Prev Page--Irrigation, Availability || Next Page--Well Records
Chemical Character of Ground Water
The general chemical character of the ground waters in Pawnee Valley is indicated by the analyses of water from 70 wells distributed as uniformly as practicable within the area and among the principal water-bearing formations (Table 20). Table 20 includes the analyses of 13 water samples from wells in the Dakota formation, 21 water samples from wells in the Greenhorn limestone, 9 water samples from wells in terrace deposits, 26 water samples from wells in alluvium, and 1 water sample from a well in the Permian rocks. The samples of water were analyzed by Howard A. Stoltenberg, chemist, in the Water and Sewage Laboratory of the Kansas State Board of Health.
Chemical Constituents in Relation to Use
Dissolved solids
When water is evaporated the residue that is left consists mainly of the mineral constituents listed in Table 20 and generally includes a small quantity of organic material and a little water of crystallization. Water containing less than 500 parts per million of dissolved solids generally is entirely satisfactory for domestic use, except for difficulties resulting from the hardness or an excessive content of iron. Water containing more than 1,000 parts per million is likely to include enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respects.
The amount of dissolved solids in the samples of ground water collected in Pawnee Valley is indicated in Table 21. Of the 35 samples of water collected from wells in the alluvium and terraces the dissolved solids in 20 samples ranged between 300 and 400 parts per million. Only two samples from wells in the alluvium (wells 21-16-33cd and 22-16-4ca) contained more than 1,000 parts per million of dissolved solids.
Hardness
The hardness of a water is commonly recognized by the increased amount of soap needed to produce a lather and by the curdy precipitate that forms before a permanent lather is obtained. Calcium and magnesium compounds cause practically all the hardness of ordinary water, and they are also the active agents in the formation of the greater part of all the scale formed in steam boilers and in other vessels in which water is heated or evaporated.
Table 20--Analyses of water from typical wells in Pawnee Valley, Kansas. Analyzed by H. A. Stoltenberg. Dissolved constituents given in parts per million,a and in equivalents per millionb (in italics)
| Well Designation (c) |
Depth (feet) |
Geologic source |
Date of collection |
Temp. (°) F |
Dissolved solids |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium and potassium (Na+K) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) |
Hardness as CaCO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
Carbonate | Noncarbonate | ||||||||||||||||
| 20-19-26cc | 160 | Greenhorn | 8-24-1944 | 60 | 354 | 4.3 | 49 2.44 |
20 1.64 |
57 2.46 |
284 4.66 |
58 1.21 |
21 .59 |
1 .05 |
2.1 .03 |
204 | 204 | 0 | |
| 20-19-29dd | 44.7 | Greenhorn | 8-24-1944 | 59 | 409 | 4.7 | 116 5.79 |
11 .90 |
14 .62 |
340 5.58 |
14 .29 |
13 .37 |
.2 .01 |
66 1.06 |
334 | 279 | 55 | |
| 20-20-32cd | 110 | Dakota | 10-11-1944 | 59 | 972 | 5.8 | 45 2.25 |
28 2.80 |
267 11.60 |
379 6.22 |
322 6.70 |
107 3.02 |
2.4 .13 |
5.3 .08 |
228 | 228 | 0 | |
| 20-21-31ad | 37 | Alluvium | 6-10-1947 | 59 | 326 | 12 | 1.8 | 90 4.49 |
12 .99 |
13 .55 |
300 4.92 |
14 .29 |
16 .45 |
.5 .03 |
21 .34 |
274 | 246 | 28 |
| 20-22-9bb | 43.6 | Greenhorn | 6-10-1947 | 58 | 652 | 5.8 | 14 | 156 7.78 |
20 1.64 |
37 1.59 |
290 4.76 |
218 4.53 |
42 1.18 |
1.4 .07 |
29 .47 |
471 | 238 | 233 |
| 20-22-9dd | 27.3 | Greenhorn | 6-10-1947 | 58 | 396 | 18 | .08 | 109 5.44 |
8.6 .71 |
12 .51 |
279 4.58 |
15 .31 |
16 .45 |
.6 .03 |
80 1.29 |
308 | 229 | 79 |
| 20-22-20ca | 48.4 | Alluvium | 7-6-1946 | 58 | 386 | 31 | .06 | 89 4.44 |
12 .99 |
28 1.23 |
301 4.94 |
54 1.12 |
16 .45 |
.4 .02 |
8 .13 |
272 | 247 | 25 |
| 20-22-24de | 38.8 | Terrace deposits | 8-12-1946 | 58 | 324 | 22 | .03 | 86 4.29 |
9.2 .76 |
16 .72 |
289 4.74 |
22 .46 |
11 .81 |
.3 .02 |
15 .24 |
252 | 237 | 15 |
| 20-22-27bb | 28.5 | Alluvium | 6-10-1947 | 57 | 365 | 20 | .84 | 92 4.59 |
13 1.07 |
22 .96 |
315 5.17 |
40 .83 |
19 .54 |
.6 .03 |
3 .05 |
283 | 258 | 25 |
| 20-23-14aa | 28.9 | Greenhorn | 6-10-1947 | 57 | 564 | 9.6 | .68 | 125 6.24 |
15 1.23 |
45 1.97 |
312 5.12 |
80 1.66 |
38 1.07 |
.6 .03 |
97 1.56 |
374 | 256 | 118 |
| 20-23-20ad | 21.7 | Greenhorn | 6-10-1947 | 55 | 48 | 15 | 9.7 | 136 6.79 |
84 .69 |
14 .61 |
322 5.28 |
27 .56 |
16 .45 |
.2 .01 |
111 1.79 |
374 | 264 | 110 |
| 2 0-23-22dc | 34.6 | Greenhorn | 8-12-1946 | 57 | 385 | 15 | .05 | 108 5.39 |
7.4 .61 |
14 .61 |
295 4.84 |
17 .85 |
12 .34 |
.3 .02 |
66 1.06 |
300 | 4 | |
| 20-23-25db | 57.6 | Alluvium | 7-5-1946 | 58 | 359 | 33 | .08 | 86 4.29 |
13 1.07 |
21 .90 |
310 5.08 |
33 .69 |
14 .39 |
.3 .02 |
5.3 .08 |
268 | 254 | 14 |
| 20-23-32ca | 69.6 | Alluvium | 8- 3-1946 | 58 | 324 | 30 | .11 | 80 3.99 |
10 .82 |
20 .85 |
288 4.72 |
24 .50 |
12 .34 |
.3 .02 |
5.3 .08 |
240 | 236 | 4 |
| 20-23-36bb | 42 | Greenhorn | 6-10-1947 | 59 | 341 | 16 | .05 | 92 4.59 |
8.8 .72 |
17 .76 |
287 4.71 |
26 .54 |
13 .37 |
.4 .02 |
27 .43 |
266 | 236 | 30 |
| 21-16-7dd | 38.8 | Terrace deposits | 10- 7-1944 | 58 | 551 | 2.7 | 89 4.44 |
14 1.15 |
102 4.44 |
299 4.90 |
27 .56 |
155 4.37 |
.3 .02 |
11 .18 |
280 | 245 | 35 | |
| 21-16-33cd | 70 | Alluvium | 8-11-1945 | 1,480 | 0 | 178 8.88 |
54 4.44 |
201 8.73 |
254 4.17 |
726 15.10 |
94 2.65 |
0 .00 |
4.9 .08 |
666 | 208 | 458 | ||
| 21-17-18dd | 42.5 | Terrace deposits | 10-10-1944 | 58 | 295 | 2.5 | 76 3.79 |
9.8 .81 |
25 1.10 |
311 5.10 |
7.4 .15 |
10 .28 |
.5 .03 |
8.4 .14 |
230 | 230 | 0 | |
| 21-18-5ad | 100 | Terrace deposits | 10-13-1944 | 59 | 287 | 13 | 75 3.74 |
11 .90 |
19 .83 |
317 5.20 |
0 .00 |
6 .17 |
.9 .05 |
3.2 .05 |
232 | 232 | 0 | |
| 21-18-17cc | 120 | Alluvium | 6-21-1945 | 272 | 10 | 74 3.69 |
14 1.15 |
13 .56 |
288 4.72 |
11 .23 |
12 .34 |
.5 .03 |
5.3 .08 |
242 | 236 | 6 | ||
| 21-18-29bb | 110 | Alluvium | 6-21-1945 | 276 | 9.4 | 76 8.79 |
14 1.15 |
13 .57 |
300 4.92 |
8.2 .17 |
11 .31 |
.5 .03 |
5.3 .08 |
247 | 246 | 1 | ||
| 21-18-32bb1 | 418 | Permian redbeds | 6-22-1945 | 8,830 | 6.1 | 628 31.34 |
284 28.34 |
2,049 89.10 |
116 1.90 |
2,990 62.21 |
2,820 79.52 |
1.8 .09 |
3.5 .06 |
2,730 | 95 | 2,640 | ||
| 21-18-32bb2 | 306 | Dakota (?) | 6-13-1945 | 423 | 17 | 56 2.79 |
17 1.40 |
86 3.73 |
293 4.80 |
37 .77 |
80 2.26 |
1.1 .06 |
2 .03 |
210 | 210 | 0 | ||
| 21-18-32cc | 120 | Alluvium | 9-25-1944 | 370 | .08 | 74 3.69 |
15 1.23 |
49 2.13 |
324 5.31 |
32 .66 |
36 1.02 |
.8 .04 |
1.4 .02 |
246 | 246 | 0 | ||
| 21-19-27cb | 42 | Alluvium | 8-24-1944 | 59 | 319 | .52 | 80 3.99 |
14 1.15 |
26 1.15 |
340 5.58 |
13 .27 |
13 .37 |
.7 .04 |
2 .03 |
257 | 257 | 0 | |
| 21-20-15da | 45 | Alluvium | 9-25-1944 | 58 | 403 | 24 | 122 6.09 |
14 .15 |
11 .49 |
405 6.64 |
19 .40 |
11 .31 |
.2 .01 |
23 .37 |
362 | 332 | 30 | |
| 21-20-20ba1 | 55 | Alluvium | 9-21-1944 | 60 | 331 | .05 | 80 3.99 |
113 1.07 |
28 1.21 |
299 4.90 |
38 .79 |
17 .48 |
.6 .03 |
4.4 .07 |
253 | 245 | 8 | |
| 21-20-28ed | 126 | Dakota | 8-24-1944 | 60 | 369 | 1.6 | 74 3.69 |
25 2.06 |
30 1.31 |
327 5.36 |
56 1.16 |
16 .45 |
1.1 .06 |
2.1 .03 |
288 | 268 | 20 | |
| 21-21-9ed | 58.7 | Terrace deposits | 6-10-1947 | 58 | 356 | 11 | 3.3 | 72 3.59 |
11 .90 |
46 1.98 |
298 4.89 |
42 .87 |
20 .56 |
1 .05 |
6.2 .10 |
224 | 224 | 0d |
| 21-21-18aa | 54.6 | Alluvium | 8-3-1946 | 58 | 414 | 26 | .15 | 97 4.84 |
15 1.23 |
31 1.35 |
356 5.84 |
52 1.08 |
16 .45 |
.4 .02 |
2 .03 |
304 | 292 | 12 |
| 21-21-22da | 51.5 | Alluvium | 6-10-1947 | 58 | 407 | 21 | .30 | 92 4.59 |
14 1.15 |
37 1.62 |
357 5,85 |
50 1.04 |
15 .42 |
.5 .03 |
1.4 .02 |
287 | 287 | 0e |
| 21-21-36ab | 93.5 | Alluvium | 8-2-1946 | 58 | 395 | 25 | .23 | 71 3.54 |
13 1.07 |
55 2.41 |
332 5.44 |
42 .87 |
23 .65 |
.8 .04 |
1.1 .02 |
230 | 230 | 0f |
| 21-22-3ab | 62.7 | Alluvium | 7-1-1946 | 58 | 379 | 31 | .48 | 94 4.69 |
11 .90 |
24 1.04 |
321 5.26 |
40 .83 |
16 .45 |
.3 .02 |
4.3 .07 |
280 | 263 | 17 |
| 21-22-4dd | 30.7 | Greenhorn | 6-10-1947 | 57 | 380 | 17 | 11 | 99 4.94 |
13 1.07 |
25 1.09 |
359 5,89 |
18 .37 |
27 .76 |
.3 .02 |
4 .06 |
300 | 294 | 6 |
| 21-22-8bb | 35.7 | Greenhorn | 6-10-1947 | 57 | 329 | 11 | 27 | 78 3.89 |
8.9 .73 |
27 1.17 |
259 4.25 |
27 .56 |
13 .37 |
.5 .03 |
36 .58 |
231 | 212 | 19 |
| 21-22-15ac | 35.2 | Greenhorn | 6-10-1947 | 57 | 397 | 16 | .11 | 124 6.19 |
8 .66 |
9.2 .40 |
372 6.10 |
18 .37 |
12 .34 |
.2 .01 |
27 .43 |
342 | 305 | 37 |
| 21-22-27cb | 285 | Dakota | 8-12-1946 | 61 | 622 | 8 | .32 | 50 2.50 |
16 1.82 |
156 6.80 |
309 5.07 |
170 3.54 |
67 1.89 |
2 .10 |
1.5 .02 |
191 | 191 | 0g |
| 22-16-4ca | 12 | Alluvium | 10-6-1944 | 59 | 1,020 | .46 | 188 9.28 |
40 3.29 |
90 3.90 |
295 4.84 |
501 10.42 |
37 1.04 |
1 .05 |
14 .22 |
634 | 242 | 392 | |
| 22-17-5ca | Alluvium | 10-10-1944 | 59 | 795 | 7 | 128 6.39 |
28 2.30 |
128 5.58 |
418 6,86 |
118 2.45 |
171 4.82 |
.5 .02 |
5.3 .08 |
434 | 343 | 91 | ||
| 22-17-11dd | 20 | Alluvium | 8-23-1944 | 58 | 981 | .72 | 169 8.43 |
34 2.79 |
102 4.44 |
239 3.92 |
492 10.23 |
38 1.07 |
.7 .04 |
25 1.40 |
561 | 196 | 365 | |
| 22-17-19cb | 215 | Dakota | 8-23-1944 | 60 | 1,480 | .58 | 42 2.10 |
26 2.14 |
491 21.36 |
337 5.53 |
129 2.68 |
610 17.20 |
2 .10 |
5.8 .09 |
212 | 212 | 0 | |
| 22-18-9ce | 26 | Alluvium | 10-11-1944 | 58 | 316 | .16 | 88 4.39 |
12 .99 |
18 .77 |
331 5.43 |
7 .15 |
12 .34 |
.3 .02 |
13 .21 |
269 | 269 | 0 | |
| 22-18-28cc | 82.5 | Terrace deposits | 10-10-1944 | 59 | 391 | 6.7 | 103 5.14 |
17 1.40 |
16 .69 |
300 4.92 |
12 .25 |
54 1.52 |
.3 .02 |
32 .52 |
327 | 246 | 81 | |
| 22-18-35dd | 56.5 | Terrace deposits | 10-7-1944 | 59 | 455 | 20 | 116 5.79 |
18 1.48 |
29 1.27 |
454 7.44 |
15 .31 |
25 .70 |
.4 .02 |
4.2 .07 |
364 | 364 | 0 | |
| 22-19-8dd | 40.5 | Terrace deposits | 10-9-1944 | 60 | 314 | .30 | 66 3.29 |
13 1.07 |
38 1.66 |
290 4.76 |
18 .37 |
26 .73 |
1 .05 |
7.1 .11 |
218 | 218 | 0 | |
| 22-19-29cc | 100 | Dakota | 10-11-1944 | 59 | 527 | 34 | 42 2.10 |
22 1.81 |
118 5.13 |
351 5.76 |
76 1.58 |
54 1.52 |
2.6 .14 |
2.5 .04 |
196 | 196 | 0 | |
| 22-20-4bb | 210 | Dakota | 10-9-1944 | 60 | 361 | 5.5 | 64 3.19 |
27 2.22 |
32 1.40 |
310 5.08 |
63 1.31 |
11 .31 |
1.4 .07 |
2.3 .04 |
270 | 254 | 16 | |
| 22-20-29ba | 28.2 | Terrace deposits | 10-9-1944 | 58 | 348 | .12 | 102 5.09 |
7.2 .59 |
15 .67 |
300 4.92 |
14 .29 |
15 .42 |
44 .71 |
.2 .01 |
284 | 246 | 38 | |
| 22-21-9cc | 67.4 | Alluvium | 8-2-1946 | 58 | 343 | 21 | 0 | 85 4.24 |
13 1.07 |
21 .92 |
300 4.92 |
30 .62 |
22 .62 |
.5 .03 |
2.7 .04 |
266 | 246 | 20 |
| 22-21-10cb | 100 | Alluvium | 6-11-1947 | 58 | 315 | 4.6 | 6.9 | 96 4.79 |
9.4 .77 |
12 .51 |
303 4.97 |
19 .40 |
23 .65 |
.3 .02 |
2.1 .03 |
278 | 248 | 30 |
| 22-21-12ab | 70.4 | Terrace deposits | 8-11-1946 | 59 | 343 | 7 | .27 | 85 4.24 |
22 1.81 |
14 .61 |
327 5.36 |
33 .69 |
18 .51 |
1.1 .06 |
2.2 .04 |
302 | 268 | 34 |
| 22-21-14aa | 67.2 | Dakota | 6-11-1947 | 58 | 240 | 5.6 | 6.3 | 60 2.99 |
10 .82 |
16 .68 |
227 3.72 |
4.1 .08 |
12 .34 |
.5 .03 |
20 .32 |
190 | 186 | 4 |
| 22-21-25aa | 126.3 | Dakota | 6-11-1947 | 59 | 111 | 2.4 | 18 | 5.6 .28 |
3.8 .31 |
33 1.44 |
72 1.18 |
9 .19 |
16 .45 |
.8 .04 |
.70 .01 |
30 | 30 | 0h |
| 22-22-13cb2 | 75.5 | Alluvium | 7-23-1946 | 58 | 363 | 31 | .29 | 86 4.29 |
12 .99 |
26 1.12 |
321 5.26 |
26 .54 |
16 .45 |
.3 .02 |
8 .18 |
264 | 263 | 1 |
| 22-22-27ca | 106 | Alluvium | 8-3-1946 | 58 | 460 | 29 | .25 | 87 4.34 |
13 1.07 |
59 2.58 |
327 5.36 |
67 1.39 |
42 1.18 |
.8 .04 |
1.3 .02 |
270 | 268 | 2 |
| 22-22-31dc | 59.4 | Alluvium | 7-9-1946 | 57 | 503 | 32 | .05 | 106 5.29 |
15 1.23 |
51 2.20 |
366 6.00 |
75 1.56 |
36 1.02 |
.6 .03 |
7.1 .11 |
326 | 300 | 26 |
| 23-21-2bb | 207.4 | Dakota | 6-11-1947 | 59 | 337 | 6.4 | 5.2 | 47 2.34 |
27 2.22 |
44 1.90 |
303 4.97 |
44 .92 |
17 .48 |
1.4 .07 |
1.2 .02 |
228 | 228 | 0i |
| 23-22-11cc | 98.1 | Dakota | 8-13-1946 | 58 | 409 | 30 | .32 | 74 3.69 |
14 1.15 |
56 2.44 |
337 5.53 |
26 .54 |
40 1.13 |
1 .05 |
1.8 .03 |
242 | 242 | 0j |
| 23-22-29dd | 248 | Dakota | 8-12-1946 | 61 | 1,610 | 5.4 | .36 | 40 2.00 |
25 2.06 |
549 23.89 |
346 5.67 |
126 2.62 |
690 19.46 |
3 .16 |
2.2 .04 |
203 | 203 | 0k |
| 23-23-15cd | 128.1 | Dakota | 8-12-1946 | 60 | 362 | 17 | 5.4 | 86 4.29 |
14 1.15 |
26 1.11 |
300 4.92 |
52 1.08 |
17 .48 |
.9 .05 |
1.1 .02 |
272 | 246 | 26 |
- One part per million is equivalent to one pound of substance per million pounds of water or 8.33 pounds per million gallons of water.
- An equivalent per million is a unit chemical equivalent weight of solute per million unit weights of solution. Concentration in equivalents per million is calculated by dividing the concentration in parts per million by the chemical combining weight of the substance or ion.
- When the number is preceded by T, the sample was collected from a test hole.
- Excess alkalinity, 20 parts per million.
- Excess alkalinity, 5 parts per million.
- Excess alkalinity, 42 parts per million.
- Excess alkalinity, 63 parts per million.
- Excess alkalinity, 37 parts per million.
- Excess alkalinity, 20 parts per million.
- Excess alkalinity, 34 parts per million.
- Excess alkalinity, 81 parts per million.
Table 21--Summary of the chemical characteristics of the samples of water collected from wells in Pawnee Valley, Kansas
| Range in parts per million | Number of samples | |||
|---|---|---|---|---|
| Dakota | Greenhorn | Terraces | Alluvium | |
| Dissolved solids | ||||
| Less than 200 | 1 | |||
| 201-300 | 1 | 2 | 2 | |
| 301-400 | 4 | 7 | 5 | 15 |
| 401-500 | 2 | 2 | 1 | 4 |
| 501-1,000 | 3 | 2 | 1 | 3 |
| More than 1,000 | 2 | 2 | ||
| Hardness | ||||
| Less than 200 | 4 | |||
| 201-300 | 9 | 3 | 6 | 19 |
| 301-400 | 7 | 3 | 3 | |
| 401-500 | 1 | 1 | ||
| More than 500 | 3 | |||
| Iron | ||||
| Less than 0.1 | 2 | 8 | ||
| 0.1-1.0 | 4 | 3 | 6 | 13 |
| 1.1-5.0 | 1 | 2 | 0 | 1 |
| 5.1-10.0 | 5 | 1 | 1 | 4 |
| More than 10.0 | 3 | 3 | 2 | |
| Chloride | ||||
| Less than 10 | 1 | |||
| 11-20 | 6 | 6 | 4 | 16 |
| 21-50 | 1 | 5 | 2 | 8 |
| 51-100 | 3 | 1 | 1 | |
| 101-500 | 1 | 1 | 1 | |
| More than 500 | 2 | |||
In addition to the total hardness, the table of analyses shows the carbonate hardness and the noncarbonate hardness. The carbonate hardness is that caused by calcium and magnesium bicarbonates and can be almost entirely removed by boiling. This type of hardness is often called "temporary hardness." The noncarbonate hardness is due to calcium and magnesium sulfates or chlorides and cannot be removed by boiling. It is sometimes referred to as "permanent hardness." With reference to use with soaps, there is no difference between the carbonate and noncarbonate hardness. In general, the noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness of less than 50 parts per million is generally rated as soft, and its treatment for the removal of hardness is rarely justified. Hardness between 50 and 150 parts per million does not seriously interfere with the use of water for most purposes, but it does increase slightly the consumption of soap; removal of the hardness by a softening process is profitable for laundries or other industries that use large quantities of soap. Treatment for the prevention of scale is necessary for the successful operation of steam boilers using water in the upper part of this range of hardness. Hardness of more than 150 parts per million can be noticed by anyone, and where the hardness is 200 or 300 parts per million it is common practice to soften water for household use or to install cisterns to collect soft rain water.
The hardness of 59 of the samples of water that were analyzed is indicated in Table 20; distribution by ranges of hardness are indicated in Table 21.
Iron
Next to hardness, iron is the constituent of natural waters that in general receives the most attention. The quantity of iron in ground waters may differ greatly from place to place, even in waters from the same formation. If a water contains much more than 0.1 part per million of iron, the excess may separate out after exposure to the air and settle as a reddish sediment. Iron, which may be present in sufficient quantity to give a disagreeable taste and to stain cooking utensils, may be removed from most waters by simple aeration and filtration, but a few waters require the addition of lime or some other substance. For the samples analyzed, 50 of the total of 60 contained more than 0.1 part per million of iron.
The iron content of the samples of ground water that were analyzed is shown in Table 21.
Water for irrigation
The suitability of water for use in irrigation is commonly believed to depend mainly on the quantity of soluble salts and on the ratio of the quantity of sodium to the total quantity of sodium, calcium, and magnesium. The quantity of chloride may be large enough to affect the use of the water, and in some areas there may be other constituents, such as boron, in sufficient quantity to cause difficulty. Magistad and Christiansen (1944, pp. 8-9) makes the following statement concerning irrigation waters:
Plants in saline soils are adversely affected by high concentrations of salts in the soil solution and by poor physical condition of the soil. Both conditions are greatly affected by the type of irrigation water used. An irrigation water having a high sodium percentage will, after a time, give rise to a soil having a large proportion of replaceable sodium in the colloid, often designated as black alkali soil. Even on sandy soils with good drainage waters of 85 percent sodium or higher will give rise to impermeable soils after prolonged use. With higher total salt content there is a flocculating action that tends to counterbalance the poor physical condition caused by a high sodium concentration in the water. On a heavy soil already high in replaceable sodium, the poorest water that one could use would be one low in total salts but having a high sodium percentage.
Magistad and Christiansen proposed standards for irrigation waters which are helpful in appraising or evaluating supplies. A class I water which is considered as excellent to good should contain not more than 700 parts per million of salt and not more than 60 percent sodium. A class 2 water which is classed as good to injurious contains from 700 to 2,000 parts per million of salt and a sodium percentage between 60 and 75. A class 2 water is probably harmful to the more sensitive crops. A class 3 water which is generally unsatisfactory contains more than 2,000 parts per million salts and more than 75 percent sodium.
It is recognized that the harmfulness of irrigation water is so dependent upon the type of land and crops, on the manner of use, and on the drainage that no specific limits can be adopted. Most of the water in the alluvium and terrace deposits of Pawnee Valley can be used safely for irrigation. Two samples contained more than 1,000 parts per million dissolved solids (wells 21-16-33cd and 22-16-4ca). The wells from which these samples were collected are in the Arkansas Valley near the mouth of Pawnee River.
Quality in Relation to Water-bearing Formations
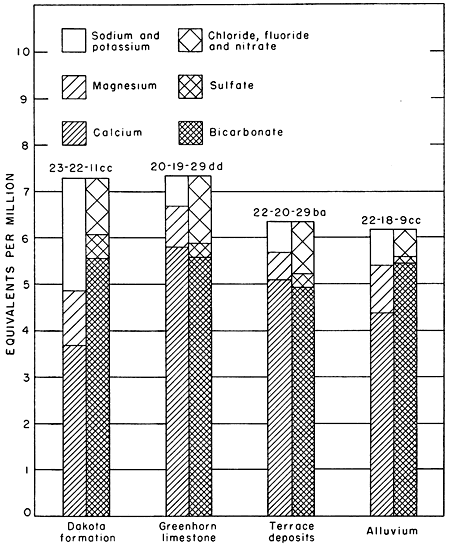
The quality of water from the alluvium, Dakota formation, and Greenhorn limestone is indicated by the analyses in Table 20, is summarized in Table 21, and is shown graphically in Figure 12. The quality of water in these formations and in the Permian redbeds is discussed below.
Figure 12--Analyses of waters from the principal water-bearing formations in Pawnee Valley.
Permian redbeds
The undifferentiated redbeds of Permian age yield little or no water to wells in this area, but the quality of the water from these beds is important because of the danger of pollution of the overlying beds that contain fresh water. Several deep wells and test holes in this area have encountered salt water under artesian pressure in these deposits. Therefore, such wells and test holes must be effectively sealed to prevent contamination of water.
The analysis of one sample of water from the Permian redbeds is given in Table 20 (21-18-32bb1). This sample contained 8,834 parts per million dissolved solids, 2,991 parts per million of sulfate, and 2,820 parts per million of chloride.
Dakota formation
Thirteen samples of water were collected from wells penetrating the Dakota formation. Seven of these samples contained more than 400 parts per million of dissolved solids, two of which had a dissolved solids content over 1,000 ppm. Water from the Dakota formation is moderately hard to hard. The 13 samples had an average hardness of 212. The maximum hardness was 288 and the minimum was 30 (well 22-21-25aa).
Greenhorn limestone
Eleven samples of water were collected from wells penetrating the Greenhorn limestone. Seven of these samples contained between 300 and 400 parts per million of dissolved solids, two contained between 400 and 500 parts per million, and two contained between 500 and 1,000 parts per million. Seven of the samples had a hardness between 300 and 400. The chloride content of all samples was less than 50 parts per million.
Terrace deposits
The dissolved solids in five of nine samples ranged from 300 to 400 parts per million. The hardness of six of the samples ranged from 200 to 300 parts per million. The other three samples had a hardness ranging from 300 to 400 parts. Seven samples had a chloride content of less than 50 parts per million, one had 54 parts per million, and one had 155 parts per million.
Alluvium
Twenty-six samples of water from the alluvium were analyzed. A few of the samples were from wells in the Arkansas Valley near the mouth of Pawnee River. The water in the alluvium of Pawnee Valley is generally of good quality, but the water in Arkansas Valley may contain a fairly high amount of dissolved solids. The dissolved solids in the 26 samples ranged from 272 to 1,480. Nineteen samples had a hardness between 200 and 300 parts per million and 7 samples had a hardness of more than 300 parts.
Prev Page--Irrigation, Availability || Next Page--Well Records
Kansas Geological Survey, Geohydrology
Placed on web May 31, 2012; originally published April 1952.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/94/10_chem.html