Prev Page--Ground water || Next Page--Irrigation
Chemical Quality of the Water
By Robert A. Krieger
The ground and surface waters of the Ladder Creek area were sampled at the 44 points shown on Figure 12, and the results of analyses of these samples are given in Table 6. The wells that were sampled are listed by well location number and under geologic source. The results in equivalents per million are given in Table 7.
Figure 12--Water-sampling sites and generalized geologic map.
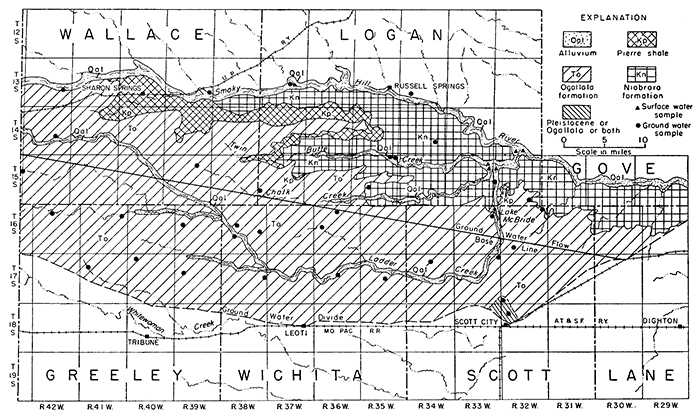
Table 6--Chemical analyses at water, in parts per million
| Location | County | Depth | Date of collection |
Temperature (°F) |
pH | Specific conductance (micromhos at 25°C) |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium (Na) |
Potassium (K) |
Carbonate (CO3) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) |
Boron (B) |
Dissolved solids |
Hardness as CaCO3 | Percent sodium | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Noncarbonate | ||||||||||||||||||||||
| Alluvium | |||||||||||||||||||||||
| 13-39-25aa | Wallace | 20 | 9/20/51 | 57 | 2,720 | 1,270 | 48 | ||||||||||||||||
| 13-42-24cc1 | Wallace | 16 | 9/20/51 | 58 | 7.6 | 498 | 31 | 1.2 | 57 | 12 | 24 | 8.8 | 0 | 227 | 19 | 13 | 0.7 | 42 | 0.09 | 324 | 192 | 6 | 20 |
| 15-35-2ab | Logan | 37 | 9/19/51 | 55 | 7.2 | 3,260 | 573 | 100 | 192 | 18 | 0 | 453 | 1,760 | 23 | 1.2 | 37 | 3,190 | 1,840 | 1,470 | 18 | |||
| 15-35-32ad | Logan | 18 | 9/19/51 | 55 | 7.3 | 2,300 | 42 | 0.3 | 256 | 77 | 172 | 17 | 0 | 348 | 770 | 185 | 1.2 | 6.7 | 0.25 | 1,700 | 956 | 671 | 28 |
| 16-38-20dd | Wichita | 30 | 9/21/51 | 56 | 7.8 | 601 | 65 | 21 | 38 | 6.3 | 0 | 316 | 44 | 15 | 1.2 | 2.2 | 386 | 248 | 0 | 24 | |||
| 17-33-1bd | Scott | 15 | 9/19/51 | 58 | 7.6 | 1,280 | 107 | 51 | 94 | 11 | 0 | 364 | 112 | 48 | 2.8 | 240 | 920 | 476 | 178 | 29 | |||
| 17-36-16aa | Wichita | 22 | 9/21/51 | 56 | 7.6 | 506 | 52 | 18 | 32 | 5.4 | 0 | 257 | 40 | 11 | 1.6 | 5.2 | 334 | 205 | 0 | 25 | |||
| Sanborn and Meade formations | |||||||||||||||||||||||
| 13-37-23bb | Logan | Spring | 9/20/51 | 55 | 7.6 | 863 | 21 | 0.03 | 81 | 21 | 73 | 8.9 | 0 | 255 | 212 | 18 | 1.1 | 1.6 | 0.17 | 581 | 288 | 79 | 35 |
| 17-32-31cb | Scott | 82 | 9/18/51 | 58 | 687 | 80 | 46 | ||||||||||||||||
| 18-32-7ac2 | Scott | 98 | 9/19/51 | 7.6 | 471 | 52 | 0.04 | 47 | 18 | 24 | 5 | 0 | 226 | 39 | 9.5 | 2 | 7 | 0.12 | 326 | 193 | 8 | 21 | |
| Ogallala formation | |||||||||||||||||||||||
| 14-42-23bc | Wallace | 180 | 9/20/51 | 58 | 7.7 | 350 | 31 | 0.03 | 35 | 11 | 24 | 3.4 | 0 | 170 | 28 | 5.5 | 1 | 6.6 | 0.1 | 230 | 133 | 0 | 27 |
| 15-32-26da | Logan | 84 | 9/18/51 | 58 | 8.2 | 491 | 54 | 18 | 23 | 4.4 | 5 | 196 | 60 | 15 | 1 | 7.8 | 338 | 209 | 40 | 19 | |||
| 15-37-30cb | Logan | 73 | 9/21/51 | 56 | 7.61 | 411 | 27 | 2.3 | 37 | 14 | 29 | 3.8 | 0 | 204 | 29 | 5.5 | 1.8 | 8.8 | 0.14 | 258 | 150 | 0 | 29 |
| 15-39-11bb | Wallace | 130 | 9/21/51 | 56 | 381 | 32 | 5.5 | ||||||||||||||||
| 15-43-12dd | Wallace | 218 | 9/20/51 | 7.7 | 484 | 17 | 1.4 | 33 | 16 | 45 | 4.6 | 0 | 202 | 59 | 9 | 1.4 | 13 | 0.19 | 304 | 149 | 0 | 39 | |
| 16-32-2bc2 | Scott | 135 | 9/18/51 | 59 | 7.7 | 394 | 38 | 0.7 | 56 | 10 | 9.1 | 4.2 | 0 | 196 | 26 | 8.5 | 0.8 | 5.4 | 0.05 | 268 | 181 | 20 | 10 |
| 16-32-32dc2 | Scott | 127 | 9/18/51 | 58 | 8.4 | 757 | 84 | 21 | 42 | 7 | 180 | 118 | 35 | 68 | a464 | 296 | 137 | 23 | |||||
| 16-33-1cc | Scott | Spring | 9/19/51 | 59 | 7.6 | 509 | 41 | 0.04 | 56 | 16 | 28 | 4.1 | 0 | 248 | 40 | 9.5 | 2.4 | 8.3 | 0.11 | 340 | 206 | 3 | 22 |
| 16-33-18dd2 | Scott | 141 | 9/19/51 | 61 | 440 | 41 | 8.5 | ||||||||||||||||
| 16-36-3cc | Wichita | 130 | 9/21/51 | 57 | 7.5 | 556 | 53 | 22 | 34 | 4.4 | 0 | 215 | 70 | 19 | 2.8 | 11 | 0.19 | 384 | 221 | 45 | 25 | ||
| 16-36-18ad | Wichita | 59 | 9/21/51 | 55 | 475 | 44 | 8 | ||||||||||||||||
| 16-38-16bb | Wichita | 200 | 9/21/51 | 58 | 386 | 41 | 8.5 | ||||||||||||||||
| 16-38-24bc | Wichita | 78 | 9/21/51 | 7.6 | 491 | 26 | 1.7 | 48 | 14 | 33 | 4.6 | 0 | 214 | 40 | 15 | 1.6 | 12 | 0.17 | 316 | 179 | 4 | 28 | |
| 16-39-18da | Greeley | 118 | 9/20/51 | 58 | 457 | 51 | 11 | ||||||||||||||||
| 16-41-12bd | Greeley | 199 | 9/20/51 | 404 | 32 | 8 | |||||||||||||||||
| 16-42-9ab | Greeley | 213 | 59 | 8.6 | 384 | 40 | 12 | 17 | 9 | 171 | 17 | 3.5 | 9.9 | a192 | 149 | 0 | 20 | ||||||
| 17-34-3bb | Scott | 132 | 9/19/51 | 59 | 7.5 | 448 | 40 | 0.04 | 42 | 16 | 30 | 4.4 | 0 | 210 | 40 | 9 | 1.8 | 8.5 | 0.14 | 300 | 171 | 0 | 27 |
| 17-35-22bb | Wichita | 118 | 9/21/51 | 58 | 492 | 60 | 17 | ||||||||||||||||
| 17-38-24ac | Wichita | 210 | 9/21/51 | 58 | 8.1 | 525 | 46 | 0.03 | 44 | 23 | 32 | 4.2 | 0 | 198 | 71 | 20 | 2 | 7.8 | 0.15 | 360 | 204 | 42 | 25 |
| 17-39-17cb | Greeley | 110 | 59 | 505 | 85 | 16 | |||||||||||||||||
| 17-40-5cb | Greeley | 162 | 9/20/51 | 63 | 452 | 26 | 5 | ||||||||||||||||
| 17-41-8cc | Greeley | 118 | 9/20/51 | 58 | 8.1 | 461 | 41 | 0.1 | 57 | 17 | 14 | 5 | 0 | 229 | 26 | 6.5 | 0.6 | 20 | 0.1 | 316 | 211 | 23 | 12 |
| Niobrara formation | |||||||||||||||||||||||
| 13-34-29bd | Logan | 9/19/51 | 61 | 8.2 | 831 | 91 | 27 | 52 | 8 | 5 | 249 | 177 | 29 | 0.6 | 16 | 568 | 340 | 128 | 24 | ||||
| 14-34-26aa | Logan | 156 | 9/19/51 | 59 | 2,410 | 1,440 | 24 | ||||||||||||||||
| 15-32-19bd3 | Logan | 21 | 9/18/51 | 58 | 7.2 | 2,560 | 477 | 83 | 92 | 17 | 0 | 270 | 1,330 | 31 | 1.8 | 44 | 2430 | 1530 | 1310 | 11 | |||
| 15-35-28bd | Logan | 70 | 9/19/51 | 58 | 7.5 | 686 | 26 | 0.24 | 98 | 19 | 27 | 3.8 | 0 | 334 | 89 | 5 | 2.4 | 2.7 | 0.2 | 446 | 324 | 50 | 15 |
| Streams | |||||||||||||||||||||||
| Ladder Creek at Wichita-Greeley County line | Wichita | 9/21/51 | 55 | 8.7 | 539 | 52 | 17 | 43 | 14 | 205 | 70 | 14 | 10 | b321 | 198 | 7 | 32 | ||||||
| Lake McBride on Ladder Creek | Scott | 66 | 8.6 | 681 | 63 | 22 | 57 | 16 | 242 | 104 | 23 | 5 | b409 | 247 | 22 | 33 | |||||||
| Ladder Creek above confluence with Chalk Creek | Logan | 9/19/51 | 74 | 8.5 | 705 | 60 | 23 | 61 | 11 | 230 | 130 | 24 | 3.1 | b425 | 246 | 39 | 35 | ||||||
| Chalk Creek above mouth | Logan | 9/19/51 | 76 | 8.2 | 1,620 | 156 | 51 | 139 | 0 | 101 | 713 | 55 | 0.5 | b1160 | 600 | 517 | 34 | ||||||
| Twin Butte Creek above mouth | Logan | 9/19/51 | 77 | 8 | 2,850 | 440 | 89 | 185 | 0 | 88 | 1,650 | 53 | 1.2 | b2,460 | 1,460 | 1,390 | 22 | ||||||
| Ladder Creek above mouth | Logan | 9/19/51 | 71 | 8.3 | 876 | 80 | 28 | 74 | 7 | 233 | 222 | 27 | 2.6 | b556 | 313 | 110 | 34 | ||||||
| Smoky Hill River below Hinshaw Spring | Logan | 9/19/51 | 75 | 8.1 | 1,050 | 81 | 28 | 111 | 0 | 207 | 338 | 24 | 1.2 | b685 | 316 | 146 | 43 | ||||||
| Smoky Hill River Elkader, Kansas | Logan | 9/19/51 | 70 | 8.2 | 966 | 89 | 30 | 75 | 0 | 158 | 325 | 27 | 1.6 | b626 | 345 | 215 | 32 | ||||||
| 1. Alluvium or Sanborn and Meade formations or all three. 2. Sanborn and Meade formations or Ogallala formation or all three. 3. Niobrara formation or alluvium or both. a. Sum of determined constituents. b. Sum of determined constituents. |
|||||||||||||||||||||||
Table 7--Chemical analyses of water, in equivalents per million.
| Source and Location | Date of collection |
Calcium (Ca) |
Magnesium (Mg) |
Sodium (Na) |
Potassium (K) |
Carbonate (CO3) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alluvium | |||||||||||
| 13-39-25aa | 9-20-1951 | 26.44 | 1.35 | ||||||||
| 13-42-24cc | 9-20-1951 | 2.84 | 1.00 | 1.04 | 0.23 | 0.00 | 3.72 | 0.40 | 0.37 | 0.04 | 0.68 |
| 15-35-2ab | 9-19-1951 | 28.59 | 8.21 | 8.35 | .46 | .00 | 7.42 | 36.64 | .65 | .06 | .60 |
| 15-35-32ad | 9-19-1951 | 12.77 | 6.35 | 7.48 | .43 | .00 | 5.70 | 16.03 | 5.22 | .06 | .11 |
| 16-38-20dd | 9-21-1951 | 3.24 | 1.72 | 1.65 | .16 | .00 | 5.18 | .92 | .42 | .06 | .04 |
| 17-33-1bd | 9-19-1951 | 5.34 | 4.18 | 4.09 | .28 | .00 | 5.97 | 2.33 | 1.35 | .15 | 3.87 |
| 17-36-16aa | 9-21-1951 | 2.59 | 1.51 | 1.39 | .14 | .00 | 4.21 | .83 | .31 | .08 | .08 |
| Sanborn and Meade formations | |||||||||||
| 13-37-23bb | 9-20-1951 | 4.04 | 1.72 | 3.17 | .23 | .00 | 4.18 | 4.41 | .51 | .06 | .03 |
| 17-32-31cb | 9-18-1951 | 1.67 | 1.30 | ||||||||
| 18-32-7ac | 9-19-1951 | 2.35 | 1.51 | 1.04 | .13 | .00 | 3.70 | .81 | .27 | .11 | .11 |
| Ogallala formation | |||||||||||
| 14-42-23bc | 9-20-1951 | 1.75 | .91 | 1.04 | .09 | .00 | 2.79 | .58 | .16 | .05 | .11 |
| 15-32-26da | 9-18-1951 | 2.69 | 1.49 | 1.00 | .11 | .17 | 3.21 | 1.25 | .42 | .05 | .13 |
| 15-37-30cb | 9-21-1951 | 1.85 | 1.15 | 1.26 | .10 | .00 | 3.34 | .60 | .16 | .09 | .14 |
| 15-39-11bb | 9-21-1951 | .67 | .16 | ||||||||
| 15-43-12dd | 9-20-1951 | 1.65 | 1.33 | 1.96 | .12 | .00 | 3.31 | 1.23 | .25 | .07 | .21 |
| 16-32-2bc | 9-18-1951 | 2.79 | .83 | .40 | .11 | .00 | 3.21 | .54 | .24 | .04 | .09 |
| 16-32-32dc | 9-18-1951 | 4.19 | 1.73 | 1.81 | .23 | 2.95 | 2.46 | .99 | 1.10 | ||
| 16-33-1cc | 9-19-1951 | 2.79 | 1.33 | 1.22 | .10 | .00 | 4.06 | .83 | .27 | .13 | .13 |
| 16-33-18dd | 9-19-1951 | .85 | .24 | ||||||||
| 16-36-3cc | 9-21-1951 | 2.64 | 1.78 | 1.48 | .11 | .00 | 3.52 | 1.46 | .54 | .15 | .18 |
| 16-36-18ad | 9-21-1951 | .92 | .23 | ||||||||
| 16-38-16bb | 9-21-1951 | .85 | .24 | ||||||||
| 16-38-24bc | 9-21-1951 | 2.40 | 1.18 | 1.43 | .12 | .00 | 3.51 | .83 | .42 | .08 | .19 |
| 16-39-18da | 9-20-1951 | 1.06 | .31 | ||||||||
| 16-41-12bd | 9-20-1951 | .67 | .23 | ||||||||
| 16-42-9ab | 2.00 | .98 | .73 | .30 | 2.80 | .35 | .10 | .16 | |||
| 17-34-3bb | 9-19-1951 | 2.10 | 1.32 | 1 30 | .11 | .00 | 3.44 | .83 | .25 | .09 | .14 |
| 17-35-22bb | 9-21-1951 | 1.25 | .48 | ||||||||
| 17-38-24ac | 9-21-1951 | 2.20 | 1.88 | 1.39 | .11 | .00 | 3.24 | 1.48 | .56 | .11 | .13 |
| 17-39-17cb | 1.77 | .45 | |||||||||
| 17-40-5cb | 9-20-1951 | .54 | .14 | ||||||||
| 17-41-8cc | 9-20-1951 | 2.84 | 1.38 | 61 | .13 | .00 | 3.75 | .54 | .18 | .03 | .32 |
| Niobrara formation | |||||||||||
| 13-34-29bd | 9-19-1951 | 4.54 | 2.26 | 2.26 | .20 | .17 | 4.08 | 3.68 | .82 | .03 | .26 |
| 14-34-26aa | 9-19-1951 | 29.98 | .68 | ||||||||
| 15-32-19bd | 9-18-1951 | 23.80 | 6.80 | 4.00 | .43 | .00 | 4.42 | 27.69 | .87 | .09 | .71 |
| 15-35-28bd | 9-19-1951 | 4.89 | 1.59 | 1.17 | .10 | .00 | 5.47 | 1.85 | .14 | .13 | .04 |
| Streams | |||||||||||
| Ladder Creek at Greeley-Wichita County line | 9-21-1951 | 2.59 | 1.37 | 1.88 | .47 | 3.36 | 1.46 | .39 | .16 | ||
| Lake McBride on Ladder Creek | 3.14 | 1.80 | 2.46 | .53 | 3.97 | 2.17 | .65 | .08 | |||
| Ladder Creek above confluence with Chalk Creek. | 9-19-1951 | 2.99 | 1.93 | 2.66 | .37 | 3.77 | 2.71 | .68 | .05 | ||
| Chalk Creek above mouth | 9-19-1951 | 7.78 | 4.22 | 6.06 | .00 | 1.66 | 14.84 | 1.55 | .01 | ||
| Twin Butte Creek above mouth | 9-19-1951 | 21.96 | 7.28 | 8.06 | .00 | 1.44 | 34.35 | 1.49 | .02 | ||
| Ladder Creek above mouth | 9-19-1951 | 3.99 | 2.27 | 3.21 | .23 | 3.82 | 4.62 | .76 | .04 | ||
| Smoky Hill River below Hinshaw Spring | 9-19-1951 | 4.04 | 2.28 | 4.81 | .00 | 3.39 | 7.04 | .68 | .02 | ||
| Smoky Hill River at Elkader, Kansas | 9-19-1951 | 4 44 | 2.46 | 3.25 | .00 | 2.59 | 6.77 | .76 | .03 | ||
Chemical Constituents in Relation to Use
Hardness
The most important basic ion in the ground waters of the area is calcium. Calcium and magnesium are the cause of most hardness of water. These two ions combine with soap to form an insoluble curd or scum, and therefore the use of hard waters results in excessive soap consumption. Calcium in these waters ranged from 33 to 573 ppm and magnesium from 10 to 100 ppm. The bicarbonate ion, which is the principal anion in the ground water, ranged from 170 to 453 ppm. Hardness equivalent to the bicarbonate is carbonate ("temporary") hardness; the rest is noncarbonate ("permanent") hardness. Noncarbonate hardness results from the solution of compounds other than the bicarbonates of calcium and magnesium. Sulfate, chloride, and nitrate ranged from 17 to 1,760 ppm, 3.5 to 185 ppm, and 1.6 to 240 ppm, respectively. A high sulfate content may indicate that the water has dissolved gypsiferous materials.
Nitrate
Excessive nitrate content in water may be an indication of pollution. Whether polluted or not, water high in nitrate is undesirable for domestic supplies because of its toxic effect on some infants (Comly, 1945). According to Comly, waters containing nitrate amounting to more than 45 ppm should not be used in infant feeding. Only two samples analyzed (17-33-1bd and 16-32-32dc) had greater concentration of nitrate.
Fluoride
Fluoride in concentrations of about 1 ppm in drinking water used by children during the calcification of the teeth prevents or lessens the incidence of tooth decay; concentrations greater than 1.5 ppm may cause mottling of the enamel. Fluoride in waters of the Ladder Creek area ranged from 0.6 to 2.8 ppm.
Boron
Boron is essential for normal plant growth, but it is beneficial only within narrow limits. The element is very toxic to many plants, and quantities in excess of the optimum will cause serious damage. A concentration of 1.0 ppm of boron in the soil waters may cause injury to many plants. Boron concentrations in the ground waters that were analyzed ranged from 0.05 to 0.25 ppm.
Ionic Relations in the Water
The analyses in equivalents per million, expressions of the chemical combining weights of the ions, are given in Table 7 and are shown graphically in Figures 13 and 14.
Figure 13--Principal mineral constituents in ground water.
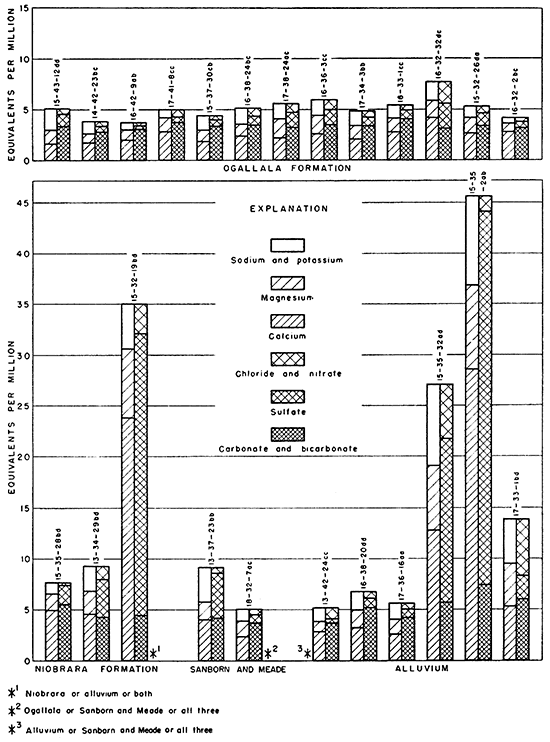
Figure 14--Principal mineral constituents in surface water.
Many natural waters that contain small or average amounts of dissolved solids are bicarbonate waters, but waters containing more than average amounts of dissolved materials contain much greater proportions of sulfate or chloride, and a correspondingly greater amount of calcium or sodium. Gypsiferous waters are high in calcium and sulfate content. An example of gypsiferous water is that found in well 15-32-19bd (Fig. 13).
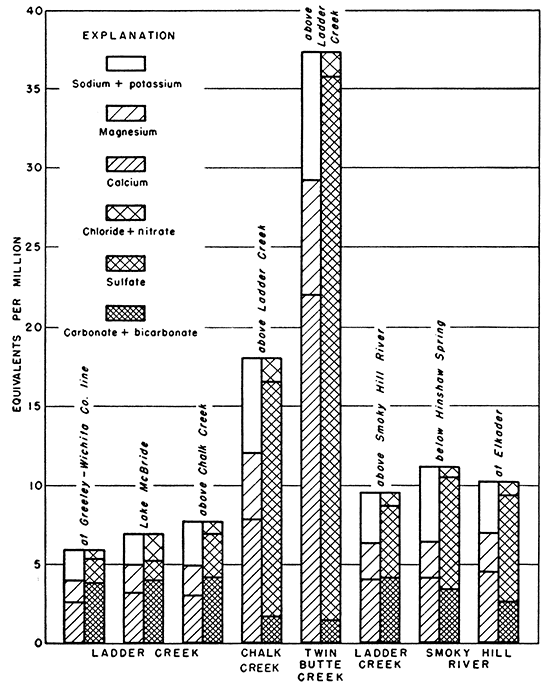
The relation between dissolved solids and sulfate is shown graphically in Figure 15. Sulfate makes up a larger part of the dissolved solids as the amount of dissolved solids increases. Although the data available for water from wells in the Niobrara formation are few, those available show that the relation of sulfate to dissolved solids is very similar to that in water from the alluvium. The proportion of sulfate to dissolved solids in the more concentrated waters from wells in the Ogallala formation is somewhat greater than in water from the Niobrara formation and alluvium.
Figure 15--Relation of sulfate to dissolved solids in ground water.
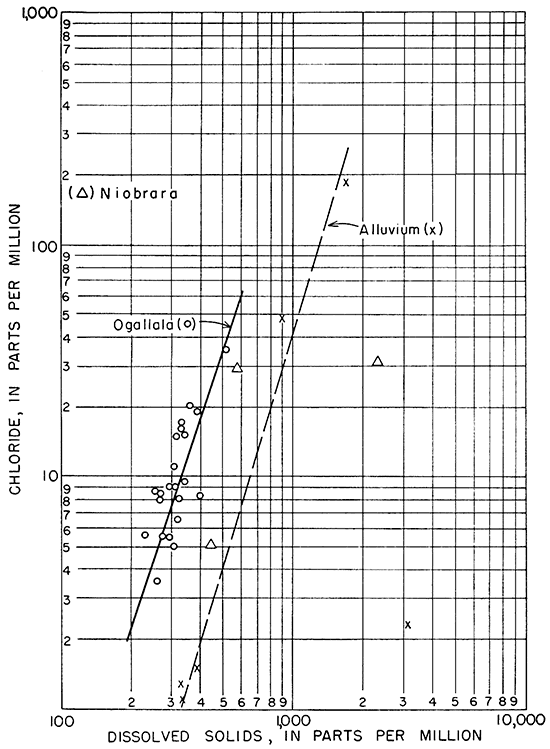
In Figure 16, the relation between chloride and dissolved solids in the ground waters is shown. The relation of chloride to dissolved solids of waters from the alluvium and the Ogallala formation is very similar.
Figure l6--Relation of chloride to dissolved solids in ground water.
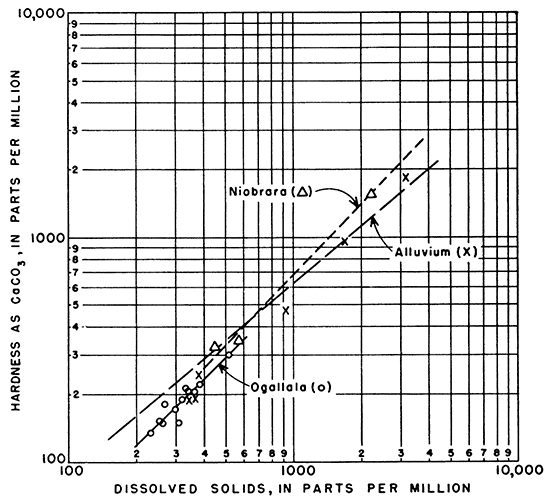
Hardness of the waters from the Niobrara and Ogallala formations and the alluvium was very similar in relation to dissolved solids, as shown in Figure 17.
Figure l7--Relation of hardness as CaCO3 to dissolved solids in ground water.
Chemical Quality in Relation to Movement of Ground Water
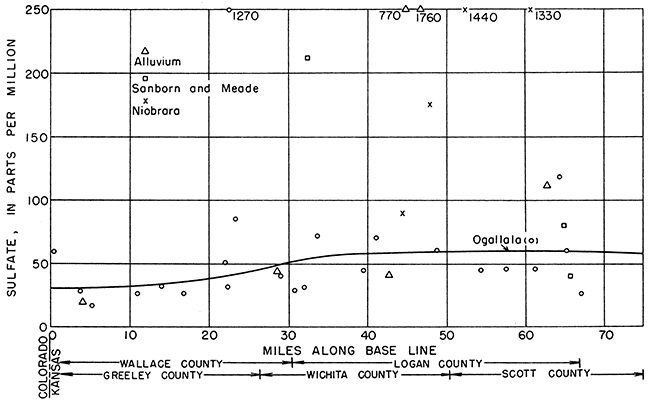
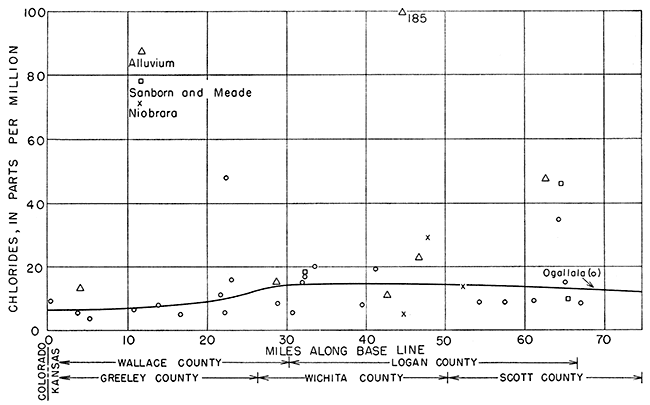
The ground water moves east by south generally in the direction of a line drawn from the northwest corner of 15-42-6 to the southeast corner of 16-31-36 (Fig. 12). The location of each well along the base line, as indicated by a perpendicular drawn from the well to the base line, was plotted as abscissa, and the concentrations in parts per million of sulfate, chloride, and hardness were plotted as ordinates in Figures 18, 19, and 20, respectively.
The concentration of sulfate increases gradually from west to east (Fig. 18). In the Ogallala formation, the sulfate shows a relatively abrupt but slight increase between 20 and 30 miles from the western edge of the state, and at 40 miles from the west border the sulfate concentration becomes approximately constant. In the alluvium, the steady increase of the sulfate content conforms with the downstream increase in Ladder Creek. The data for the Niobrara formation and Sanborn and Meade formations were not sufficiently widespread to indicate any trends.
Figure 1B--Sulfate content of ground water along direction of movement.
Chloride in water from the Ogallala formation showed trends similar to that for sulfate (Fig. 19). After the increase at 20 to 30 miles, the chloride concentration showed a slight tendency to decrease at 50 miles from the western edge of the state. This may be due either to an exchange of chloride ion for some other ion or, more likely, to a dilution by less concentrated waters. Chloride increased slightly in the alluvium with distance from the western boundary; chloride data for the Niobrara formation and Sanborn and Meade formations were too meager to show any significant trends.
Figure 19--Chloride content of ground water along direction of movement
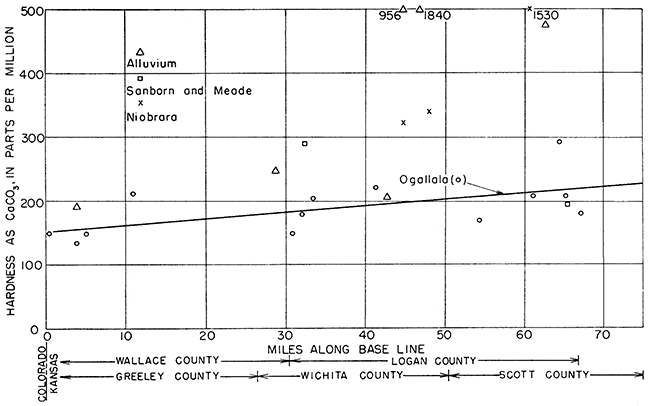
Hardness in water from the Ogallala formation and the alluvium showed a slight but steady increase with distance from west to east (Fig. 20).
Figure 20--Hardness (as CaCO3) of ground water along direction of movement.
Chemical Quality in Relation to Geologic Source
Samples of water were obtained from the Niobrara formation, Ogallala formation, the Sanborn and Meade formations, undifferentiated, and the alluvium along streams in the area. In addition, several water samples were obtained from the streams that drain the area.
Niobrara Formation
Only a few wells obtain water from the Niobrara formation. Consequently, only four water samples from this source were obtained, and it is uncertain whether well 15-32-19bd yields water from the Niobrara formation or alluvium or both. The data available show that the composition of the water from the Niobrara formation differs considerably from place to place. WeIl14-34-26aa yielded water containing 1,440 ppm sulfate, but samples from two other wells contained only 177 and 89 ppm of sulfate. Insofar as conclusions can be drawn on the basis of three samples, the water from the Niobrara formation seems to contain more dissolved material than water from the Ogallala formation. The principal anions are bicarbonate and sulfate; calcium is the principal cation.
Ogallala Formation
The Ogallala formation is very calcareous; consequently, the action of dissolved carbonic acid produces a water whose dissolved material is relatively rich in calcium, magnesium, and bicarbonate. The total amount of dissolved material in water from the Ogallala formation, however, is generally less than that in water from other sources in the area. Calcium and bicarbonate are the principal . ions in the water from the formation, ranging in concentration from 33 to 84 ppm and 170 to 248 ppm, respectively. The amount and proportions of dissolved material were nearly uniform, as shown in Figure 13. The Ogallala formation yields water of the best quality and most nearly uniform chemical composition of all the ground-water sources in the Ladder Creek area.
Sanborn and Meade Formations Undifferentiated
Two wells and one spring from which samples were obtained are believed to draw water from the Pleistocene deposits (Sanborn and Meade formations), although one of the wells, IB-32-7ac, may yield water from the Ogallala formation also. Water from this well contained 226 ppm of bicarbonate and 39 ppm of sulfate; water from the spring (13-37-23bb) contained 255 ppm of bicarbonate and 212 ppm of sulfate. The waters are hard.
Alluvium
The concentration of dissolved material in the six samples of water from the alluvium ranged from 324 to 3,190 ppm. Water in the alluvium along Ladder Creek was of better quality than that obtained from the alluvium in the valleys of Chalk and Twin Butte Creeks, as the samples from the latter two were gypsiferous in addition to containing considerable calcium bicarbonate. All the water is very hard.
Waters from the alluvium contained 0.7 to 2.8 ppm of fluoride, the maximum being somewhat more than the maximum limit set by the U. S. Public Health Service for acceptable public water supplies.
The streams in the Ladder Creek area were sampled at or near base flow stages. The chemical characteristics of the surface waters would then be similar to water from wells in the alluvium. Chalk and Twin Butte Creeks were high in sulfate and low in bicarbonate. The water in Smoky Hill River was somewhat gypsiferous and was of poorer quality than the water in Ladder Creek.
Chemical Quality in Relation to Use
The Ladder Creek area provides water for irrigation, stock, and domestic use. Water from the Ogallala formation is the best for irrigation. It is low in boron, and values for percent sodium range from 10 to 39. The specific conductance of the samples analyzed ranged from 350 to 2,720 micromhos, but values for all except one sample were below 757 micromhos. Waters from the alluvium generally are less suitable for irrigation because of high salinity. Most waters from the Niobrara formation and from the Sanborn and Meade formations can be used for irrigation but are less suitable than water from the Ogallala formation. No high-boron waters were found. All the waters are suitable for livestock except those containing excessive sulfate, which may be unpalatable.
The U. S. Public Health Service (1946), in setting up standards of quality for drinking water used on interstate carriers and for public supplies in general, stated that the following chemical substances should not exceed the stated concentrations:
| Concentration (ppm) |
|
|---|---|
| Iron and manganese together | 0.3 |
| Magnesium | 125 |
| Chloride | 250 |
| Sulfate | 250 |
| Fluoride | 1.5 |
Total solids should not exceed 500 ppm, but 1,000 ppm is acceptable if water of better quality is not available. Most of the waters meet the U. S. Public Health standards with regard to all the ions except sulfate and fluoride. The largest number fail to meet the standards because of high fluoride concentrations; of 22 samples analyzed for fluoride, 11 contained more than 1.5 ppm.
Prev Page--Ground water || Next Page--Irrigation
Kansas Geological Survey, Geology
Placed on web Jan. 30, 2013; originally published December 1957.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/126/07_chem.html