Prev Page--Contents || Next Page--Geology
Introduction
Among the most remarkable of plant fossils are the silicified grass anthoecia, and endocarps, nutlets, and achenes of other angiosperms that have been reported from the late Tertiary rock strata of the High Plains of central North America (Engelmann, 1876; Cockerell, 1909, 1914; Barbour, 1925; Berry, 1928a,b; Elias, 1931, 1932, 1935, 1942; Gregory, 1942; Frye, Leonard, and Swineford, 1956; Leonard, 1958; Segal, 1965, 1966a,b,c; Skinner et al., 1968; Voorhies, 1974; Galbreath, 1974; Hager, 1974; Thomasson, 1976 a,b, 1977, 1978a,b). Genera previously described include Berriochloa (=Stipidium), Nassella, Paleoeriocoma, Panicum, and Setaria of the Gramineae; Biorbia, Cryptantha, and Prolithospermum of the Boraginaceae; Achaenites of the Compositae; and Celtis of the Ulmaceae. Unlike other North American Tertiary fossils representing these groups, which are generally compressions or impressions and provide limited information, fossils from the High Plains are three-dimensional silifications, often having the smallest epidermal features exquisitely preserved. Coming from a stratified and nearly complete sequence of fluvial deposits of a widespread areal and temporal nature stretching from North Dakota to southern Texas and New Mexico, the fossils offer a unique opportunity to study the evolution of the represented taxa.
One of the chief obstacles to the understanding of the phylogenetic significance of the fossils is the scarcity of previous systematic and biostratigraphic studies of a detailed and comprehensive nature. That of Elias (1942) is reasonably complete both botanically and geologically, but its true importance rests in its nature as a pioneering work. Until recently (Thomasson, 1976b, 1978a,b) no attempts had been made to utilize microscopic features, such as those proposed by Prat (1932, 1936, 1960) and Clark and Gould (1975) for modern grasses and by Schuyler (1971) for certain sedges, to aid in elaborating a more accurate phylogeny of the fossil plants from the High Plains.
The evolution of the Gramineae has long fascinated botanists, and there is a rather large and ever-increasing literature on the subject. However, the many conclusions that have been drawn suffer from one serious deficiency: all the evidence has come from a single time plane--the present. Thus, authors variously utilize such features as morphology and anatomy (Klastersky, 1955; Phipps, 1967; Cronquist, 1968), cytology (Johnston, 1972; Stebbins, 1956, 1972, 1975), distribution (Hartley, 1958a,b, 1960, 1961; Beetle, 1961), or a combination of these (Soderstrom and Calderon, 1974) in elucidating the evolution of specific groups within the Gramineae. Without doubt, these studies are important and contribute greatly to our knowledge of the present status of these characters or conditions. However, in the final analysis any extrapolation of these data into the past is speculation, no matter bow much data are accumulated. It is fortunate, then, that among the most abundant of fossils from the High Plains are those of various representatives of the Gramineae. Among the classics of paleobotany is the pioneering effort by Elias (1942), the father of paleoagrostology, to compare these High Plains fossils and their modern counterparts in an evolutionary scheme. Although his study provided important answers to many questions relating to the evolution of certain Gramineae, many questions remain.
Much of stratigraphic work in the Ogallala Formation has been dependent on studies of the areal and temporal distribution of "seeds" recovered from stratigraphic sections in the High Plains. As a result, a rather detailed succession of floral zones has been established. Those biostratigraphic zones have been more or less firmly accorded equivalence with lithologic units in which the fossils are found (Elias, 1942; Frye and Leonard, 1959). Recently, this practice has come into question (Webb, 1969; Zehr, 1974; Thomasson, 1976b, 1977), and a reconsideration of this problem in light of newly found evidence indicates that the past reliance on widely spaced stratigraphic sections has greatly oversimplified an extremely complex situation.
The origin of the present grasslands of central North America is little understood. Elias (1942) has indicated the presence of widespread treeless grasslands as early as the Miocene, while MacGinitie (1962) has postulated that such grasslands are probably a result of Pleistocene influences. Gregory (1971) has presented convincing evidence for the Pliocene origin of such grasslands. By studying the combined fossil angiosperm and vertebrate evidences, it should be possible to shed new light on this problem.
Materials and Methods
Herbarium Studies
Herbarium operations consisted of examinations of both fossil and living specimens. Herbaria and museums from which these specimens were secured are listed below, using the standard abbreviations of Lanjouw and Stafleu (1964) for herbaria and common usage for museums.
FHSM--Sternberg Memorial Museum, Hays, Kansas
GH--Gray Herbarium of Harvard University, Cambridge, Massachusetts
ISC--Iowa State University, Ames, Iowa
KANU--University of Kansas, Lawrence, Kansas
KUMNH--University of Kansas Museum of Natural History, Lawrence, Kansas
MEXU--Herbario Nacional, Instituto Biologica, Mexico, D.F. Mexico
MO--Missouri Botanical Garden, St. Louis, Missouri
NY--New York Botanical Garden, Bronx, New York
TAES--Tracy Herbarium, College Station, Texas
TEX--University of Texas, Austin, Texas
UNSM--University of Nebraska State Museum, Lincoln, Nebraska
US--U.S. National Herbarium, Washington, D.C.
USNM--United States National Museum, Washington, D.C.
In addition, numerous specimens were sent as gifts to ISC from MVFA (Laboratorio Botanica, Facultad Agonomica, Montevideo, Uruguay) and SI (Instituto Botanica Darwinion, San Isidro, Argentina).
Field Studies
Field collections were made during 1972-1977 at 47 sites in Kansas, Nebraska, and Colorado. Methodology at the sites consisted of diligent searching of outcrops to remove fossils directly or to remove samples of fossil-bearing matrix. Beds were mapped in order to provide strict stratigraphic control of samples collected. Samples collected were numbered and dated as shown below:
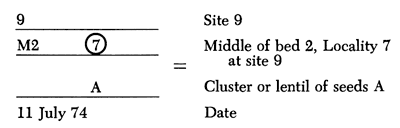
Fossils were not collected for study purposes unless they could be recovered in situ.
Polaroid Type 105 positive-negative film was used to record fossil collection locations. Detailed locations of sites have not been given except where absolutely necessary. All such information will be permanently stored with the type collections at the Sternberg Memorial Museum, Hays, Kansas. It may be obtained by contacting the director there or the author.
Laboratory Methods
The fossils were removed from their enclosing matrix by handpicking (degaging) with needles under a binocular microscope, by dissolving the cement binding the matrix, or by a combination of both methods. Handpicking was the principal method used when the matrix was consolidated but uncemented. With careful handpicking, it is possible to preserve even the finest of fossilized features such as hairs.
When the matrix containing the fossils is cemented by calcium carbonate or opal, it is necessary to employ chemical methods to separate fossils and matrix. Fossils cemented by calcium carbonate were relatively easy to free using a solution of hydrochloric acid. Solutions of 30-50% were generally employed, although solutions from 5-100% were used with success. Fossils are usually composed of opal (Frye, Leonard, and Swineford, 1956), so they are not affected by the acid. The disaggregated matrix and fossils are washed in running tap water for at least 5 minutes and then air or oven dried. Opal or chalcedonic or microcrystalline quartz (i.e., chert) cemented matrix presented a special problem because no previous attempts had been made in the literature to utilize fossils thus preserved. Experiments with heated, saturated KOH (potassium hydroxide) solutions were, in some cases, quite successful, although KOH is very corrosive and the process must be closely monitored. After treatment, fossils were washed several times in tap water. Several regimes were attempted and the results are presented in Table 1.
Table 1--KOH treatments of fossil-bearing matrix and their effects on fossils.
| Site | Taxon of Fossil |
Temp. of Solution |
Length of Treatment |
Results |
|---|---|---|---|---|
| 4 | Berriochloa | 113° C | 18 min. | Satisfactory, but loss of some surface detail |
| 4 | Berriochloa | 115° C | 2 min. | Unsatisfactory |
| 22 | Berriochloa | 113° C | 6 min. | Unsatisfactory |
| 22 | Berriochloa | 113° C | 3 min. | Excellent |
| 8 | Nassella | 160° C | 30 sec. | Unsatisfactory |
| 28 | Berriochloa | 130° C | 30 sec. | Satisfactory, but loss of some surface detail |
Generally, any methods which require agitation (i.e., screen washing, strong acids) destroy delicate features such as hairs and frequently the fossils themselves. Such methods as screen washing are routinely used by many vertebrate paleontologists. This may help to explain the absence of reports of angiosperm remains in many vertebrate studies in the High Plains Tertiary.
Scanning Electron Microscopy
Fossils used for the scanning electron microscopy (SEM) were selected under a binocular microscope and were mounted on double stick nonmetallic tape which had been previously attached to brass specimen plates. Silver cement was then applied around the contact of tape and plates and touched to the specimen at at least two points to assure good conductivity. Specimens were then coated with vacuum evaporated carbon and gold and viewed at an accelerating voltage of 15-25kV in a JOEL Stereoscan 35 electron microscope. A photographic record was kept on Polaroid Type 105 positive-negative film.
Attempts to prepare the anthoecia of modern species of grasses for examination by the SEM were not entirely successful because of the extremely thick nature of the lemma cuticle. The most successful method was that used for epidermal studies by light microscopy except that the final step was air or critical point drying. Species of Lappula, as well as Nassella, Berriochloa, and Stipa, were coated with carbon and gold in a vacuum evaporator and examined. Each specimen stub prepared for SEM has been numbered in a BP (Brass Plate) series. These are permanently stored at Sternberg Memorial Museum, Hays, Kansas.
Light Microscopy
Lemmas of modern species of the Stipeae were cleared to survey the epidermal features by light microscopy. The method used in preparing the anthoecia was modified after that of Shobe and Lersten, 1967. The steps, all done at room temperature, are: (1) specimens were placed in 5% NaOH for 24-48 hours; (2) the specimens were transferred directly to a full-strength solution of chlorine bleach for 1.5 hours; (3) cleared lemmas were washed three times (minimum 5 min. each) in distilled water; (4) a solution of 100% ethanol was used to dehydrate the specimens. At this point they could be stored in 100% ethanol; (5) the specimens were stained with chlorozol black E for 30 seconds to 5 minutes. This step must be monitored under a dissecting microscope. Too much time in the stain results in too dense preparations, whereas too little time results in poorly or irregularly stained specimens. This modified method was generally quite successful, although in certain instances considerable experimentation was necessary in order to adjust the steps to varying cuticle thicknesses.
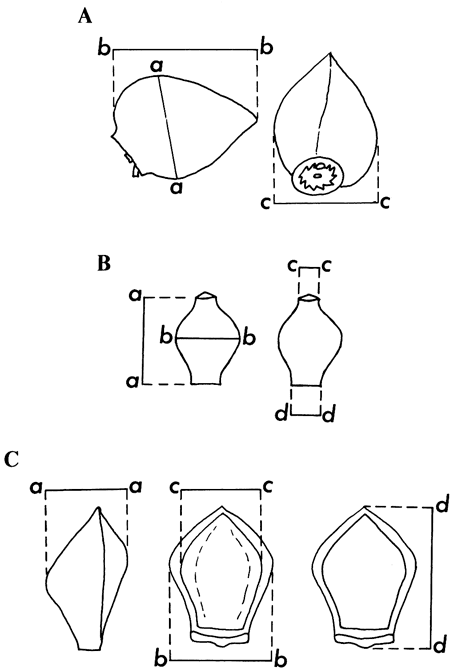
The anthoecia, nutlets, achenes, and endocarps were measured under a Bausch and Lomb dissecting microscope with a micrometer disk graduated to 0.1 mm. Characters measured on the individual taxa are represented in Figs. 1-3.
Figure 1--Manner of measurement of anthoecia of: A. Berriochloa (top row); and B. Berriochloa (Stipidium) (bottom row).
a--a: length of anthoecia; b--b: length of callus; c--c: width of awn scar; d--d: width of anthoecia; e--e: depth of anthoecia; f--f: distance from rear of awn scar to dorsal rib; g--g: width of awn scar; h-h: width of palea exposed; i--i: length of anthoecium and callus. Measurements of Nassella are taken as for those of Berriochloa.
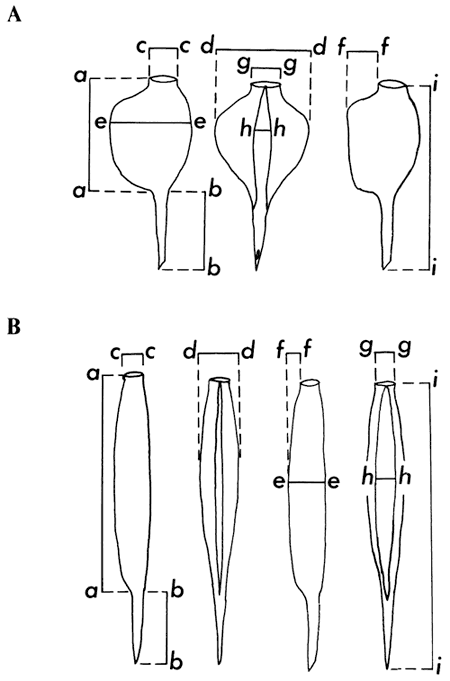
Figure 2--Manner of measurement of: A. Eliasiana (top row), B. Cryptantha (middle row), and C. Prolithospermum (bottom row).
a--a: depth (height) of nutlet; b--b: length of nutlet; c--c: width of nutlet; d--d: basal width of scar of attachment. Measurements of Prolappula taken as for those of Cryptantha.
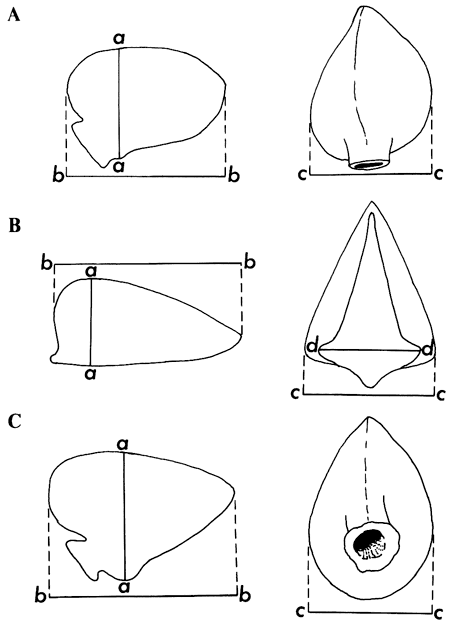
Figure 3--Manner of measurement of: A. nutlets of Biorbia
(top row), B. the achenes of Eleofimbris (middle row) and C. anthoecia of Panicum (bottom row).
A. a--a: depth (height) of nutlet; b--b: length of nutlet; c--c: width of nutlet.
B. a--a: length of achene; b--b: width of achene across broadest face; c--c: width of style base scar; d--d: width of base.
C. a--a: depth of anthoecia; b--b: width of anthoecia; c--c: width of palea exposed; d--d: length of anthoecia.
Prev Page--Contents || Next Page--Geology
Kansas Geological Survey, Geology
Placed on web May 1, 2009; originally published September 1979.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/218/02_intro.html