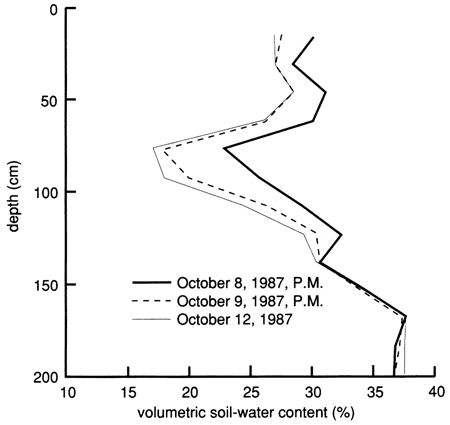
Because of the much sandier nature (and the expected higher hydraulic conductivity under wet conditions) of the soil profile at site 7 compared with site 6, the site was not flooded with Stafford municipal water before applying the chemical solution, as was done at site 6. Instead, 3.79 m3 (1000 gal) of chemical solution of the same composition as that used at site 6 was used to flood the site after prewetting the perimeter. The flooded area was nearly rectangular and enclosed 13.5 m2 (145 ft2) of surface. The flooding of the site started on October 7, 1987 (10:22 A.M.), and was completed by the next morning. The soil-moisture profile immediately before flooding and immediately after flooding is shown in fig. 42. The flooding caused wetting of the soil profile down to at least 3 80 cm (12.5 ft). The clayey layer from 165 cm to 240 cm (65-95 in.) was wet throughout the study period and did not show much change during flooding; the sandier soil layers both above and below the clayey layer showed large moisture changes during flooding and the subsequent drainage period. Soil-moisture drainage curves for 15-cm (6-in.) depth intervals since covering the site with plastic, shown in fig. 43, indicate soil-water drainage down to the 380-cm (150-in.) plotted depth. The clayey layer from 165 cm to 240 cm (70-95 in.) did not show any significant drainage.
Figure 42--Soil-water profiles before and during flooding at site 7.
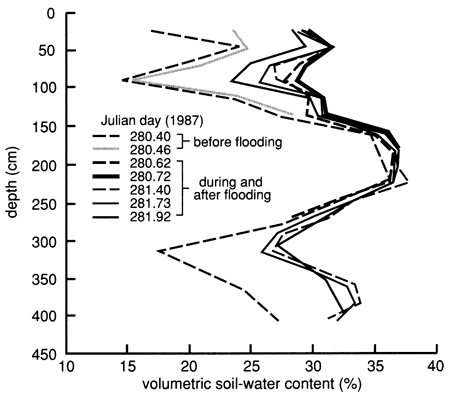
Figure 43--Soil-water content drainage curves for five depths at site 7. The curves were derived by means of a least-squares fit using a drying model.
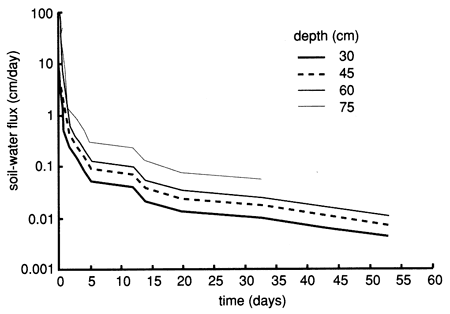
Figures 44, 45, and 46 show the instantaneous profile analysis results for the water characteristic curves, the hydraulic conductivity functions, and the Darcy water fluxes for various soil depths, respectively. The sandier nature of site 7 is reflected in the shape of the water characteristic curves (fig. 44), which show that the water content decreases much more with an increase in capillary pressure or suction than the more -clayey site 6. The sandier texture of site 7 is also reflected in higher saturated hydraulic conductivities compared with site 6 (fig. 45).
Figure 44--Field-measured water-retention curves for site 7.
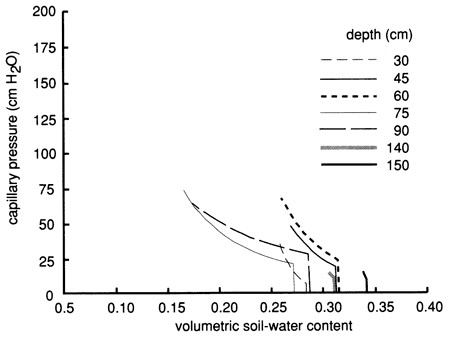
Figure 45--Hydraulic conductivity curves derived by means of the instantaneous profile technique for site 7.
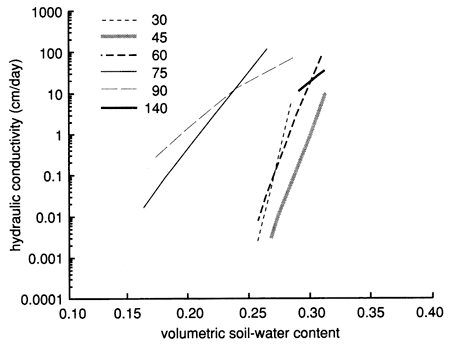
Figure 46--Soil-water flux time distributions (for 1987) derived by means of the instantaneous profile technique for site 7.
The chloride contents at all the soil depths sampled were appreciably less than the chloride concentration of the added chemical flood solution. The chemical solution was added in two 1.89-ml (500-gal) mixes. The first contained 103 mg/L chloride, and the second contained 140 mg/L chloride. The result is that the chloride in the chemical floodwaters acts as a minor tracer.
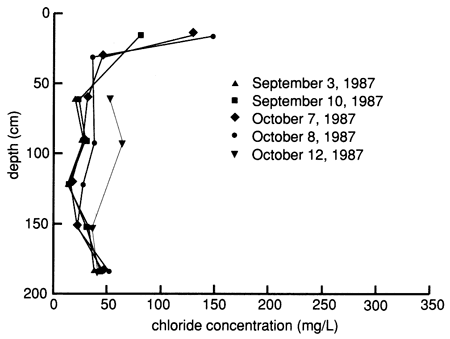
After the chemical flood solution was added, the chloride concentrations increased when the slug of actual floodwater reached the sampled depth (fig. 47). Chloride concentrations in samples from the shallowest lysimeter were above the chloride concentration in the floodwater, indicating dissolution of near-surface salts in the soil. In general, the deeper the lysimeter, the longer the time before the chloride concentration increased to a value near that in the flood solution. However, the rate of chloride increase resulting from the movement of floodwaters was not uniform with depth and time, suggesting that preferential flow through macropores and fractures in the soil occurred. The chloride concentration began to decrease in the shallowest lysimeter after several days and in the next shallowest lysimeter after several months. Later increases and decreases in chloride concentrations were probably related to changing conditions resulting from irrigation and evapotranspiration at the site. The results indicate that movement of the chemical floodwaters and later irrigation waters exerts more control over chloride concentration than displacement of saline soil solutions in the profile. The distribution of chloride from the suction lysimeters as a function of depth and time (fig. 48) further supports the lack of significant salt displacement, in contrast to what was observed at site 6.
Figure 47--Chloride time-series distribution from suction lysimeters before, during, and after flooding at site 7.
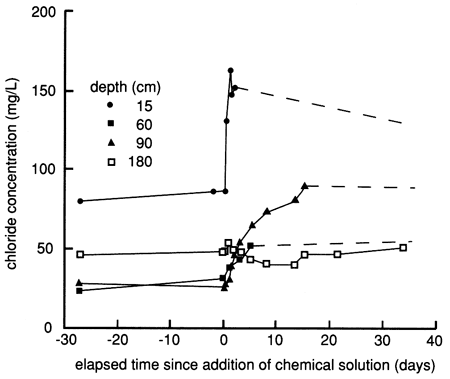
Figure 48--Profiles of dissolved chloride sampled from suction lysimeters before, during, and after flooding at site 7.
In addition, we observed no significant change in specific conductance and chloride content matching the chemical flooding in the samples from the wells at site 7 (see figs. 37 and 38). Changes in specific conductance and chloride just after flooding were of the order of variations observed at other times during the monitoring. The trends in the specific conductance and chloride content of ground water from the 9.14-m (30-ft) well actually decreased during the period from before flooding to about 1 year after flooding. Thus no substantial salt displacement occurred at this site in contrast to site 6. Apparently there was no appreciable accumulation of salts in the unsaturated zone between the section of soil sampled by the lysimeters and the water table. The greater permeability of the unsaturated zone and the lower dissolved solids concentration of the irrigation waters are probably the main reasons for the smaller salt accumulation at site 7 than at site 6.
Bromide
Bromide versus time breakthrough curves through the soil profile are shown in fig. 49. The bromide tracer concentrations for the two 1.89-m3 (500-gal) portions of the chemical floodwaters were 430 mg/L and 457 mg/L, giving an average bromide concentration of 444 mg/L. The added bromide penetrated to all site 7 soil depths sampled by the lysimeter within 1.3 days after flooding began, as indicated by bromide concentrations that were at least 50 times the background level. Sampling problems similar to those described for site 6 prevented a detailed set of bromide observations for determining tracer velocity (depth of tracer peak divided by sampling time) and decay.
Figure 49--Bromide concentration breakthrough curves based on suction lysimeter sampling for site 7. Bromide is expressed as a ratio of bromide corrected for background levels (Brc), over bromide tracer concentration in the flooding solution (Br0).
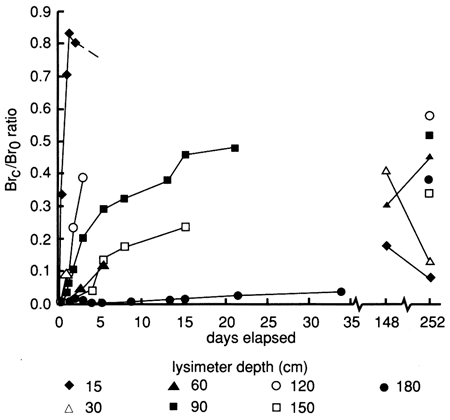
The bromide concentration distribution in the soil profile during the first few weeks after flooding shows a sigmoidal distribution with peaks at the uppermost lysimeter depth [15 cm (0.5 ft)] and at the 120-cm (4-ft) depth (fig. 50). Such a distribution indicates preferential flow, whereby diffuse flow through porous media is bypassed by flow through macropores, resulting in higher tracer concentrations at depth than normally expected. A plot of water content versus depth (fig. 51) has several peaks that approximately match the observed bromide peaks in fig. 50. This probably indicates that the increase in water content throughout the soil profile is due mainly to the applied water solution moving through the profile, not to displacement of much of the initial water.
Figure 50--Bromide concentration profiles since chemical flooding was initiated at site 7.
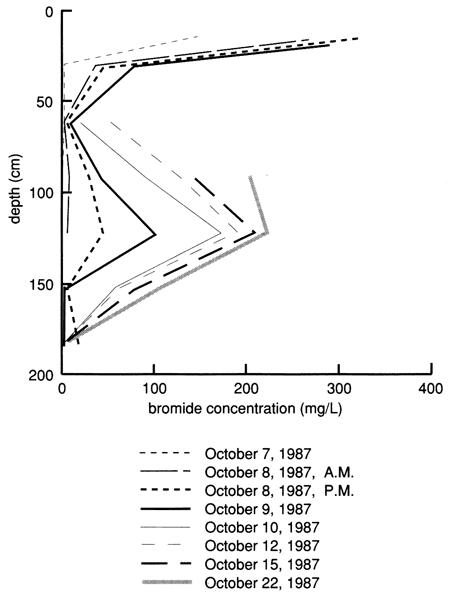
Figure 51--Soil-water content profiles since chemical flooding was initiated at site 7.
Bromide concentrations in water sampled by the shallowest lysimeter reached a peak two days after flooding (see fig. 49), whereas values for all the other depths continued to increase for several months. Maximum bromide contents were measured at 148 days for the 30-cm (1-ft) lysimeter depth and at 252 days for the 60-cm and 90-cm (2-ft and 3-ft) lysimeter depths. The time and bromide concentrations for the maximum peaks at these depths were probably not observed because of lysimeter sampling problems. Bromide values for samples from the lysimeters at greater depths generally were still increasing slowly after a year after chemical flooding, indicating the great persistence of soil solutions in the profile. The maximum tracer concentrations observed to date for all lysimeter depths would show a general persistence in the sigmoidal pattern of fig. 50 if plotted as bromide concentration versus depth. For example, the highest bromide concentration measured at the 120-cm (4-ft) depth was higher than that found for any of the other depths except the shallowest.
Although bromide tracer contents in solutions extracted from the lysimeter at the 180-cm (6-ft) depth had reached nearly 200 mg/L a year after flooding, no detectable tracer bromide had yet reached the shallowest screened interval [6.1-9.1 in (20-30 ft)] of the aquifer at the site. All bromide data are tabulated in tables C.2 and C.4.
Atrazine and atrazine metabolites
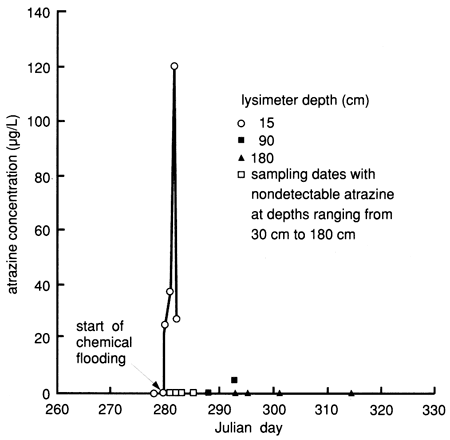
Many of the suction lysimeters did not work properly at site 7 most of the time. In addition to the reasons given in discussing the results of site 6, a vacuum could not be maintained at site 7 because of the porous, sandy nature of the soil. Only the 180-cm (6-ft) deep lysimeter completed in the immediate vicinity of the clayey layer was functional most of the time. Therefore we used soil core data to generate the atrazine breakthrough curves. Although the soil core data indicate the presence of atrazine within the soil profile down to the 120-135-cm (48-54-in.) sampled interval within the first 5 days after flooding, suction lysimeter sampling during that same time interval did not detect atrazine below the upper 15-cm (0.5-ft) depth interval (fig. 52). This may indicate that much of the atrazine movement bypassed the areas around the lysimeters, probably because of the reduced wetness around the porous cups caused by repeated attempts to induce a high vacuum (60-70 kPa) in the lysimeters or because of preferential flow through macropores, also indicated by the bromide data (see fig. 50).
Figure 52--Atrazine breakthrough curves for site 7 based on suction lysimeter sampling.
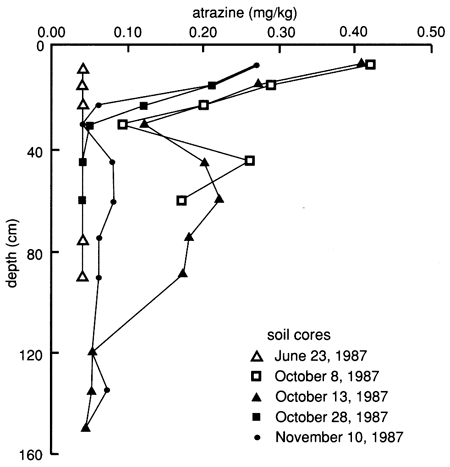
The soil core data exhibit extreme variability within the flooded area, and reliable breakthrough curves could not be ascertained (fig. 53). This spatial variability probably indicates that the applied water solution was not moving as a front through the soil horizons but was moving according to the spatial variability and soil-water transmission characteristics of the soil pores. However, one definitive observation is that atrazine penetrated to the 120-135-cm (48-54-in.) level within 5 days after flooding and no detectable atrazine was found at the 135-150-cm (54-60-in.) depth level. The vertical distribution of atrazine in the soil shows a sigmoidal bimodal distribution (fig. 54), with peaks at the 15-cm (0.5-ft) and 45-60-cm (1.5-2-ft) depths. This distribution supports evidence of preferential flow, discussed earlier. All atrazine data are tabulated in appendix D.
Figure 53--Atrazine breakthrough curves for site 7 based on soil cores. Detection limit is 0.04 mg/kg.
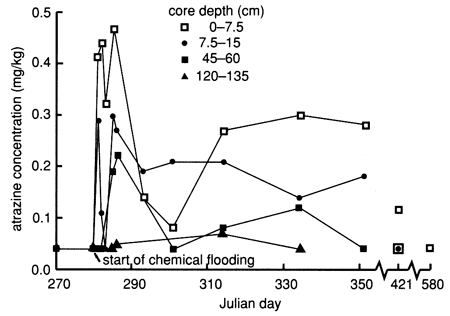
Figure 54--Atrazine profiles at site 7 based on soil cores.
To determine the possibility of atrazine hydrolysis at this site, we chemically analyzed two 15-cm (6-in.) long soil cores from the 60-75-cm (24-30-in.) and 75-90-cm (30-36-in.) depth intervals, sampled on February 24, 1988. The results of all atrazine degradation by-product analyses are tabulated in appendix E. Dealkylated by-products, indicative of biodegradation, were found below the detection limit (0.05 mg/kg), as was the case at site 6. The reported hydroxyatrazine concentrations were 0. 172 mg/kg and 0.408 mg/kg, respectively, and the reported parent atrazine concentrations were 0.056 mg/kg and <0.05 mg/kg (detection limit), respectively. This may indicate that hydrolysis of atrazine still takes place, even in soils with only slightly acidic conditions (see fig. 33), low organic matter contents, and low CEC (see fig. 30). As was done at site 6, additional soil samples collected in August 1988 were sent for atrazine metabolite analyses, especially to check for biodegradation by-products. The results showed appreciable amounts of both ethylatrazine and isopropylatrazine (appendix E), indicating microbial decomposition of atrazine.
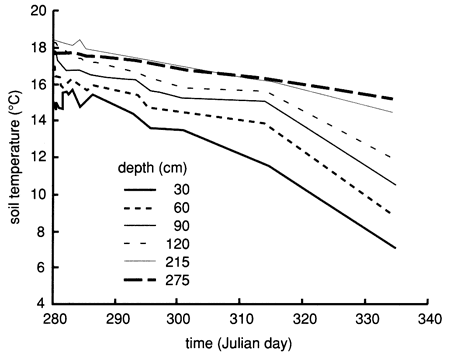
The soil profile temperatures during the flooding experiment (October-November 1987) are shown in fig. 55. The soil temperature increases with depth but decreases with time. However, the temporal temperature decreases at a lower rate compared to site 6 (see fig. 19).
Figure 55--Soil-temperature time-series distribution for six depths at site 7.
The sandy nature of the soil profile, the doubling of the amount of chemical flood solution, the much lower organic carbon content (see fig. 30), and the near neutral pH of the soil profile (see fig. 33), compared to site 6, are the apparent reasons for the deeper penetration of atrazine to the 120-135-cm (48-54-in.) level. It should also be noted that, similar to site 6, no atrazine was observed in the shallow or deeper observation wells of site 7.
Prev Page--Site 6 || Next Page--Site 10
Kansas Geological Survey, Geohydrology
Placed on web Aug. 23, 2010; originally published 1990.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/GW12/05_site7.html