Prev Page--Contents || Next Page--Installation
Statement of the problem
Recent incidents of chemical contamination of ground water by pesticides and other agricultural chemicals, such as nitrogen fertilizers, in surrounding states and in Kansas have increased concern for long-term environmental consequences of current agricultural practices on ground-water quality, especially in regard to the high toxicity and health implications of such agrichemicals on humans and animals. A recent epidemiologic study of occurrences of non-Hodgkin lymphoma (NHL) in Kansas found farm herbicide use to be highly correlated with NHL. The study suggests that NHL occurrence is associated with the number of days of exposure to herbicides per year (Hoar et al., 1986). A Kansas farmstead study (Steichen et al., 1988) indicated that 29 of 103 statistically representative samples collected statewide had nitrate levels above the maximum contaminant level for drinking water and 8 had detectable pesticides, with atrazine being the only pesticide detected more than once (present in 4 wells). However, the sources of these contaminants have not been determined. Studies in Iowa indicate an increase in statewide average nitrate concentration from 3 mg/L to 10.3 mg/L nitrate-nitrogen from the 1950's to 1983 (Hallberg, 1986b). Also, pesticides and elevated nitrate concentrations were present in all types of aquifer statewide in Iowa--glacial, alluvial, carbonate, and bedrock--indicating a non-point-source problem (Hallberg, 1985). Atrazine persisted in ground water year-round in both Iowa and Nebraska (Hallberg, 1985; Wehtje et al., 1983). Pesticides also were detected in public water supplies, particularly those in shallow alluvial systems and surface waters in Iowa and Ohio (Kelley and Wnuk, 1986; Baker et al., 1985).
Recently discovered incidences of ground-water pollution from agrichemicals throughout the United States and the resulting series of environmental laws regulating the use and disposal of chemicals on land surfaces have created a new surge of interest in field-scale solute transport studies. Table 1, adapted from the Council for Agricultural Science and Technology (CAST) (1985) report, summarizes some facts about the constituents of principal concern in croplands overlying significant ground-water aquifers. The transport of dissolved chemicals with percolating water has been studied for many years under controlled laboratory conditions but has received relatively little experimental study under field conditions until recently (Biggar and Nielsen, 1967; Boast, 1973). As a result, most chemical transport models developed over the last decade for use in field-scale transport simulations use laboratory results to formulate the transport equations (Anderson, 1979).
Table 1--Summary of prominent facts about the principal constituents of ground water underlying croplands.
| Constituent | Sources | Soil processes affecting the amount lost to ground water | Tendency to move downward through soils in percolating water | Comments |
|---|---|---|---|---|
| Soluble salts | Irrigation water Organic materials Fertilizers Weathering of minerals Rainfall Absorption and cation exchange |
Precipitation and dissolution of carbonates and sulfates Weathering of soil minerals |
High | Must be leached from most irrigated soils to maintain crop production |
| Nitrate Nitrogen fertilizers Organic materials Atmospheric nitrogen fixed by legumes Rainfall |
Production and removal by microorganisms Removal by plants |
High | Loss to ground water is incidental to water movement and is a loss to crop production potential | |
| Pesticides Commercial products Decomposition Volatilization |
Retention by soil Removal by plants |
Varies widely among pesticides and soils | Principal occurrences in ground water result from pesticides that remain in solution in the soil, are not decom- posed rapidly, and are applied to sandy soils with ground water near the surface and with much water movement | |
| Adapted from CAST (1985). | ||||
It is evident from the 1985 CAST report that our knowledge of the behavior of agrichemicals in the environment and specifically the mechanisms for moving to ground water is mostly qualitative. The retardation processes and factors are poorly understood. We designed the present study to address some of these problems.
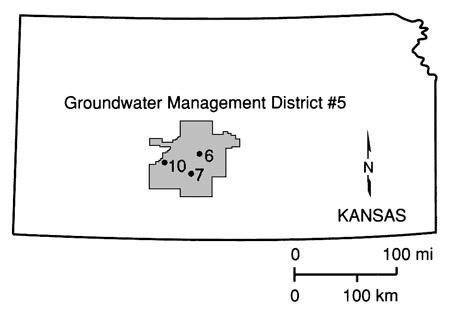
The Great Bend Prairie is a prime agricultural area in Kansas where agrichemicals have been used for decades. However, until recently, no study had been conducted in that area to assess the impact of these practices on the Great Bend aquifer, which yields water for irrigation, industrial, municipal, and domestic uses. [The recently completed phase of the chemigation well water quality study in Kansas by the Kansas State Board of Agriculture (Anderson, 1989) included a 1988 summer survey of 38 wells from the Big Bend Groundwater Management District No. 5 (GMD5), but no pesticide was detected from that area. Also, according to the low sample density farmstead study (Steichen et al., 1988), no pesticide was detected in south-central Kansas.] Water resources for most of the Great Bend Prairie are locally managed by the GMD5, whose goal is to maintain an adequate amount of good quality water for present and future needs (fig. 1). Recent studies that indicate aquifer pollution from agrichemicals in neighboring states with similar agricultural practices raised considerable concern in Kansas. Our initial conception was that the shallow aquifer of the region would either contain appreciable amounts of various agrichemicals, such as nitrogen fertilizer derivatives and atrazine, or the agrichemicals would be held "in transit" in the unsaturated zone on top of the shallow clay layers in the region if they were not present in the aquifer. This study was undertaken to provide some answers in this regard so that, if significant concentrations of pesticides are found in the soils and ground water, proper measures, such as improved nutrient, pesticide, and water management, can be encouraged.
Figure 1--Location of field sites.
Purpose and objectives
The purpose of this project is to determine the fate of agrichemicals commonly used in Kansas croplands in general and in the Great Bend Prairie in particular and to evaluate their potential to move through the soil profile into the underlying aquifer.
Atrazine is one of the most widely used herbicides in the Great Plains. According to the June 1987 issue of Chemical and Engineering News, 36 million kilograms (79 million pounds) of atrazine active ingredient are used annually for weed control on corn and sorghum crops and for nonselective weed control on grain and pasturelands. In Kansas atrazine is the most commonly used herbicide; nearly 2.5 million kilograms (5.5 million pounds) of atrazine or combinations including atrazine were applied to Kansas farmlands in 1978 (Nilson and Johnson, 1980). During the last decade, atrazine has been identified in ground water taken from aquifers underlying com-growing areas in the Great Plains. It is also the most commonly detected pesticide in municipal and domestic wells in Kansas and Nebraska. Therefore this herbicide was the obvious choice for further study.
The specific objectives of this project are (1) to measure and evaluate the movement of a routinely applied agrichemical in the soil profile, the herbicide atrazine, and its ability to reach the underlying freshwater aquifer; (2) to test the validity and predictive capabilities of existing numerical simulation approaches to subsurface organic chemical transport; and (3) to assess the relationships among land use, groundwater recharge, and movement of agrichemicals in the unsaturated zone and aquifer.
Related research
For a detailed literature review, the reader is referred to appendix A. A brief summary account of atrazine and nitrate is presented here.
Atrazine
The movement of atrazine and other herbicides depends on a variety of factors, for example, organic matter and clay content, pH of the soil and water, temperature, presence or absence of bacteria, and soil texture. Past studies have generally been conducted under laboratory conditions using repacked soil columns or small soil samples as medium. These studies are useful for demonstrating basic chemical processes that affect the movement of herbicide chemicals; however, they are not a substitute for the field situation. In a farm field other factors come into play, such as the presence or absence of routes of preferential flow (macropores caused by worms, plant roots, desiccation cracks, or small fractures), incompletely mixed parcels of water and chemical solutions, and slug and blob flow.
Degradation of atrazine strongly depends on the soil environment (Sheets, 1970). The physical characteristics of the soil (aeration, exposure to light, inorganic and organic nutrients, soil pH, quantity of clay and organic matter) play a major role in the chemical and biologic degradation of atrazine. Microbial degradation results in nitrogen dealkylation of atrazine side chains (see fig. A. 1) to produce deethylated atrazine and/or deisopropylated atrazine (Kaufman and Kearney, 1970). Microbial degradation is temperature and moisture dependent and increases with the amount of soil organic carbon and associated nutrients (Skipper and Volk, 1972), which are the major constituents of microbial food. Microbial community size in mineral soils has been directly related to organic matter content (Alexander, 1977). Chemical degradation occurs as a hydrolysis reaction at the number 2 carbon of the atrazine ring, producing hydroxyatrazine. Adsorption onto soil colloids is a prerequisite for hydrolysis (Armstrong et al., 1967). Atrazine adsorption onto clays and organic matter increases markedly as pH decreases below 6 (Bailey et al., 1968). Chemical hydrolysis of atrazine has been shown to be the predominant degradation route, especially at pH levels less than 6 (Skipper et al., 1978). Appendix A summarizes most aspects of atrazine degradation and movement in soils.
As the literature review (appendix A) indicates, there is much controversy over the movement of herbicides through the soil to ground water. Many states (e.g., Nebraska and Iowa) are finding the chemicals in their alluvial aquifers but are only beginning to evaluate the factors responsible for preventing or permitting the movement. Studies in Kansas indicate that atrazine enters surface waters through attachment to soil particles, which are carried by runoff water from fields (Gilliom et al., 1985). The occurrence of atrazine in ground water is limited, and the chemical is usually not detected unless there is a spill or some other mitigating circumstance.
Nitrate
Studies of the movement of nitrate in soils and ground water indicate that there is a growing problem throughout the United States. Much of the research on the occurrence and migration of nitrates through soil to ground water indicates that nitrate contamination in many areas is a non-point-source problem. Much of the work cited in appendix A suggests that fertilizer use in conjunction with excess precipitation and/or irrigation practices is a primary source of the nitrate. In several states, such as Nebraska and Iowa, the combination of well-drained sandy to silty soil conditions, fertilizer use, and irrigation has caused a widespread problem. Work in Kansas indicates that there is a trend toward increased nitrate concentrations in ground water.
Studies in Nebraska and California indicate that contamination from oxidation of nitrogen bound up in sediments also is probable. Sediment type, potential for oxidizing conditions, and quantity of organically bound nitrogen are the major controls on the formation and movement of nitrate from sediments.
The occurrence of nitrate in ground water is a potential public health problem and a potential irrigation problem for economical crop management. In addition, the occurrence of pesticides in ground water may mirror the nitrate problem. That nitrates move through the system may indicate that pesticides can also move under similar circumstances.
Site selection and instrumentation
We instrumented three field sites in the Great Bend Prairie to monitor the in situ processes involved in the movement and concentration of agrichemicals in soils and the underlying aquifers (see fig. 1). Two sites [site 6 (NE SW SE NW sec. 36, T. 23 S., R. 12 W.) and site 7 (NW NE SE NE sec. 11, T. 26 S., R. 14 W.); fig. 1], characterized by different soil types but used for growing the same crop (irrigated corn), were examined in detail. A third site [site 10 (SE SW SE SE sec. 1, T. 25 S., R. 19 S.); fig. 1], which is not cultivated (pastureland) and on which agrichemicals have never been used, was selected as the control (pristine) site.
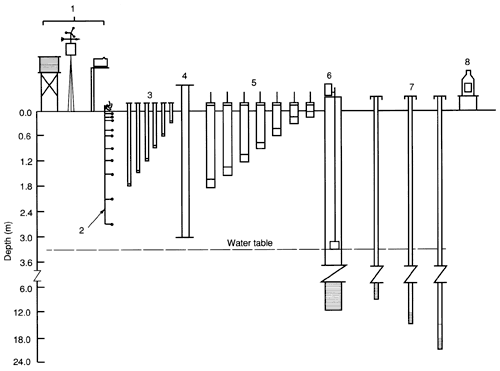
All three sites were outfitted with a number of observation wells screened at different depth intervals, a neutron-probe access tube for monitoring soil moisture, tensiometers at various depths, and a recording rain gauge. This instrumentation was the minimum necessary for characterizing soil-water movement (the agrichemical transporting vehicle) through the soil to the underlying aquifer. The two sites located in cornfields had additional instrumentation: a weather station, suction lysimeters installed at depths of 15, 30, 60, 90, 120, 150, and 180 cm (0.5, 1, 2, 3, 4, 5, and 6 ft), and 11 thermistors to measure soil temperatures from the near-surface to 275 cm (9.0 ft) below ground surface. Figure 2 illustrates the instrumentation at each site.
Figure 2--Schematic layout of site instrumentation. 1, Weather station; 2, thermistors; 3, tensiometers; 4, neutron-probe access tube; 5, suction lysimeters; 6, observation well; 7, piezometers; 8, recording rain gauge.
Methodology
Because of their chemical nature, organic and other chemicals interact with soil, water, and biota when applied to farmland. It is generally useful to study the behavior of both reactive and nonreactive or conservative chemicals, such as bromide, to contrast and elucidate this interactive behavior. Bromide is superior to other halogens as a tracer in soil-water systems (Jester and Uhler, 1974). Some of its advantages are its low background level in the environment and its low toxicity to plants and animals. Therefore we studied the simultaneous movement of both an organic chemical (atrazine) and a conservative inorganic tracer (bromide) by running flooding experiments at sites 6 and 7. The purpose of the flooding experiments was not necessarily to simulate actual field conditions (although flood irrigation may be reasonably simulated with our flooding experiments) but to evaluate the potential of atrazine to be leached into the water table under highly favorable conditions.
Because of the importance of the soil environment in controlling the movement and degradation of atrazine, a detailed study of the soil profiles of the selected sites was conducted before the flooding experiments. Trenches for detailed soil sampling and analysis were excavated to at least a 2-m (7-ft) depth next to the test sites. With the assistance of the Soil Conservation Service (SCS) Lincoln Laboratory, we determined the following soil- and water-related properties: detailed and complete soil description, textural analysis, organic carbon content, cation-exchange capacity (CEC), bulk density, water content at various applied pressures, extractable ions, soil pH, electrical conductivity, clay mineralogy, and various other physical and chemical soil characteristics.
The flooding experiments had a dual purpose: (1) to contrast the relative movement of a conservative tracer and an organic chemical in two different soil profiles and (2) to derive the hydraulic conductivity function of the soil profile through the instantaneous profile method.
We used the following procedure for the flooding experiments. The grassy area around the tensiometers, suction lysimeters, thermistors, and neutron-probe access tube was mowed as close to the ground as possible. Planks [244 x 40 cm (8 x 1.3 ft)] were connected with door hinges to form a hexagon (site 6) or a rectangle (site 7) around the site. The structure was secured with a 5-8-cm (2-3-in.) groove dug around the inner perimeter and wooden stakes placed outside the planks at the mowed site, forming a diked enclosure of 14-15 m2 (150-160 ft2) around the instrumentation and the shallowest observation well. The planks were covered with 3-5-mil-thick plastic sheeting. The outer perimeter of the diked area, which was also mowed, was wetted to reduce subsurface lateral fluid movement from the area to be flooded and covered with heavy black plastic. Then the diked area was flooded with the prepared chemical solution (site 7) or preflooded with municipal water from Stafford, Kansas, the nearest city, followed by the chemical solution (site 6). When the readings from the deepest tensiometers [150-180 cm (5-6 ft)] indicated near steady-state conditions, addition of the chemical solution to the diked area was stopped. At that time the chemical solution ponded to 5-10 cm (2-4 in.). This ponding head differential was due to the uneven or nonlevel surface of the flooded sites. As soon as the ponded solution infiltrated, the planks were removed and the flooded area was covered with heavy black plastic to prevent evapotranspiration and infiltration of rain.
Before, during, and especially immediately after covering the site with plastic, frequent instrument readings and sampling were conducted for a period of two to three months, as can be inferred from the various time-series plots and from appendixes C and D. Soil cores [2.5 cm (1 in.) in diameter and 30 cm (I ft) long] were collected periodically for measurement of atrazine concentrations. The surface 30-cm (1-ft) core was divided into four 7.5-cm (3-in.) core increments, and all the rest into 15-cm (6-in.) long increments for analysis. Field measurements of water conductance, temperature, and pH accompanied most water sampling. For logistic reasons, the flooding experiments were initiated on September 9, 1987, for site 6 and on October 7,1987, for site 7 after the corn had been harvested. The plastic cover was removed from both sites in December 1987, after snow had covered the sites.
Prev Page--Contents || Next Page--Installation
Kansas Geological Survey, Geohydrology
Placed on web Aug. 23, 2010; originally published 1990.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/GW12/02_prob.html