Gypsum in Kansas
by Robert O. Kulstad, Paul Fairchild, and Duncan McGregor

Originally published in 1956 as Kansas Geological Survey Bulletin 133. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (19 MB) is also available, with plates available separately.
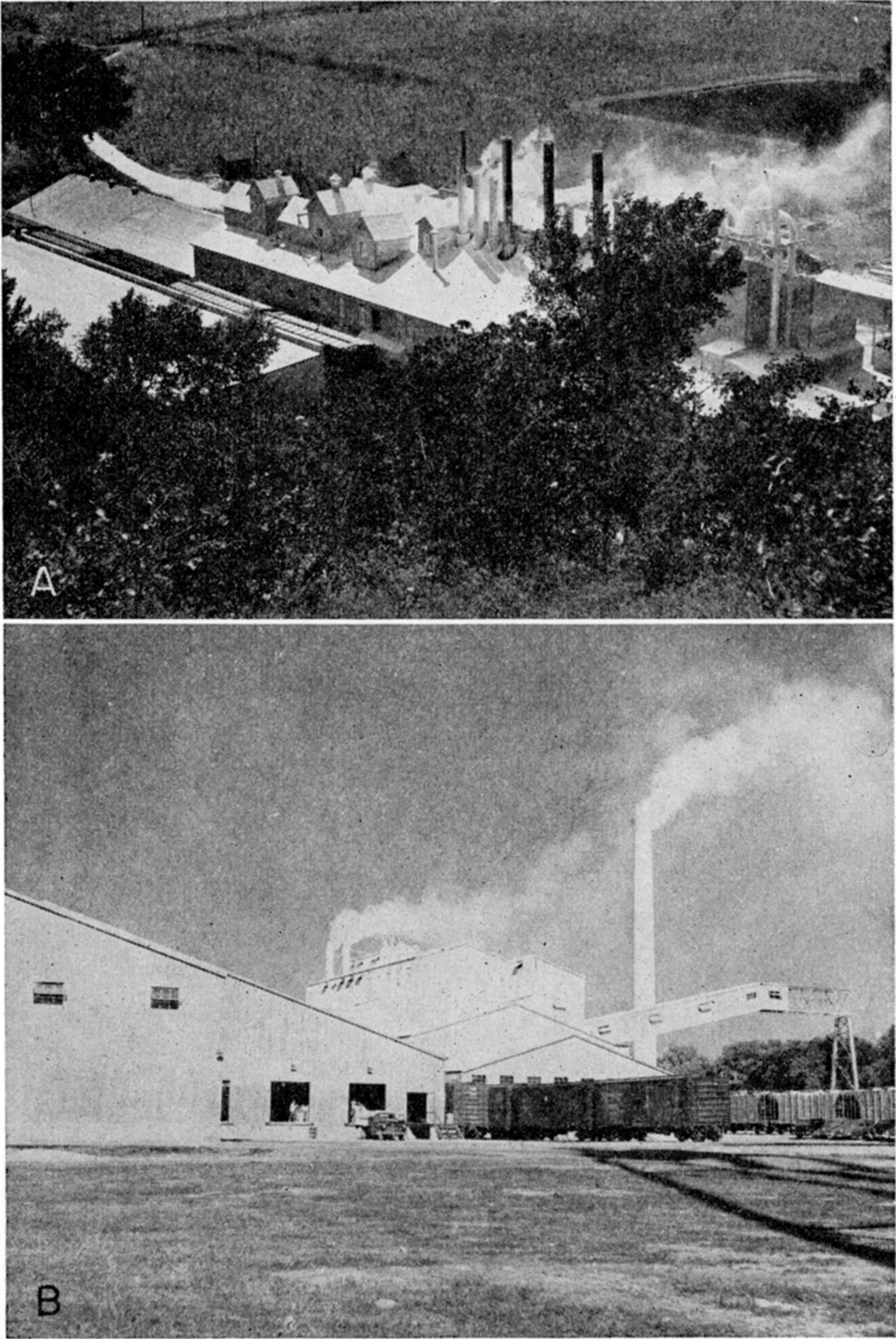
Frontispiece—A. Gypsum processing plant, Certainteed Products Company, Blue Rapids, Kansas. B. Gypsum processing plant, National Gypsum Company, Medicine Lodge, Kansas.
Abstract
Calcium sulfate occurs naturally in two forms, gypsum (CaSO4 · 2H2O) and anhydrite (CaSO4). Additional forms can be obtained by the application of heat to gypsum. The hemihydrate (CaSO4 · 1/2H2O) or "plaster of paris" is the most common of these additional forms. Either gypsum or anhydrite may be deposited from an evaporating body of sea water. Temperature and salinity of water from which the calcium sulfate is depositing are factors that determine which mineral is deposited. Either form may alter to the other, subsequent to deposition.
The known gypsum deposits in Kansas are found in rocks of Permian age. A brief description of Kansas Permian rocks is included in the report.
The gypsum mined near Sun City by the National Gypsum Company is obtained from the Medicine Lodge member, Blaine formation, Nippewalla group, in the upper part of the Kansas Permian section. Gypsum is crushed at the mine and transported by rail 20 miles to the plant at Medicine Lodge for processing. The gypsum beds are found at the surface in much of Barber and Comanche Counties. The Medicine Lodge member consists of gypsum and anhydrite in a single bed, which has a maximum known thickness in Barber County of approximately 30 feet. The anhydrite occurs in lenses in the middle of the bed. Both gypsum and anhydrite are thought to have been formed by original deposition from sea water, although some of the anhydrite has subsequently been hydrated to gypsum.
Gypsum is found in the Wellington formation, which is exposed almost continuously from Nebraska to Oklahoma, but is no longer mined, although several mines operated in the past in the Wellington outcrop area. Wellington gypsum is exposed at few places on the surface, but evidence of gypsum throughout the outcrop area is provided by surface slump structure, old gypsum mines, and subsurface information obtained from oil exploration. The Wellington formation contains a considerable amount of relatively soluble evaporites; consequently its true character is destroyed by weathering. Subsurface information reveals that the Wellington, where undisturbed, contains a salt sequence several hundred feet thick. Directly below the salt is an anhydrite zone 100 to 150 feet thick in the area studied. This anhydrite zone seems to correlate with the gypsum deposits found at or near the surface. Gypsum probably was formed by the hydration of this anhydrite by the action of ground water, although not enough is known about the gypsum deposits to eliminate the possibility that some of them may have been deposited directly as gypsum contemporaneously with the anhydrite.
Gypsum is mined at Blue Rapids by the Certainteed Products Company from the only known deposits of gypsum in the Easly Creek shale, in the Council Grove group of Permian rocks. The company produces gypsum products in its plant at the mine site. The average thickness of the gypsum bed is between 8 and 9 feet. Petrographic evidence is interpreted as showing that the calcium sulfate was deposited from sea water as gypsum. Some subsequent recrystallization could have resulted from diagenetic processes, some from the action of ground water.
Other gypsum-anhydrite deposits are found in the Day Creek dolomite near the top of the Kansas Permian, in the Stone Corral dolomite of the Sumner group, and in the Oaks shale member of the Hamlin shale of the Admire group.
Most gypsum is calcined for use in the building industry as base coat and finishing plaster or in prefabricated products such as wall board. Calcined gypsum or "plaster of paris" is used also in small amounts as cast plaster, pottery plaster, industrial and molding plaster, dental and orthopedic plaster, and in the manufacturing of plate glass. Raw gypsum is used in the manufacture of portland cement and as fertilizer. Gypsum is also a possible source of sulfur. Gypsum is calcined in kettles or in rotary kilns. Special types of plaster require special handling, and prefabricated gypsum products require special machinery in their manufacture.
Kansas Geological Survey, Geology
Placed on web Feb. 20, 2018; originally published March 21, 1956.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/133/index.html