Prev Page--Stratigraphy || Next Page--Sedimentologic analysis
Petrographic analysis
For this study I examined 152 thin sections of sandstone and siltstone samples from the Rock Lake Shale Member of the Stanton Limestone. The siliciclastic components of these rocks consist mainly of monocrystalline and polycrystalline quartz, with minor amounts of orthoclase, plagioclase, microcline, muscovite, zircon, and tourmaline. Petrographically, sandstones of the Rock Lake Shale Member are all quartzarenites [classification of Folk (1974)], with quartz grains averaging more than 97% of all detrital grains (table 1).
Table 1--Modal analysis data from 25 selected thin sections (200 points per thin section) representing different sandstone facies in the Rock Lake Shale Member.
| Sample | Quartz | Feldspar | Chert | Muscovite | ||||
|---|---|---|---|---|---|---|---|---|
| Monocrystalline unit extinction |
Monocrystalline undulatory extinction |
Polycrystalline | Orthoclase | Plagloclase | Microcline | |||
| Southern Montgomery Co. | ||||||||
| R.3-4 | 43.0 | 14.0 | 2.0 | T | 0.5 | - | 1.0 | T |
| R.3-7 | 59.0 | 32.0 | 1.5 | T | T | - | 1.0 | T |
| R.3-11 | 58.5 | 27.5 | 1.5 | 1.5 | 1.0 | T | 1.0 | T |
| R.5-1 | 63.5 | 31.5 | 1.0 | 0.5 | T | - | T | T |
| R.5-2 | 65.0 | 29.5 | 1.5 | 0.5 | T | - | 0.5 | T |
| R.5-3 | 62.5 | 32.5 | 1.5 | T | 0.5 | - | 0.5 | T |
| R.8-4 | 51.5 | 34.5 | 4.0 | 2.0 | 0.5 | - | 1.0 | 0.5 |
| R.15-2 | 30.0 | 18.5 | 4.0 | 1.0 | 0.5 | - | 1.0 | - |
| R.15-4 | 61.5 | 22.5 | 1.0 | 2.5 | 0.5 | - | 0.5 | T |
| Average | 54.88 | 26.94 | 2.0 | 0.88 | 0.38 | T | 0.72 | 0.05 |
| Onion Creek | ||||||||
| R.27-1 | 47.5 | 34.0 | 3.0 | 0.5 | T | - | 0.5 | T |
| R.27-2 | 46.5 | 22.5 | 2.5 | T | T | - | 1.0 | - |
| R.20-1 | 43.0 | 16.0 | 4.0 | T | T | - | 1.0 | T |
| R.20-6 | 62.0 | 24.0 | 2.0 | 0.5 | T | - | 2.0 | T |
| R.20-7 | 63.0 | 26.5 | 2.0 | 0.5 | T | - | 2.0 | T |
| R.20-8 | 59.5 | 29.0 | 1.5 | 1.0 | T | - | 1.5 | T |
| R.2A-430 | 73.0 | 14.0 | 3.0 | T | T | - | 1.0 | T |
| Average | 56.35 | 23.71 | 2.57 | 0.35 | T | - | 1.28 | T |
| Wilson Co. channel | ||||||||
| R.28-1 | 53.5 | 16.0 | 2.5 | T | 0.5 | - | 1.5 | T |
| R.28-2 | 60.0 | 26.0 | 2.5 | 0.5 | T | - | 2.0 | T |
| R.28-3 | 58.5 | 33.5 | 0.5 | T | T | - | 0.5 | T |
| K-39 | 42.0 | 29.5 | 3.0 | 0.5 | T | - | 0.5 | T |
| K-40.5 | 49.0 | 37.5 | 1.5 | 1.0 | 1.0 | - | 0.5 | 0.5 |
| Average | 52.6 | 28.5 | 2.0 | 0.4 | 0.3 | - | 1.0 | 0.1 |
| Woodson Co. channel | ||||||||
| I-37.4 | 40.5 | 20.0 | 1.0 | T | T | - | 0.5 | T |
| I-44.4 | 36.5 | 21.0 | 0.5 | 0.5 | T | - | T | T |
| J-28.7 | 37.5 | 22.5 | 1.0 | T | 0.5 | - | 1.0 | T |
| J-31 | 41.0 | 22.5 | 3.0 | 1.0 | 0.5 | - | 0.5 | T |
| Average | 38.87 | 24.0 | 1.37 | 0.37 | 0.25 | - | 0.5 | T |
| Total Average | 52.28 | 25.88 | 2.06 | 0.56 | 0.24 | - | 0.9 | 0.04 |
| Sample | Heavy Minerals | Skeletal fragments |
Oolite | Coated grains |
Cement | Clay | Percentage of quartz within total detrital grains in each thin section |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zircon | Tourmaline | Calcite | Limonite | Sericite | Silica | ||||||
| Southern Montgomery Co. | |||||||||||
| R.3-4 | T | - | 0.5 | - | - | 39.0 | - | - | - | - | 97.5 |
| R.3-7 | T | T | - | - | - | - | 3.5 | 1.0 | 0.5 | 1.5 | 98.9 |
| R.3-11 | 0.5 | T | - | - | - | 0.5 | 3.0 | 2.5 | 0.5 | 2.0 | 95.6 |
| R.5-1 | T | - | - | - | - | - | 1.0 | 2.0 | - | 0.5 | 99.48 |
| R.5-2 | T | T | - | - | - | - | T | 1.5 | 1.0 | 0.5 | 98.96 |
| R.5-3 | T | T | - | - | - | - | T | 1.5 | - | 1.0 | 98.97 |
| R.8-4 | T | - | - | - | - | - | 1.5 | 2.5 | 1.0 | 1.0 | 95.7 |
| R.15-2 | T | - | 1.5 | 2.5 | 5.0 | 37.0 | - | - | - | - | 95.4 |
| R.15-4 | T | - | - | - | - | - | 6.0 | 3.0 | 1.0 | 2.0 | 96.0 |
| Average | 0.05 | T | 0.22 | 0.27 | 0.55 | 8.5 | 1.66 | 1.55 | 0.44 | 0.94 | 97.39 |
| Onion Creek | |||||||||||
| R.27-1 | T | - | - | - | - | 7.5 | 4.5 | 2.0 | - | 0.5 | 98.83 |
| R.27-2 | T | T | - | - | - | 24.5 | 2.5 | 0.5 | - | - | 98.62 |
| R.20-1 | T | - | T | - | - | 36.0 | T | - | - | - | 98.4 |
| R.20-6 | T | - | - | - | - | - | 5.5 | 2.5 | 1.5 | - | 97.2 |
| R.20-7 | T | - | - | - | - | - | 2.0 | 3.0 | - | 1.0 | 97.3 |
| R.20-8 | T | T | - | - | - | - | 3.5 | 2.0 | 0.5 | 1.5 | 97.3 |
| R.2A-430 | T | - | - | - | - | 9.0 | T | T | - | - | 98.9 |
| Average | T | T | - | - | - | 11.0 | 2.57 | 1.35 | 0.35 | 0.42 | 98.07 |
| Wilson Co. channel | |||||||||||
| R.28-1 | T | T | T | - | - | 21.0 | 4.5 | 0.5 | - | - | 97.2 |
| R.28-2 | T | - | - | - | - | - | 4.0 | 4.5 | 0.5 | - | 97.25 |
| R.28-3 | T | - | - | - | - | - | 4.0 | 1.5 | 0.5 | 1.0 | 99.4 |
| K-39 | - | - | T | - | - | 22.0 | - | 1.0 | 1.5 | - | 98.93 |
| K-40.5 | - | - | - | - | - | 1.5 | - | 3.0 | 3.5 | 1.0 | 96.7 |
| Average | T | T | T | - | - | 8.9 | 2.5 | 2.1 | 1.2 | 0.4 | 97.87 |
| Woodson Co. channel | |||||||||||
| I-37.4 | T | - | - | - | - | 38.0 | - | - | - | - | 99.19 |
| I-44.4 | - | - | - | - | - | 41.0 | - | - | 0.5 | - | 99.14 |
| J-28.7 | T | - | - | - | - | 37.5 | - | T | - | - | 97.6 |
| J-31 | T | - | - | - | - | 16.0 | - | 1.5 | 2.5 | 1.5 | 97.45 |
| Average | T | - | - | - | - | 33.12 | - | 0.37 | 0.75 | 0.37 | 98.34 |
| Total Average | 0.02 | T | 0.08 | 0.1 | 0.2 | 13.22 | 1.82 | 1.44 | 0.62 | 0.6 | 97.83 |
Components
Grains
Monocrystalline quartz is the dominant component, averaging more than 78%. These grains vary in average size among samples from very fine grained to medium-grained sand. Most grains are clear, with less than 10% containing inclusions or fractures. About two-thirds of the quartz grains show unit extinction, and the remainder show undulatory extinction. Silt-size quartz grains are the dominant component of the siltstone beds.
The polycrystalline quartz grains are well rounded and vary from very fine grained to fine-grained sand, making up 2% of the sandstones (table 1). Most of the polycrystalline grains are smaller crystals of uniform size, but some contain several crystals of different sizes that are stretched (foliated) and contain strongly sutured intercrystalline boundaries. These foliated textures suggest a metamorphic origin.
The only other significant detrital rock fragment type in Rock Lake sandstones are well-rounded, fine-grained sand size chert grains, which constitute about 1% of the gains in the sandstones.
Feldspar is the second most abundant mineral in the sandstones but constitutes only up to 3% of the grains in any one sample (table 1). Untwinned orthoclase, twinned plagioclase, and microcline are all present in small amounts. Most of the feldspar grains show various stages of alteration to clay minerals.
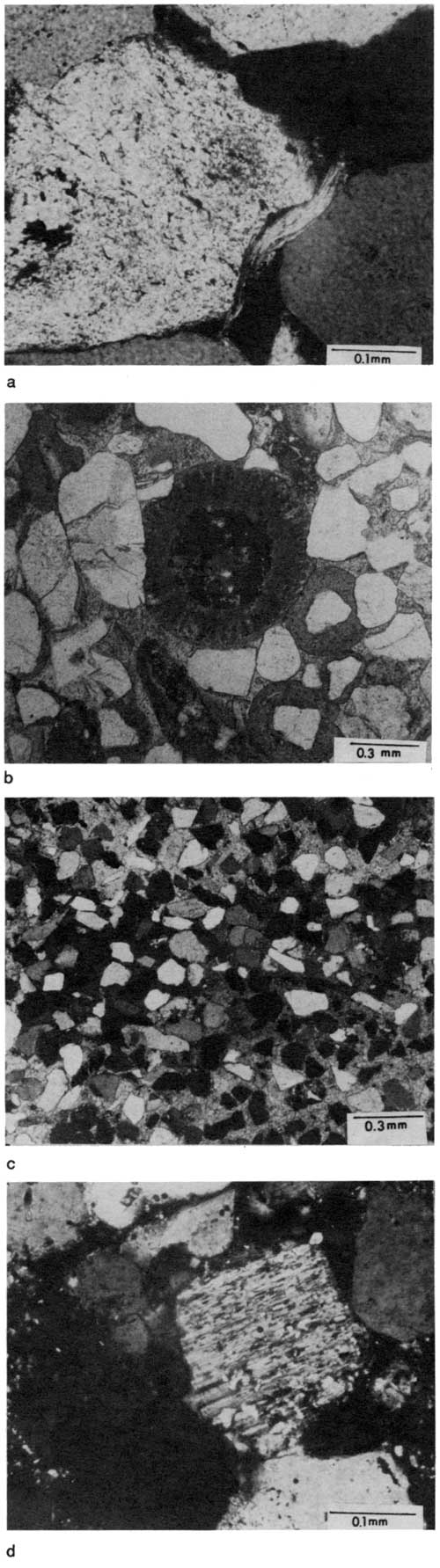
Muscovite commonly occurs as an accessory mineral in Rock Lake sandstones but never exceeds 0.5% of any one sample (table 1). Elongated rectangular flakes of muscovite, ranging from 0.05 mm to 0.2 mm, are usually found oriented parallel to bedding planes. However, some of the flakes are bent around more compact siliciclastic grains, indicating that they were deformed during compaction (fig. 15a).
Figure 15--Photomicrographs showing petrographic characteristics of Rock Lake Sandstone units. (a) Deformed muscovite flake bent around quartz grains; sample K-39; cross-polarized light. (b) Pervasive calcite-cemented quartzarenite with echinoderm fragments and coated grains; note replacement of calcite by pyrite in middle of echinoderm fragment and fining of fractures in quartz grain with calcite cement; sample R.9-2; plane-polarized light. (c) Poikiloblastic cementation texture; many detrital grains appear to be "floating" in single crystal of coarse calcite cement; sample R.3-4; cross-polarized light. (d) Very fine sand-sized plagioclase feldspar grain partially altered to sericite; sample J-31; cross-polarized light.
Zircon and tourmaline are the only heavy minerals found in the sandstones. Zircon occurs in trace amounts in most samples; tourmaline (in trace amounts) was found in only 7 of 25 samples analyzed (table 1).
Carbonate grains, such as oolites, coated grains, and skeletal fragments of marine organisms (mostly echinoderm, brachiopod, bryozoan, and mollusk), are present in a few samples of fine-grained sandstones (fig. 15b) in the lower part of the Rock Lake Shale Member in southern Montgomery County (table 1). Pyrite has replaced calcite in some of the skeletal fragments (fig. 15b). Carbonate grains were not found in the upper massive sandstones of southern Montgomery County nor in the Onion Creek sandstone. Thin sections of core samples from the northern channels of Wilson and Woodson counties show a few scattered skeletal fragments. These were observed in outcrop samples only in SE sec. 26, T. 26 S., R. 16 E., in the Woodson County channel and in the basal channel lag from a small side channel to the Wilson County channel in section K-47 (Moussavi-Harami, 1980, appendix C, section 28).
In terms of grain components, the Rock Lake sandstones are quite mature compared with other sandstones recently studied in the midcontinent. Missourian sandstones from lower cycles (Wyandotte, Iola) in the Kansas City region (Verhulst, 1970) and Desmoinesian sandstones from much older cycles in southeastern Kansas (Reinholtz, 1982; Worthington, 1982) contain significantly greater amounts of rock fragments, feldspar, and mica relative to quartz.
Cements
Four different types of cement are present in Rock Lake sandstone units: calcite, iron oxide (limonite), sericite, and silica (table 1). Calcite is the dominant cement in many of these rocks, accounting for up to 41 % of some samples. It is more abundant in surface samples of the lower sandstones than in the upper massive sandstones there and the Onion Creek sandstone in Montgomery County. Thin sections from cores I, J, and K (in the northern channels in Wilson and Woodson counties) and from the Trico Production Company (Onion Creek sandstone), however, show that calcite cementation is also important in the upper rocks in the subsurface. Much of the calcite cement shows a poikiloblastic fabric, in which single cement crystals encompass numerous sand grains (fig. 15c). In some samples detrital grains seem to "float" within the calcite cement, indicating that early cementation preceded compaction.
Iron oxide (limonite) is the second most abundant cement, occurring in most samples in amounts up to 4.5%. It forms coatings around grains and fills pore spaces. Limonite is relatively abundant in thin sections from surface exposures but is quite rare in subsurface samples. This indicates that iron oxide may be primarily a modern weathering product.
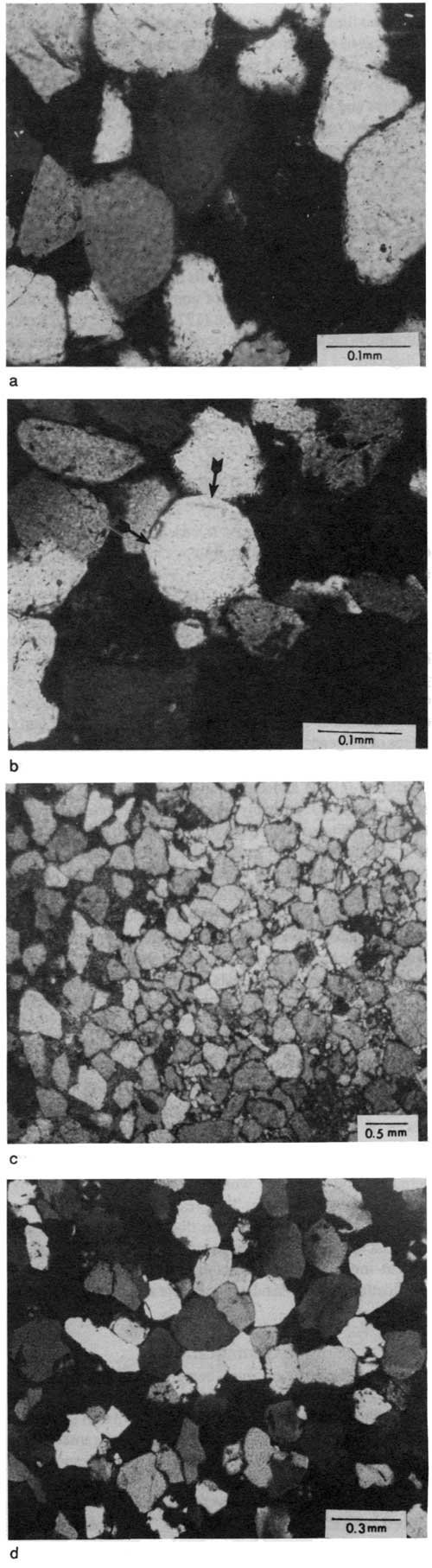
Sericite is present in most of the thin sections studied, occurring mainly as coatings around quartz grains. It also fills some of the pore spaces between detrital grains and is associated with altered feldspar (fig. 15d). Silica is present as quartz overgrowths on detrital grains (fig. 16a) and as chert replacing calcite.
Figure 16--Photomicrographs showing characteristics of silica and carbonate cements in Rock Lake sandstone units. (a) Subrounded to rounded detrital quartz grains with authigenic silica cement forming overgrowths; most of the dark areas are pore spaces filled with epoxy; sample R.3-7; cross-polarized light. (b) Well-rounded quartz grains with recycled (rounded) overgrowths, shown by "ghost" rims (arrows); sample R.20-4; cross-polarized light. (c) Pervasive calcite (lower relief, lighter color) and iron-rich dolomite (higher relief, darker color) cements in sample TPC-2A, 422.1; plane-polarized light. (d) Syntactic overgrowths of quartz forming a mosaic of interlocking crystals; sample R.26-4; cross-polarized light.
Matrix
Clay is present as a matrix. It makes up as much as 2.0% of some thin sections but averages less than 1% of the rock (table 1). It is possible that most of the clay in these rocks is authigenic and formed during the alteration of feldspar. Most of the apparently authigenic clay in these rocks occurs as pore fillings, pore linings, and pseudomorphic replacements of feldspar grains similar to those illustrated by Wilson and Pittman (1977). Schroeder (1967) reported that some clay minerals in algal-mound facies and associated limestones of Woodson and Wilson counties are dickite and kaolinite. X-ray diffraction analyses indicate that the white powdery clay in the sandstones of cores I, J, and K is dickite.
Thin sections from two core samples from a well in the northern end of the detrital facies belt (SE sec. 14, T. 32 S., R. 12 E.) supplied by the Trico Production Company show the presence of intergranular oil.
Textures
I measured the average grain size petrographically in 88 samples of Rock Lake sandstone using the loose-grain analysis method of Griffiths (1967). Measurement of 50 monocrystalline quartz grains in each sample indicates that the average grain size in these rocks varies between 0.0625 mm (very fine grained sand) and 0.5 mm (medium-grained sand). These analyses indicate that the lower sandstone unit exposed in southern Montgomery County, the lower sandstone beds in the Onion Creek area, and some of the sandstone beds in the northern channels are predominantly fine to very fine grained. In contrast, the upper massive sandstones of southern Montgomery County, the upper Onion Creek body, and most of the northern channel fillings are fine to medium grained.
Mineralogically and texturally, these sandstones are submature to mature (Folk, 1951, 1974). The siliciclastic grains probably have been subjected to several cycles of erosion and sedimentation. Grains in these units are predominantly moderately to well sorted, except in conglomeratic lenses at the base of channel deposits. Grain shape varies from subangular to rounded, and, less commonly, angular. Where observable in thin section, contacts between grains are mainly tangential and longitudinal.
In many sandstones detrital grains float within coarser crystals of calcite cement in a poikilotopic fabric (Fuhrmann, 1968; Pettijohn et al., 1973; Scholle 1979, p. 119). Authigenic silica overgrowths are present as rims around quartz grains. On some of these gains the silica overgrowths are well rounded, indicating that overgrowth occurred during a previous cycle of deposition (fig. 16b). Because authigenic overgrowths may have modified original grain shape and size, care must be used when interpreting textural features (Jacka, 1970).
Diagenesis
In what follows I discuss a variety of postdepositional events that affected the petrographic characteristics of these rocks.
Compaction
Friability of exposed and core-sample sandstones of the Rock Lake Shale Member renders them unamenable to packing calculations. Compaction in these sandstones is related to lithostatic pressure (overburden) and to concomitant mechanical rearrangement of grains during the early stages of diagenesis. Because these rocks were probably not buried deeply in the nonorogenic cratonic setting, high temperatures and pressures would not have been controlling factors in their diagenesis. The presence of concave-convex grain-to-grain contacts indicates that some compaction did take place. However, detrital grains "floating" in calcite cement indicates that cementation was a major factor in lithification of some sandstones before any significant compaction.
Cementation
Calcite, the most common cement, averages more than 13% of these rocks (table 1). Calcite-cement crystals are predominantly large, ranging from 1 mm to 3 mm in diameter. Boundaries between adjacent cement crystals are straight.
It is likely that calcite cement in Rock Lake sandstones formed in two different generations, similar to the situation in the Lower Cretaceous Muddy Sandstone of Wyoming described by Almon and Davies (1979). Early generation of calcite cement occurred before much compaction. The cement exhibits poikilotopic texture (grains "floating" among large calcite-cement crystals). Early cementation is probably related to solution of unstable carbonate fossil fragments in percolating meteoric water during regression and the emergence of the sand bodies, as outlined by Heckel (1983) for regressive carbonates. This resulted in supersaturation of the percolating water with respect to calcium carbonate. Crystallization of poikilitic calcite crystals probably resulted from slow precipitation from a dilute solution with few nuclei rather than from rapid crystallization around many nuclei, such as what occurs when many carbonate grains are present. Poikilitic cement is observed mostly in the lower sandstone facies in southern Montgomery County. This level is low enough in the sand sequence for percolating meteoric water to have become saturated with dissolved carbonate from higher levels.
The poikilotopic cementation texture is also observed in some outcrop and core samples from the Trico Production Company well at the northern end of the detrital facies belt. Here it is probably related to dissolution of unstable carbonate grains from the southern end of the algal-mound facies belt. Poikilitic cementation has also been observed in some thin sections of outcrop and core samples from the northern channels (in Wilson and Woodson counties), where it is probably related to solution of adjacent emergent carbonate buildups and reprecipitation as sparry calcium carbonate in the channel sandstones.
The later generation of calcite cement, which partially replaces silica overgrowths, occurs in more compacted sediments and was probably precipitated in response to supersaturation of deeper connate waters with calcium carbonate. A sample from the Trico core at the northern end of the detrital facies belt displays two different cement types (fig. 16c): ferroan dolomite, which fills most of the pore space in one thin section, and patches of nonferroan calcite in small areas. Al-Shaieb and Shelton (1978) state that the ferroan dolomite in the shallow-burial sediments of the Chadra Sands (Oligocene of Libya) formed in a meteoric-connate mixing environment similar to the situation described by Folk and Siedlecka (1974) and Folk and Land (1975) and that the source of iron was probably alteration of glauconite. Because the Rock Lake sandstones were buried only to shallow depth, it is possible that the ferroan dolomite formed from the mixing of meteoric and connate waters, probably during cementation of calcite, similar to the situation described by Al-Shaieb and Shelton (1978). The source of iron was possibly dissolved iron in the low-oxygen connate water, or the iron may have come from adjacent shales during diagenetic alteration of clay minerals, for example, chlorite.
The second most abundant cement in Rock Lake sandstones, iron oxide (limonite), constitutes an average of less than 2% of the total rock (table 1). It fills some of the pore space between grains and is also seen as coatings around some detrital grains in outcrop samples. Because iron oxide occurs as a cement in surface exposures compared to only traces of iron oxide present in shallow core samples and because there is no evidence of iron-bearing minerals (e.g., hornblende, chlorite, or magnetite) in subsurface rocks, the major source of iron oxide is probably modern soil-forming processes, such as hydration of brown amorphous ferric oxide to limonite. The presence of iron oxide in shallow core samples suggests that another source of iron may be dissolution of ferroan dolomite. It is also possible that a small amount of iron was dissolved in the connate water and precipitated as iron oxide in the subsurface.
Sericite, the third most abundant cement in these rocks, occurs most commonly as a pore filling but also occasionally as a grain coating. Sericite cement may form as a result of recrystallization of clay minerals or hydrolysis of feldspar during compaction. It is more likely that most of the sericite cement formed from alteration of feldspar because most feldspar grains show some form of incipient alteration, especially along cleavage traces.
Silica cement, the least abundant type of cement in Rock Lake sandstones, averages less than 1% of the total rock (table 1). The most common form of silica cementation is syntactic overgrowths that form euhedral crystals or a mosaic of interlocking overgrowths (fig. 16d). The well-rounded silica overgrowths in these rocks are related to previous cycles of sedimentation and therefore are not counted as diagenetic components in the present study.
Chert is another form of silica cement that has been observed as a void filling in some sandstone units. Chert cement is found in a few subsurface samples of the northern channels (Wilson and Woodson counties) but has not been observed in either surface or subsurface samples from the Onion Creek sandstone or southern Montgomery County sandstones. The presence of scattered calcareous fossil fragments and the size of chert-filled areas in sandstone suggest that chert may have replaced some fossil fragments.
The sources of silica cement in sandstone have been reviewed by Waldschmidt (1941), Heald (1955, 1956), Siever (1959, 1962), Siever et al. (1965), Dapples (1971), Thompson (1959), Weyl (1959), Towe (1962), Phillip et al. (1963), and Füchtbauer (1967). Blatt (1979) suggested several possible sources of silica overgrowths: (1) dissolved silica in pore spaces from pressure solution along grain boundaries, (2) precipitation of silica produced from hydrolysis of feldspar grains by a weak carbonic acid solution, (3) release of silica from conversion of clay minerals (e.g., smectite and interlayered smectite-illite to pure illite), (4) diagenetic alteration of volcanic rock fragments within sandstone, and (5) dissolution of opaline siliceous skeletal fragments, such as diatoms and radiolarians. All possibilities except item 4 are likely sources of silica in the Rock Lake sandstones.
Replacement
Quartz-calcite cement contact relationships indicate that replacement of quartz by calcite has taken place in sandstones of the Rock Lake Shale Member. One of the most conspicuous contact features is the etching of quartz grains with embayments filled with calcite cement. Such replacement cementation is related mainly to chemical instability of quartz grains relative to calcite. Dapples (1971) suggested that replacement of quartz by calcite in certain sandstones is related to pH changes, which is controlled by carbon dioxide changes near the outcrop. Silica solution takes place contemporaneously with calcite cementation if the rate of dissolution is slow (Dapples, 1971). Blatt et al. (1980) suggested that both pH and temperature are important controlling parameters in the replacement of quartz by calcite. As both pH and temperature increase, the solubility of silica tends to increase and the solubility of calcite decreases. Nevertheless, it is likely that pH rather than temperature was the major controlling factor of replacement in the Rock Lake sandstone because these rocks are located in a tectonically inactive area and were not buried deeply and thus were not subjected to high temperatures.
Replacement of calcite by chert is suggested in a few core-sample thin sections from the northern channels. The evidence for this is a few small crystals of calcite that occur in patches of chert. It is possible that a decrease in pH caused calcite to dissolve and silica to precipitate.
Pyrite occurs as a partial replacement of calcite is some shell fragments. Berner (1971) reported that stable pyrite can form under conditions of low Eh and moderate to low sulfur content. Such an environment is found where organic matter is abundant, that is, where sulfur is reduced by bacteria. The presence of scattered fossils in the Rock Lake sandstones provides a source of sulfur in the organic compounds of decaying organisms. The iron may have been brought into the system by low-oxygen meteoric waters that leached soils or may have been derived later from dissolution of ferroan dolomite.
Alteration
The most common diagenetic alteration in the Rock Lake sandstones is decomposition of feldspar to clay. Both plagioclase and alkali feldspar grains are partially altered to sericite. The localization of sericite in areas of feldspar decay suggests recrystallization of the alumina and silica components. Sericite and other clay minerals were probably a product of partial hydrolysis of feldspar grains along surfaces of exposure, such as grain surfaces and cleavage traces. Füchtbauer (1967) suggested that, during the early stages of coalification, water with dissolved humic acid and CO2 migrated from coal beds into Upper Carboniferous sandstones of northern Germany, forming an acidic environment in which feldspars were hydrolyzed and replaced by kaolinite. The presence of organic matter, such as plant fragments, in the Rock Lake sandstones supports formation of an acidic environment in this way, creating the proper conditions for hydrolysis of feldspar grains.
Paragenesis
Definitive paragenetic relations among all the diagenetic events in the Rock Lake sandstones are complicated by present-day weathering of the outcrop and the relatively small number and local distribution of subsurface samples. A hypothetical sequence of diagenetic events can be proposed based on the criteria and relations discussed previously (fig. 17).
Figure 17--Probable sequence of diagenetic events (paragenesis) occurring since deposition of Rock Lake sandstones.
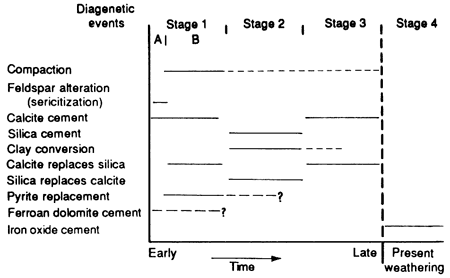
Stage 1--The first stage took place during shallow burial with only minimal compaction, indicated by a loosely packed grain fabric. Early calcite cementation produced a poikiloblastic texture with grains "floating" in calcite cement. It probably occurred during a regression, when meteoric water replaced marine connate water in the sediment, dissolved carbonate fossil fragments in the sandstones, and began to dissolve emergent carbonate buildups along the northern channels until the water became supersaturated with calcium carbonate, which then precipitated as cement (Longman, 1980; Heckel, 1983). Replacement of quartz by calcite, indicated by corroded quartz grains with embayments filled with calcite cement, may have occurred during early calcite cementation, as both pH and temperature increased (fig. 17, substage B). This caused silica to be dissolved and calcite to be deposited.
Alteration [hydrolysis of feldspar to sericite (sericitization) and other clay minerals] along the surface of grains is also probably a precompaction phenomenon (fig. 17, substage A) that released some silica into the connate water. Both processes may have aided later silica cementation. In one core ferroan dolomite in a poikiloblastic cementation texture possibly formed during early cementation in a meteoric-connate mixing environment before the South Bend Limestone Member was deposited. Replacement of calcite by pyrite in carbonate shell fragments may have been contemporaneous with compaction and cementation in low Eh microenvironments created by the decay of organic matter within the skeletal fragments.
Stage 2--Conversion of clay minerals, such as smectite to illite, is considered to correspond in time with compaction and would have provided some silica for formation of silica cement. Silica cement (quartz overgrowths) formed after silica was released into the connate water by the processes discussed earlier. Replacement of calcite by silica, as shown by a few crystals of calcite left within the chert cement, probably is related to a decrease in pH that caused calcite to dissolve and silica to precipitate.
Stage 3--Second-generation or late calcite cement formed in overcompacted pore space in more deeply buried sediments and is probably related to the supersaturation of later connate water. Replacement of silica by calcite occurred along the margins of quartz grains in sediments with tighter packing modes than those discussed in stage 1.
Stage 4--Iron oxide cements, which surround most of the detrital grains in outcropping rocks, probably formed from the migration of modern oxygenating meteoric ground water through the rocks as they were exhumed. This process dissolved ferroan carbonate cements and oxidized the released ferrous ions, which then precipitated as ferric oxide coatings on grains. The formation of iron oxide cements probably is continuing at present.
Prev Page--Stratigraphy || Next Page--Sedimentologic analysis
Kansas Geological Survey, Geology
Placed on web Nov. 4, 2010; originally published 1990.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/GS5/04_petro.html