Prev Page--Geology || Next Page--Conclusions
Ground Water
Source, Occurrence, and Movement
The following discussion on the source, occurrence, and movement of ground water has been adapted from Meinzer (1923, p. 2-102), who gives a comprehensive treatment of the subject. Moore (1940) summarized ground-water conditions in Kansas, and detailed cooperative ground-water reports have been published by the State Geological Survey of Kansas for many counties in Kansas. Ground-water reports of nearby areas have been made by Lohman (1941), Davis and Carlson (1952), and Fischel (1948), including the Lawrence area, the Lawrence-Topeka area, and the Kansas City-Bonner Springs area, respectively.
Ground water is water that occupies open spaces or pores of rocks within the zone of saturation. The zone of saturation is the zone in rocks in which all pores or interstices are completely filled. The upper surface of the zone of saturation is called the water table, except where the zone intersects an impermeable bed. The water in the upper part of the zone of saturation is derived almost entirely from precipitation in the form of rain or snow. A large part of the water that falls on the surface is lost as surface runoff, which replenishes the streams. Part of the water is absorbed by vegetation and is returned to the atmosphere by transpiration. A small amount of the water percolates downward through the soil and rock until it reaches the zone of saturation.
Nearly all of the ground water in the Bonner Springs-Lawrence area consists of water that has fallen as rain or snow within or adjacent to the area. Heavily pumped areas, such as the valley flat near De Soto, may have a water table lower than the water surface of the river. Where such conditions prevail, the zone of saturation is replenished by river water. In some upland areas minor tributary streams lie at altitudes higher than the water table; if the underlying rock is permeable, water percolates to the water table from the channels of these streams.
The amount of ground-water in any region depends upon the character of the rocks. The porosity, or percentage of open space in a rock, determines the amount of water a rock can hold. The permeability of a rock is the ability of the rock to transmit water. An aquifer is a rock that has sufficient permeability to allow ground water to move rapidly enough and in sufficient quantities to supply water to wells. Many rocks that have a high porosity are not aquifers, because they have a low permeability. Permeability is determined by the size, shape, and arrangement of the pore spaces. As molecular attraction retains a thin layer of water on the surface of each grain, only rocks containing interconnected openings larger than the space occupied by this retained water are permeable. Thus beds of rock containing an appreciable percentage of fine silt or clay are not good aquifers. As the shape and arrangement of the openings also influence permeability, well-sorted sand, gravel, poorly cemented sandstone, and limestone containing abundant solution cavities provide the best sources for ground water. The degree of cementation within sandstone formations may be of considerable importance in delimiting bedrock aquifers in this area. This is suggested by the erratic nature of the Tonganoxie Sandstone as an aquifer. Although the Tonganoxie is generally a well-sorted friable sandstone where observed in outcrop in the area, information provided by local residents indicates that wells drilled into the Tonganoxie Sandstone yield water of variable quantity and nonuniform quality.
Ground-water movement is controlled by the shape of the water table. The water table in homogeneous materials is a subdued reflection of the topography. As most rock formations are not homogeneous, the water table generally has an irregular shape controlled by differences in permeability and rates of groundwater discharge. Records of typical wells in the area are compiled in Table 6. Figure 3 shows the configuration of the water table in Kansas River valley alluvium. The water table has been contoured at 5-foot contour intervals, and minor irregularities due to differences in permeability, recharge, or discharge generally are not indicated. As ground water moves in the direction of the greatest slope of the water table, the movement is at right angles to the contour lines shown on the map. The generalized contour map was based on information obtained during an abnormally dry year, during which time elevations of the river surface and of the ground-water table progressively declined. Therefore, the depths to water are probably at least 3 feet greater than would be expected during a year having normal precipitation. The river stage is represented on the map by uncorrected altitudes taken at different times during the winter of 1952 and may not represent true relationships that existed on November 1, 1952, the date to which all water-table altitudes have been corrected. Regardless of these abnormal relationships, the generalized contour map roughly shows the normal configuration of the water table. Kansas River is effluent; that is, it gains water from the water table, as is illustrated by the direction of movement perpendicular to the contour lines. Contours in the divide area between Kansas River and Wakarusa River show that both of these streams are effluent, movement being away from the interstream ground-water ridge and toward the rivers.
The movement of ground-water into Wakarusa River is very slow, and the slope of the water table is fairly steep (Geologic cross section A-A', Fig. 2) owing to the relatively impervious nature of the silts that constitute a significant thickness of surficial material. underlying the Newman Terrace.
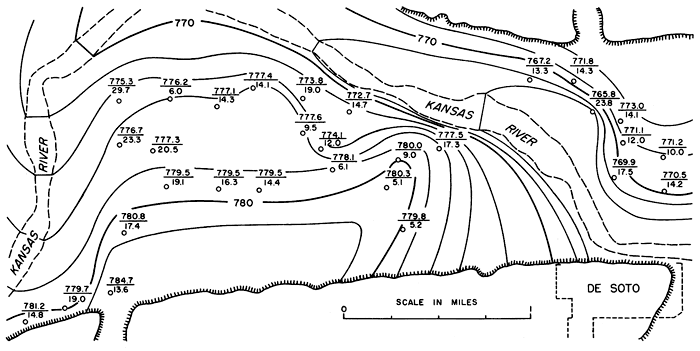
A detailed contour map of the water table in Kansas River valley alluvium in the De Soto area is shown in Figure 9. It has been prepared from information obtained from logs of test holes drilled during July and August 1942 for the Sunflower Ordnance Plant. Altitudes of the ground surface (surveyed to tenths or hundredths of a foot) and depth to water (measured to tenths of a foot or inches) were included with the logs. The contour interval of 2 feet makes possible a clearer observation of the configuration and movement of the water table in Kansas River valley alluvium. The movement of ground water primarily is toward the river. The configuration of the water table shows a greater tendency for movement at right angles to the valley bluff than downstream toward the east. This results in a greater gradient on the water table near the river at places where the course of the river is at right angles to the valley bluff. Discharge into the river at this position is greater than where the course of the river parallels the valley sides. Since 1942, 12 wells have been pumped heavily in the area, and the river in the De Soto area has been influent during periods of heavy pumpage.
Figure 9--Detailed contour map of water table in alluvium in part of Kansas River valley near De Soto during summer of 1942. Contour interval 2 feet. Number above line is altitude of water table, and number below line is depth to water below ground surface at test hole or well indicated by circle.
Recharge
Ground-water recharge is the addition of water to the aquifer. Water is added by the percolation of local precipitation, through infiltration from streams or ponds, and by subsurface inflow. The amount of recharge in the Bonner Springs-Lawrence area is variable, owing to the diversity of geologic formations. Areas underlain by shale, clay, or glacial till receive little recharge by the downward percolation of local precipitation, as such fine-grained materials are almost impervious. Kansas River valley alluvium in the mapped area offers excellent conditions for recharge by percolation of local precipitation.
The silts and sandy silts underlying the intermediate surface complex and the modern active floodplain allow large quantities of recharge by slow percolation from the surface to the water table. Newly formed scour pits and recently abandoned channels that have cut deep enough into the alluvium to truncate sand are excellent sites of recharge because much of the surface runoff is concentrated in these areas. Older features of similar origin generally have become filled with fine silts deposited by vertical accretion (the so-called "clay plugs"), and the efficiency of recharge is correspondingly reduced. The silty clay underlying the Newman Terrace is not favorable for recharge through additions of local precipitation, although small sandy areas of former natural levees of the Newman Terrace permit local increments of recharge.
The amount of recharge from ponds and streams in the area normally is small, as most streams are effluent. In upland areas having abundant stock ponds and intermittent streams, however, the water table may receive moderate recharge after rainstorms that follow prolonged periods of dryness. Of course, the floor of well-constructed stock ponds is composed of impervious material, making recharge from this source negligible.
One of the most important sources of recharge in Kansas River valley alluvium is the infiltration of river water to the ground-water body during periods of heavy pumpage in certain areas. This increases the amount of available ground water severalfold and helps to insure abundant supplies of water from wells in Kansas River valley alluvium.
Discharge
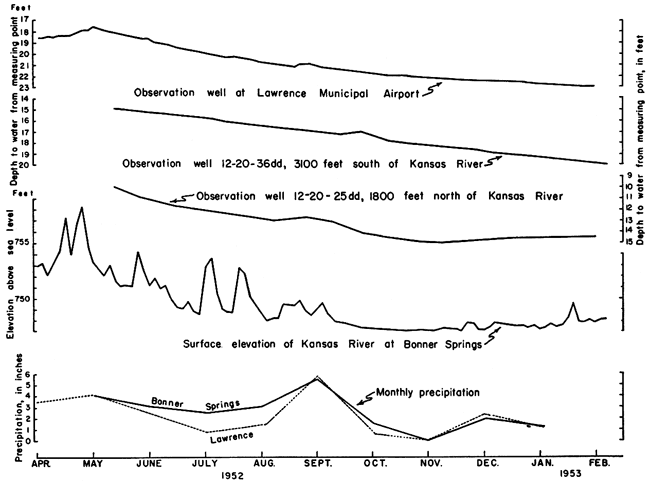
Ground-water is discharged by transpiration by plants whose roots tap the zone of saturation, by discharge into streams through bank seepage or springs, and by the pumpage of wells. Transpiration by plants probably accounts for little discharge in the area except after floods or during long periods of heavy precipitation when the water table may be near the surface in local areas. Discharge through springs is important in bedrock and Menoken Terrace deposits. Bank seepage in Kansas River valley alluvium provides the means for natural discharge from the underground reservoir to Kansas River. This is especially true after the valley has been flooded, when great quantities of ground water are locked up as bank storage. A comparison of the hydrographs of three observation wells in Kansas River valley alluvium with river stage fluctuations and monthly precipitation during the period from April 1952 to February 1953 shows a marked correlation between the progressive lowering of the water table and the gradual decrease in base flow of Kansas River (Fig. 10). Inasmuch as the area received subnormal precipitation during that period, the general decline in water table and river surface elevations must be attributed to the dissipation of bank storage after the 1951 flood. The significant amount of local precipitation during August 1952 is reflected in the hydrographs of all three observation wells by a slight rise in the water table after a short lag. The observation well closest to the river (12-20-25dd) is the only one whose hydrograph shows any tendency of the water table to reverse the downward trend. This is probably due to the combined influence of local precipitation and slightly rising river stage.
Figure 10--Graphs showing fluctuations of water level in observation wells and in Kansas River and monthly precipitation at Lawrence and Bonner Springs.
One of the principal means of ground-water discharge is through the pumpage of wells. The effect this discharge has upon the ground-water body of any aquifer depends upon the size, extent, and permeability of the aquifer and the quantity of water pumped from all wells penetrating it. Discharge from domestic and stock wells driven into Kansas River alluvium produces little effect upon that ground-water reservoir, whereas discharge from similar wells in bedrock or in discontinuous remnants of terrace deposits may exhaust the entire supply temporarily.
The total lowering of the static water table in the area during the period of investigation was more than 6 feet. A graphic illustration showing the nature of this phenomenon is at least partly recorded in the deepest scour pits excavated in Kansas River valley alluvium during the 1951 flood. Total fluctuations of the static water table in the alluvium during a normal year probably do not exceed 4 feet.
Utilization
Domestic and Stock
Three types of domestic and stock wells are found in the area: driven wells, drilled wells, and dug wells. Most older wells in upland areas or in tributary valley alluvium are shallow dug wells. Most of the more recent wells in Pennsylvanian bedrock are drilled. Nearly all of the domestic wells in Kansas River valley alluvium and a few in older terrace deposits are driven wells.
Most driven wells are constructed in unconsolidated deposits by augering to the first saturated sand layer and driving the sand point and pipe several feet into the water-bearing sand or gravel. Hand pumps may be installed in such a way that the cylinder is in a pit or buried a few feet below the ground surface. Many electrically operated pumps for shallow wells are placed in basements of houses. These practices place the cylinders as close to the water table as possible and prevent freezing during the winter. A few pitcher pumps are found in the area. The convenience and inexpensiveness of constructing driven wells makes them particularly attractive for domestic and stock use, where relatively small quantities of water are needed.
Drilled wells may be required where moderate supplies of ground water are desired for domestic and stock use. Both rotary and percussion methods of drilling have been employed to construct wells of various types. Most bedrock wells are open-end construction; casing is used only to prevent inflow of surface water, caving of higher wall materials, or seepage from higher undesirable aquifers. Most wells drilled in alluvium or terrace deposits are screened to prevent caving of the unconsolidated sand and gravel. If maximum efficiency is desired from a well drilled in almost any formation, gravel packing is an essential part of the construction, but probably few residents in the area need large enough quantities of water for domestic and stock use to make the added expense economically worthwhile.
Most of the dug wells were constructed before modern methods of well drilling came into general use. Most of the dug wells are shallow, but some have been dug to depths exceeding 47 feet. Diameters of dug wells range from 2 to 15 feet. Brick or limestone curbs line the walls, and mortar is used to seal the upper portion, preventing the inflow of surface water. The amount of ground water pumped for domestic and stock wells in this area is estimated at about 300,000 gallons per day.
Irrigation
Irrigation using ground water has not been extensive in this area even during recent years. Local residents report that an early irrigation project using ground water (a large potato farm near Bonner Springs) was abandoned several years ago. Large quantities of ground water in Kansas River valley alluvium are available for irrigation, as the construction of large wells becomes economically feasible for that purpose. The period of prolonged drought in the area beginning in 1952 emphasized the need for having good wells available for irrigation in preparation for future droughts.
Municipal
The four towns in the area obtain their entire city water supplies from gravel-packed wells in valley alluvium. Linwood provides its municipal supply from one well drilled in Stranger Creek valley alluvium, and each of the other towns has two wells in Kansas River valley alluvium.
After the 1951 flood two new wells were drilled on the north side of Kansas River for Bonner Springs. The old wells were located just south of Kansas River and are described by Fishel (1948). About 4,500,000 gallons of water per month is pumped from the two wells, which are equipped with electrically driven turbine pumps having a capacity of 500 gallons per minute. The wells are 85 and 90 feet deep, and the deeper one penetrates 46 feet of saturated sand and gravel. Chlorination is the only treatment that this water receives before entering the storage tank. Chemical analyses of water samples collected from these wells soon after drilling show dissolved solids of 603 and 534 parts per million and total hardness of 436 and 409 ppm. Neither contains much iron.
Pumping tests of both municipal wells at Bonner Springs were made soon after drilling was completed. The pumping test of the east well is shown in Table 4. The specific yields of the wells are 65 and 68 gallons per minute per foot of drawdown. The average specific yield of representative industrial and irrigation wells in Kansas River alluvium between Lawrence and Topeka was reported to be 53 gallons per minute per foot of drawdown (Davis and Carlson, 1952).
Table 4--Pumping test of the east municipal well of Bonner Springs, conducted by Layne-Western Company on September 6, 1951
| Time | Discharge, gallons a minute |
Pumping level | Drawdown | ||
|---|---|---|---|---|---|
| feet | inches | feet | inches | ||
| 11:00 a.m. | 0 | 23 | 2 | 0 | 0 |
| 12:00 noon | 457 | 29 | 11 | 6 | 9 |
| 12:30 p.m. | 457 | 30 | 0 | 6 | 10 |
| 1:00 | 457 | 30 | 1 | 6 | 11 |
| 1:30 | 453 | 30 | 1 | 6 | 11 |
| 2:00 | 453 | 30 | 1 | 6 | 11 |
| 2:30 | 453 | 30 | 2 | 7 | 0 |
| 3:00 | 453 | 30 | 2 | 7 | 0 |
| 3:30 | 457 | 30 | 2 | 7 | 0 |
| 4:00 | 457 | 30 | 3 | 7 | 1 |
| 4:30 | 457 | 30 | 3 | 7 | 1 |
| 5:00 | 457 | 30 | 4 | 7 | 2 |
| 5:30 | 200 | 26 | 10 | 3 | 8 |
| 6:00 | 300 | 28 | 2 | 4 | 0 |
| 6:30 | 400 | 29 | 10 | 6 | 8 |
| 7:00 | 500 | 32 | 0 | 9 | 10 |
| 7:05 | 0 | 24 | 7 | 1 | 5 |
| 7:10 | 0 | 24 | 0 | 10 | |
De Soto derives its water supply from two wells located near the mouth of Kill Creek valley. An average of 70,000 gallons per day is pumped from these wells. Electrically operated turbine pumps having a capacity of 115 gallons per minute pump water from these wells, which are 59 and 57 feet deep. The water is aerated and filtered through a hermetically sealed tank containing white limestone and a tank containing river gravel. Then it is softened by means of the zeolite process. The water is treated with chlorine and soda ash.
Eudora has two wells about 65 feet deep, which supply an average of 60,000 gallons per day for the city water supply. The Wells are pumped by electrically driven turbine pumps having capacities of 150 gallons per minute. This water is aerated in a settling basin, filtered through 4 feet of sand and gravel, and chlorinated. Iron content of a water sample from these wells is 9.5 ppm, and total hardness is 446 ppm.
Linwood receives its municipal supply from a well drilled after the flood in 1951. It is 66 feet deep and is cased with 8-inch iron pipe. An electrically operated turbine pump supplies about 200,000 gallons per month. The water is aerated and chlorinated.
A total of 287,000 gallons per day generally is pumped from all seven municipal wells in the area.
Industrial
The largest industrial supply of ground water in the area is that obtained from twelve gravel-packed wells owned by Sunflower Ordnance Plant near De Soto. These wells were drilled in Kansas River alluvium to depths ranging from 40 to 60 feet. The wells are used to supplant large quantities of water obtained from Kansas River; the average daily pumpage of well water is about 2,000,000 gallons. About 8,000,000 gallons per day of river water was being used in 1953. The water from wells generally has a total hardness of about 400 parts per million. Total hardness of river water is variable but averaged about 350 ppm during March 1953. Well water is aerated and softened to a total hardness of about 70 or 80 ppm. The slaked lime and soda ash process is used to soften the water. Finished well water is also subjected to the zeolite process. All water is chlorinated and stabilized. Water is used at the Sunflower Plant in boilers and for making high explosives.
The Lone Star Cement Company, at the extreme eastern edge of the area, pumps about 350,000 gallons per day of ground water from wells in Kansas River valley alluvium (Fishel, 1948). The combined use of ground water for industrial purposes in the mapped area is about 2,350,000 gallons per day. The total average utilization of ground water for all purposes in the area covered by this report is estimated to be about 3,000,000 gallons per day.
Quality
The quality of ground water in the area has been determined from chemical analyses of samples of water collected from 14 representative wells and one spring. Nine of these wells are distributed as uniformly as possible throughout the valley flat of Kansas River. The others are completed in typical upland water-bearing formations. Analyses of samples from municipal wells of four towns in the area are included in the discussion. All analyses were made by Howard Stoltenberg in the Water and Sewage Laboratory of the Kansas State Board of Health. Only dissolved mineral contents were determined. Analyses are tabulated in Table 5.
Table 5--Chemical analyses of water from 14 typical domestic wells, four municipal wells, and one spring in the Kansas River valley between Bonner Springs and Lawrence, Kansas
| Well location |
Depth, feet |
Geologic source |
Date collected |
Dissolved solids |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium (Na) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) |
Hardness as CaCO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Carbonates | Noncarbonates | |||||||||||||||
| 13-21-17dc | 0.0 | Meade Group | 2-24-1953 | 220 | 8.8 | 0.06 | 34 | 7 | 35 | 176 | 14 | 7 | 0.1 | 27 | 114 | 114 | 0 |
| 13-21-5db | 65 | Kansas River alluvium |
2-27-1950 | 990 | 23 | .58 | 240 | 16 | 49 | 417 | 250 | 100 | .1 | 35 | 665 | 342 | 323 |
| 13-20-11ba | 41 | Newman Terrace deposits |
12-29-1952 | 516 | 20 | .08 | 153 | 14 | 14 | 451 | 31 | 27 | .1 | 35 | 439 | 370 | 69 |
| 12-23-5ca | 36 | Kansas River alluvium |
2-7-1953 | 444 | 23 | 17 | 120 | 21 | 14 | 448 | 37 | 60 | .4 | 1.8 | 386 | 368 | 18 |
| 12-23-7ba | 47 | Kansas City Gr. | 2-7-1953 | 867 | 25 | .37 | 223 | 19 | 27 | 418 | 85 | 56 | .1 | 226 | 634 | 343 | 291 |
| 12-23-18cc | 28 | Kansas River alluvium |
2-7-1953 | 585 | 27 | .94 | 165 | 21 | 10 | 449 | 99 | 23 | .3 | 19 | 498 | 368 | 130 |
| 12-22-9cd | 55 | Stanton Ls. | 2-24-1953 | 412 | 9.8 | .31 | 100 | 15 | 26 | 304 | 84 | 12 | .3 | 15 | 311 | 250 | 61 |
| 12-22-9dc | 41 | Kansas Till | 2-24-1953 | 486 | 18 | .69 | 117 | 17 | 23 | 323 | 11 | 35 | .2 | 106 | 362 | 265 | 97 |
| 12-22-23ca | 35 | Kansas River alluvium |
2-1-1953 | 553 | 36 | .08 | 145 | 13 | 10 | 314 | 65 | 14 | .3 | 115 | 416 | 258 | 158 |
| 12-22-27bc | 58 | Kansas River alluvium |
2-27-1952 | 568 | 27 | 9.5 | 149 | 23 | 20 | 511 | 60 | 19 | .4 | 1.3 | 466 | 419 | 47 |
| 12-22-30aa | 29 | Kansas River alluvium |
1-3-1953 | 485 | 32 | .14 | 130 | 14 | 13 | 351 | 57 | 13 | .3 | 53 | 382 | 288 | 94 |
| 12-21-13cc | 66 | Stranger Creek alluvium |
5-15-1952 | 450 | 16 | 2.6 | 114 | 12 | 13 | 337 | 53 | 21 | .2 | .88 | 334 | 276 | 58 |
| 12-21-25bb | 28 | Kansas River alluvium |
2-1-1953 | 364 | 17 | 3.9 | 106 | 10 | 12 | 333 | 47 | 6 | .3 | 1.3 | 306 | 273 | 33 |
| 12-21-27dc | 22 | Kansas River alluvium |
12-29-1952 | 569 | 22 | .11 | 168 | 15 | 14 | 468 | 75 | 21 | .2 | 23 | 480 | 384 | 96 |
| 12-21-30cb | 27 | Meade Group | 2-7-1953 | 155 | 21 | .08 | 25 | 5.4 | 17 | 105 | 9.1 | 9 | .1 | 16 | 84 | 84 | 0 |
| 12-21-31dc | 18 | Kansas River alluvium |
12-29-1952 | 504 | 34 | .08 | 138 | 14 | 17 | 422 | 40 | 15 | .3 | 38 | 402 | 346 | 56 |
| 12-20-25aa | 108 | Tonganoxie Ss. | 2-19-1953 | 212 | 14 | .41 | 49 | 10 | 16 | 222 | 4.1 | 7 | .1 | 2.8 | 163 | 163 | 0 |
| 12-20-35dc | 25 | Kansas River alluvium |
12-29-1952 | 639 | 24 | 1.4 | 187 | 20 | 18 | 515 | 67 | 65 | .1 | 4.2 | 548 | 422 | 126 |
| 11-23-28cc | 85 | Kansas River alluvium |
9-6-1951 | 602 | 15 | .10 | 155 | 12 | 16 | 306 | 177 | 16 | .2 | 15 | 436 | 251 | 185 |
Dissolved Solids
Solids that are dissolved in natural water are left as a residue of mineral matter after the water has been evaporated. Some organic materials and a small amount of water of crystallization may be included in the residue. Water containing less than 500 ppm of dissolved solids is satisfactory for domestic use and most industrial purposes, except for difficulties that result from hardness or a possible excess of iron content. Water containing more than 1000 ppm of dissolved solids generally contains enough of certain constituents to produce a noticeable taste or to make the water unsuitable in other respects.
The dissolved solids in samples of water from municipal and private wells in this area ranged from 155 to 990 ppm. Water from 13 wells in the valley flat contained more than 364 ppm of dissolved solids and that from nine contained more than 500 ppm of dissolved solids. Three samples of water from Menoken Terrace deposits and Tonganoxie Sandstone contained less than 220 ppm of dissolved solids.
Hardness
The hardness of water is generally recognized by the amount of soap needed to produce a lather, and by the curdlike scum that forms before a permanent lather is obtained. Calcium and magnesium are the constituents that cause almost all of the hardness of ordinary water and are the active agents that form most of the scale in steam boilers and other containers in which water is heated or evaporated.
The tables of analyses show not only total hardness but also carbonate hardness and noncarbonate hardness. The presence of calcium and magnesium bicarbonates causes carbonate hardness. Inasmuch as boiling removes most of the calcium and magnesium bicarbonates, this type of hardness is called temporary hardness in some reports.The noncarbonate hardness is due to the presence of chlorides and sulfates of calcium and magnesium, which cannot be removed by boiling. This type of hardness has been called permanent hardness. Either type of hardness will produce similar reactions with soap, but the noncarbonate hardness usually forms a harder adhering scale in steam boilers.
Water that has hardness of less than 50 ppm generally is regarded as soft, and its treatment for the removal of hardness usually is not necessary. Water having hardness between 50 and 150 ppm generally is satisfactory for most purposes, but its treatment by a softening process is profitable for laundries or other industries that use large quantities of soap. Hardness in the upper part of this range produces considerable scale in steam boilers and should be reduced. Hardness of more than 150 ppm is apparent to anyone, and most water having a hardness in the ranges of 200 to 300 ppm is softened for domestic purposes or replaced by soft rain water collected in cisterns.
Water samples collected from 15 wells and 4 municipal water supplies ranged in hardness from 84 to 665 ppm. All water samples collected in the Kansas River valley flat had a hardness of more than 306 ppm; eleven samples contained less than 500 ppm and four had less than 400 ppm. A water sample from a spring in the Meade Group was the softest water analyzed; it had a hardness of only 84 ppm.
Iron
Iron is present in various amounts in most aquifers; the quantity of iron may differ greatly from place to place in the same formation. If more than about 0.2 ppm of iron is present, the excess iron in the water may separate as a reddish precipitate. The presence of iron is undesirable because it may stain containers or clothing and may give a disagreeable taste. Iron can be removed from most waters by aeration and filtration, although some water may require the addition of lime or some other substance.
Analyses of all but seven water samples listed in Table 5 show more than 0.2 ppm of iron. Iron content of water samples collected from wells in the Kansas River valley flat ranged from 0.08 to 17 ppm. The lowest iron content, however, was 0.06 ppm, from a water sample obtained from a well in the Meade Group.
Chloride
Water that contains less than 150 ppm of chloride is satisfactory for most uses, but that which has more than 350 ppm is objectionable for irrigation or industrial use. If water contains more than about 500 ppm, it has a disagreeable taste.
All the water samples analyzed contained 100 ppm or less of chloride. All but three samples contained less than 50 ppm of chloride; in 14 water samples it ranged from 6 to 23 ppm.
Fluoride
The fluoride content of most ground water is very small, but it is desirable to know the exact amount of fluoride in water that is used by children. Water that contains 1.5 ppm or more of fluoride is likely to produce mottled enamel on the teeth of children who drink the water during the formation of their permanent teeth. If the water contains as much as 4 ppm of fluoride, 90 percent of the children exposed are likely to have mottled enamel, and at least 35 percent of the cases will be classified as moderate or worse (Dean, 1936). Dean and others (1941) have found that small quantities of fluoride that are not sufficient to cause mottled enamel are actually beneficial in decreasing dental cavities.
The fluoride content of 15 water samples collected from domestic wells in this area ranged from 0.1 to 0.4 ppm. Of these samples, six contained 0.1 ppm, two contained 0.2 ppm, and six contained 0.3 ppm. The fluoride content of a water sample from one municipal water supply was 0.4 ppm.
Nitrate
A knowledge of the nitrate content of water is important because an unusually large amount of nitrate in well water used in the preparation of a baby's formula may cause cyanosis. The nitrate content differs greatly from well to well and seemingly is not related to any aquifer. The presence of large amounts of nitrate in well water may be due to the inflow of surface water into the well or to the movement of the water through soils that have been enriched in nitrate by certain plants, particularly legumes. Shallow wells that are not properly sealed and wells that do not have surface water properly cased off may yield water containing excessive nitrate.
Although all the water samples analyzed contained some nitrate, only three samples had more than 90 ppm, the lower limit of the range that the Kansas State Board of Health regards as likely to cause infant cyanosis. Four samples from municipal water supplies contained nitrate ranging from 0.08 to 35 ppm.
Prev Page--Geology || Next Page--Conclusions
Kansas Geological Survey, Geology
Placed on web March 29, 2012; originally published April 15, 1958.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/130_1/06_water.html