Water Resources of the Dakota Aquifer in Kansas
by Donald O. Whittemore, P. Allen Macfarlane, and Blake B. Wilson
Originally published in 2014 as Kansas Geological Survey Bulletin 260. This is, in general, the original text as published. An Acrobat PDF version (4 MB) is also available.
Acknowledgments
We are grateful for the help of several individuals at the Kansas Geological Survey in producing this document. Rogheyeh Eskrootchi assisted in the development of a data base for Dakota water chemistry. John Woods and Rogheyeh Eskrootchi helped in the development of the regional water-quality maps of the aquifer. Others who participated in mapping and data activities included student assistants Elizabeth Greive, Xiaodong Jian, and Brian Wardlow. Mark Schoneweis provided graphics for some of the figures. Tyan-ming Chu conducted his Ph.D. dissertation on hydrogeochemical processes in the Dakota aquifer, including numerical modeling of the evolution of groundwater geochemistry, especially as affected by cation exchange. He also participated in the collection of samples from water-supply wells in the Dakota aquifer. Roger Boeken determined resistivities from geophysical logs in northwest Kansas for estimating total dissolved solids concentration in the Dakota aquifer where there are no or very little sample data and used the study for his M.S. thesis. Lawrence Hathaway, Karmie Galle, Truman Waugh, and Masato Ueshima analyzed water samples collected by or sent to the KGS. Julie Tollefson edited and produced the final publication. Jim Butler reviewed and commented on a draft of this document. The authors appreciate the comments and edits of Scott Ross, retired, Division of Water Resources, Kansas Department of Agriculture, Susan Stover, Kansas Water Office, and Mark Rude, Southwest Kansas Groundwater Management District No. 3, who reviewed the current document, and also those of Robert Cullers, retired, Kansas State University, and Daniel Schurr, GeoSchurr Resources LLC, Ellsworth, Minnesota, who reviewed an earlier, expanded version of the geochemistry section.
Abstract
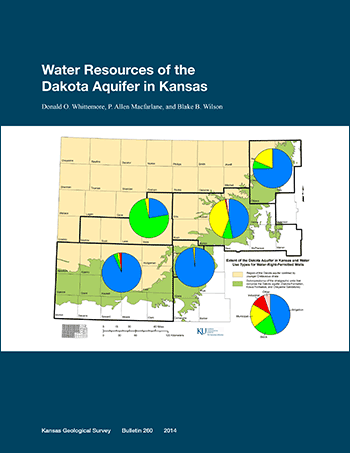
The Dakota aquifer system underlies most of the western two-thirds of Kansas and includes sandstone units in the Cretaceous Dakota, Kiowa, and Cheyenne Sandstone formations. The underlying Jurassic Morrison Formation in southwest Kansas is also considered by state statute to be part of the Dakota system. The Dakota aquifer has been developed as a water-supply source where the groundwater is fresh or only slightly saline and where other more easily obtained water supplies are not available. A total of 2,237 wells with active water rights and active uses made of water as of the end of 2011 were determined to produce greater than 5% of their total yield from the Dakota aquifer. Most of these wells are located where the Dakota aquifer underlies the High Plains aquifer (HPA) in southwest Kansas. In the 36 counties in which water-right-permitted wells pump partially or solely from the Dakota aquifer, the wells with Dakota yield are estimated to comprise 9% of the total of wells with water-right permits in all aquifers. Most (78%) of the water-right-permitted wells that draw part or all of their water from the Dakota aquifer are used for irrigation. Stock, municipal, and industrial wells comprise nearly all of the other uses (9.6%, 8.9%, and 2.2%, respectively, of the wells with some Dakota yield).
The mean annual volume of water used from the Dakota aquifer by water-right-permitted wells in Kansas is estimated to have been 117,000 acre-ft/yr (1.44 x 108 m3/yr) from 2006 to 2010. The use was greatest in southwest Kansas (approximately 86% of the total Dakota use). The mean annual use for other regions ranged from approximately 0.5% of the total Dakota use for west-central Kansas, to 2.4% for central, 2.9% for south-central, and 8.1% for north-central Kansas. Although Dakota water use in north-central Kansas was much lower than in southwest Kansas, the percent Dakota use relative to total use from all aquifers was the highest (nearly 20%) of all the regions. The percent Dakota use compared to total use from all aquifers for the other regions is 5.2% for southwest, 2.5% for central, 2.0% for south-central, and 0.4% for west-central Kansas. About 90% of the mean annual use from the Dakota aquifer during 2006-2010 was for irrigation, most of which was in southwest Kansas. For stock and municipal purposes, water usage was nearly 4% each of the total volume pumped from the Dakota aquifer. However, municipal demands accounted for 41% and 18% of the total use from the Dakota in central and north-central Kansas, respectively.
The total number of "domestic" wells, defined as those for which water-right permits are not required, that currently produce most or all of their water from the Dakota aquifer in Kansas is estimated to be more than 11,000 (about 8,000 for north-central and central Kansas and nearly 3,200 for south-central, west-central, and southwest Kansas). Water use from the Dakota aquifer by "domestic" wells is estimated to be 4,800 acre-ft/yr (5.9 x 106 m3/yr) in central Kansas, 1,500 acre-ft/yr (1.9 x 106 m3/yr) in north-central Kansas, and a total of 1,700 acre-ft/yr (2.1 x 106 m3/yr) in south-central, west-central, and southwest Kansas. The total "domestic" well use (about 8,000 acre-ft/yr) is about 6.4% of the approximately 125,000 acre-ft/yr (1.54 x 108 m3/yr) pumped from the Dakota aquifer by both permitted and "domestic" wells in Kansas.
The processes of mixing, reactive cation exchange, and mineral dissolution and precipitation have produced a complex range of chemical characteristics for groundwater in the Dakota aquifer. Water quality in the aquifer ranges from very fresh (<300 mg/L total dissolved solids [TDS]) to saltwater (>10,000 mg/L TDS). Freshwaters in the outcrop and subcrop portions of the Dakota aquifer in north-central and central Kansas are usually calcium-bicarbonate type waters. Calcium-sulfate type water in some regions can result from one of two processes: (1) weathering of pyrite in shales in Dakota strata and concomitant dissolution of calcite or dolomite and (2) recharge from upper Cretaceous strata that was affected by the same processes or by dissolution of gypsum. Large areas of the Dakota aquifer contain saline water (sodium-chloride type water) that was derived from the upward intrusion of saltwater from underlying Permian units, especially the Cedar Hills Sandstone in central and north-central Kansas. The saltwater is derived from the dissolution of evaporite deposits containing rock salt (halite) in the Permian. The salinity of groundwater in the Dakota aquifer generally increases with depth, particularly across substantial shale units of appreciable lateral extent that confine or separate aquifer units. Sodium-bicarbonate type water, which exists in parts of the confined Dakota aquifer in central and west-central Kansas, is generated by the flushing of saline water from the aquifer by groundwater recharge of calcium-bicarbonate or calcium-sulfate types. During this process, calcium (and magnesium) in the freshwater is exchanged for sodium on clays in Dakota strata. Fluoride concentrations increase in the sodium-bicarbonate water as a result of dissolution of calcium-containing fluoride minerals during the decrease in calcium in the groundwater caused by the exchange process.
Fluoride concentrations exceed the maximum contaminant level (MCL) of 4 mg/L for public drinking water supply in some areas of the confined Dakota aquifer. About 10% of the sample records for the Dakota aquifer exceed the MCL for arsenic and the action level for lead, although some of the high lead values could be related to lead in plumbing systems. Uranium concentration and the radioactivity from radium isotopes and alpha particles exceed the MCL for public drinking waters in a small percentage of Dakota groundwaters. Many other natural constituents and properties in Dakota waters exceed recommended or suggested levels for drinking water, such as TDS, chloride, sulfate, iron, manganese, and ammonium ion concentrations, especially in saline water in the confined aquifer and in groundwaters that have chemically reducing conditions. The main contaminant from anthropogenic activities in Dakota groundwater is nitrate. Nitrate-nitrogen concentrations exceeding the MCL of 10 mg/L primarily occur in shallow wells in the unconfined aquifer in central and north-central Kansas. The expected sources are animal and human waste and fertilizer that enter groundwaters by shallow recharge or through the annular space of poorly constructed wells.
Development of the Dakota aquifer has been dependent on both the hydrogeologic properties of the aquifer and the salinity of the groundwater. The Kansas Geological Survey has identified an area of nearly fresh to slightly saline waters in upper Dakota strata that could be important for future water supplies. The area is triangular in shape, with its base along the south lines of Sheridan and Graham counties and its northern extent into south-central Norton County. Another factor in aquifer development is the decline in the water table in the HPA where it overlies and is hydraulically connected to the Dakota aquifer in southwest Kansas. Many new wells have been completed in both the HPA and underlying Dakota strata. In cases in which the new construction is a replacement well, the previous well was often only completed in the HPA. Thus, the percentage of wells completed in both aquifers is increasing. Continued assessment of the water resources potential of the Dakota aquifer is especially needed in southwest Kansas but is difficult due to the very limited data for depth-to-water measurements in the Dakota in that area. A selected group of wells across the Dakota in southwestern Kansas should be equipped with continuous monitoring equipment so that a better understanding of the relationship between the Dakota and the overlying HPA can be obtained.
Kansas Geological Survey, Geohydrology
Placed on web Nov. 6, 2014; originally published Fall 2014.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/260/index.html