Prev Page--Introduction || Next Page--Blaine Formation
Geology and Properties of the Calcium Sulfate Minerals
Properties of Gypsum
Physical properties
Gypsum (CaSO4 · 2H2O) has a specific gravity of 2.32 and hardness of 2. It is commonly colorless, white, or gray. Shades of yellow, red, and brown result from impurities. Crystals, when present, are generally of tabular or prismatic habit and crystallize in the monoclinic system. "Swallowtail" twins, with the orthopinacoid (100) as the twinning plane, are relatively common.
Cleavage is present in two directions: perfect cleavage parallel to the clinopinacoid (010); less perfect parallel to the orthopinacoid (100); there is fibrous fracture to the negative hemipyramid (111).
Varieties of gypsum
The most common variety of gypsum is massive or rock gypsum. It consists of an intimate intergrowth of small crystals, and ranges from finely granular to coarsely crystalline in texture. It is widely distributed as a sedimentary rock interstratified with limestone and shale. Selenite is a colorless variety of gypsum, occurring as transparent crystals or foliae. Satin spar is a finely fibrous variety filling veins in massive gypsum or other rocks.
Properties of Anhydrite
Anhydrite (CaSO4) has a specific gravity of 2.93 and a hardness of 3 to 3.5. It is commonly white, but a grayish, bluish, or reddish tinge may result from the presence of small amounts of impurities.
The common form is massive; crystals, which are rare, may be lamellar, tabular, or elongate parallel to the "b" crystallographic axis, and crystallize in the orthorhombic system. Cleavage is parallel to the basal pinacoid (001), brachypinacoid (010), and macropinacoid (100). Crystals may be twinned parallel to the macrodome (101).
Relationships of the Calcium Sulfate Minerals
Calcium sulfate occurs naturally as gypsum (CaSO4 · 2H2O) and anhydrite (βCaSO4). Three additional forms are known but are not found in nature. These are the hemihydrate or "plaster of paris" ( CaSO4 · 1/2H2O ), formed at 128° C., "soluble anhydrite" (γCaSO4), formed at 163° C., and an extremely high temperature (1,200° C.) form, which decomposes rapidly into CaO and SO3 (Posnjak, 1938; Newman, 1941). These other forms can be produced by heating gypsum, and these transformations must follow a definite succession. All but the last state are obtained at temperatures below 200° C. The order of succession is as follows: gypsum, hemihydrate, "soluble anhydrite", and anhydrite.
The reactions that alter gypsum to the other calcium sulfate minerals are reversible. If water is added to the hemihydrate, gypsum is again formed. The fine needlelike crystals produced by this reaction form an interlocking mass that has cementitious properties. "Soluble anhydrite" is so easily hydrated that if exposed to air it will react with water vapor to form gypsum. Anhydrite reacts with water much more slowly, but laboratory and geological evidence indicates that it too will alter to gypsum.
The development of a multimillion-dollar gypsum industry was made possible by the following factors: (1) Gypsum is an abundant, naturally pure raw material. (2) It can be dehydrated to the hemihydrate at relatively low temperature using relatively simple equipment. (3) The reaction is virtually 100 percent complete, as is the reverse reaction. (4) The product of the reaction (plaster) is stable for a long period of time if stored in a dry place.
Deposition of Gypsum and Anhydrite
The association of calcium sulfate (gypsum-anhydrite) and halite affords evidence that these beds have been deposited by evaporation of sea water within a restricted basin. Table 2 (modified from Clarke, 1924, p. 220), shows the depositional sequence of common evaporite salts precipitated from evaporating sea water. Further evaporation of the sea water precipitates magnesium, potassium, and bromide salts.
Table 2—Salts laid down in concentration of sea water (in grams) (Modified from Clarke, 1924, p. 220)
| Density, gram/liter |
Volume, liters |
Iron oxide |
Calcium carbonate |
Calcium sulfate |
Sodium chloride |
Magnesium sulfate |
Magnesium chloride |
Sodium bromide |
Potassium chloride |
|---|---|---|---|---|---|---|---|---|---|
| 1.0258 | 1.000 | ||||||||
| 1.0500 | .533 | 0.0030 | 0.0642 | ||||||
| 1.0836 | .316 | Trace | |||||||
| 1.1037 | .245 | Trace | |||||||
| 1.1264 | .190 | .0530 | 0.5600 | ||||||
| 1.1604 | .1445 | .5620 | |||||||
| 1.1732 | .131 | .1840 | |||||||
| 1.2015 | .112 | .1600 | |||||||
| 1.2138 | .095 | .0508 | 3.2614 | 0.0040 | 0.0078 | ||||
| 1.2212 | .064 | .1476 | 9.6500 | .0130 | .0356 | ||||
| 1.2363 | .039 | .0700 | 7.8960 | .0262 | .0434 | 0.0728 | |||
| 1.2570 | .0302 | .0144 | 2.6240 | .0174 | .0150 | .0358 | |||
| 1.2778 | .023 | 2.2720 | .0254 | .0240 | .0518 | ||||
| 1.3069 | .0162 | 1.4040 | .5382 | .0274 | .0620 | ||||
| Total deposit | .0030 | .1172 | 1.7488 | 27.1074 | .6242 | .1532 | .2224 | ||
| Salts in last bittern | 2.5885 | 1.8545 | 3.1640 | .3300 | 0.5339 | ||||
| Sum | .0030 | .1172 | 1.7488 | 29.6959 | 2.4787 | 3.3172 | .5524 | .5339 | |
Calcium sulfate may be deposited from sea water either as gypsum or as anhydrite, depending on the temperature of deposition and the concentration of other sea salts (mainly sodium chloride) in the water from which the calcium sulfate is deposited. Posnjak (1940) showed that 42° C. is the critical temperature above which anhydrite is deposited and below which gypsum is deposited. Increased concentration of other sea salts in the aqueous solution lowers this. temperature and affects the solubility of both gypsum and anhydrite at 30° C., as shown in Figure 2 (from Posnjak, 1940).
Figure 2—Graph showing solubility of gypsum and anhydrite in sea water of various concentrations (from Posnjak, 1940).
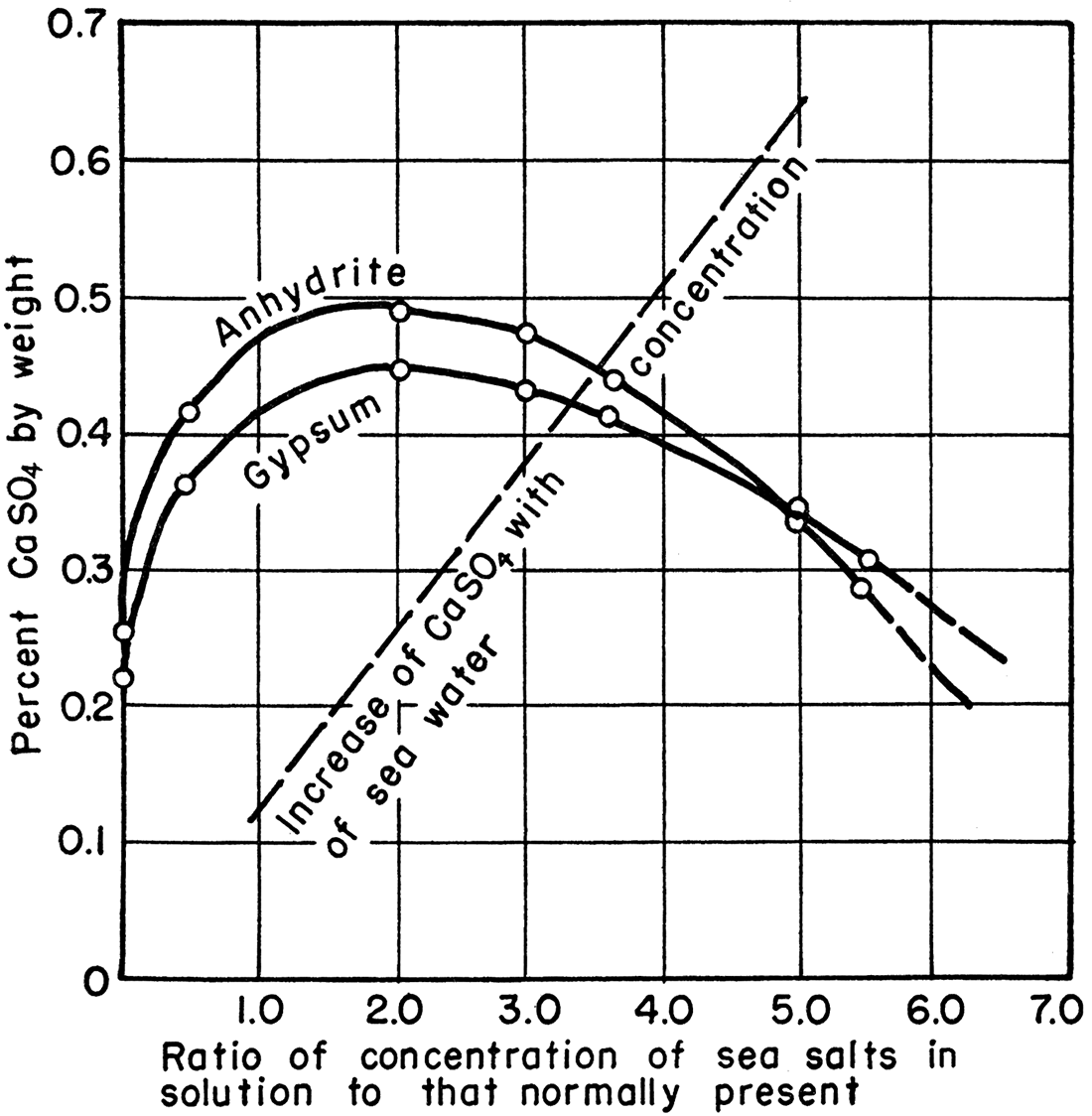
Thick gypsum beds such as are found in Kansas present further problems. For example, deposition of a 30-foot bed of gypsum in the Blaine formation would require the evaporation of more than 8 miles of sea water, assuming that sea water contained the same amount of calcium sulfate in Blaine time as it does now. So deep a basin of evaporation is incredible; the formation of such thick beds of gypsum required a process more complicated than single-stage evaporation of water in an enclosed arm of the sea.
Several theories have been advanced to account for the origin of various evaporite deposits found in different parts of the world. Ochsenius proposed the now classic theory of evaporation in basins that are partly cut off from the sea by a bar or barrier (Grabau, 1920). As evaporation progressed these basins would periodically receive new supplies of water, as at high tides. Such conditions would result in a more or less continuous "salt pan" so shallow basins would suffice for the accumulation of thick beds of salt or gypsum. Branson (1915) presented a modification of Ochsenius' theory, assuming the existence of sub-basins separated by low barriers, so that new supplies of concentrated brine from the seaward basins overflow into the landward basins. Under such conditions gypsum would be deposited in the seaward basins and salt in the landward basins. Fulda (1935) suggested that thick evaporites can be formed in basins adjacent to the sea if sea water is added only by seepage through the barrier into the basin. Continued seepage and evaporation would thereby build up the deposit. King (1947) proposed a theory whereby continued restricted access to the open sea is maintained. Under these conditions sea water enters the basin at or near the surface of the water. Upon partial evaporation in the basin it deposits its least soluble constituents. Simultaneously it becomes more dense, sinks below the incoming sea water, and eventually passes to the sea at depth below the incoming current.
Subsequent Alteration of the Calcium Sulfate Minerals
The stability relationships of gypsum and anhydrite through a range of temperature and pressure have been studied by Bowles and Farnsworth (1925). They found that anhydrite was unchanged at a pressure of 19 atmospheres and temperature of 210° C. Gypsum subjected to the same pressure changed to anhydrite at 160° C. Such a temperature can be produced in nature only by extremely deep burial or by intrusion of igneous rock. They proposed that temperature was more effective than pressure in the inversion of gypsum.
McCormak (1926) likewise concluded that temperature was a more important factor than pressure in the dehydration of gypsum. He added, however, that pressure plus time in the geologic sense is an unknown factor and may have more influence on the dehydration of gypsum than any laboratory experiments could indicate.
Newland (1921, p. 141) stated that some economically important gypsum deposits in New York were originally anhydrite. He considers gypsum to be unstable under conditions of permanent load, whereas anhydrite is stable. Evidence for this is the fact that gypsum grades into anhydrite down dip. Muir (1934, p. 1297-1318) stated that the gypsum members of the Blaine formation of Oklahoma were originally beds of sedimentary anhydrite. He based his conclusions on two factors: (1) petrographic evidence shows that gypsum has replaced anhydrite, indicating that anhydrite was the original precipitate; (2) anhydrite is more often encountered than gypsum in drill cores and cuttings at lower depths, that is, down dip away from the outcrop.
Summarizing, either gypsum or anhydrite may be deposited as a result of the evaporation of sea water, depending upon the temperature and salinity of the water. As salinity increases, the temperature above which anhydrite will be deposited and below which gypsum will be deposited is lowered. The Kansas deposits could not have originated from one single body of water because of the tremendous depth of water required; therefore, these beds must have been deposited in a gradually sinking basin of evaporation receiving several influxes of sea water. After deposition either gypsum or anhydrite can alter to the other, under suitable conditions. Temperature, pressure, and the presence of water determine the stability of these minerals.
The foregoing discussion deals in a general way with the deposition from sea water and subsequent alteration of gypsum and anhydrite. Evidence will be presented to show that individual gypsum deposits in Kansas originated in the following manner: (1) The gypsum in the Easly Creek shale was deposited directly from sea water and was later recrystallized, possibly in part during diagenesis, but chiefly by ground water. (2) By far the major portion and probably all of the calcium sulfate in the lower Wellington formation was deposited as anhydrite. The gypsum found in the Wellington was probably formed by the alteration of anhydrite, although the possibility of direct deposition cannot be entirely eliminated. (3) The calcium sulfate deposits in the Medicine Lodge were deposited both as anhydrite and as gypsum, the anhydrite making up the middle portion of the bed. Subsequent alteration has transformed some of the anhydrite to gypsum.
Permian Rocks in Kansas
Virtually all the known gypsum deposits in Kansas are of Permian age. The following general description of the character and surface distribution of rocks belonging to the Permian System in Kansas is given by Moore and others (1951, p. 33).
"Rocks of Permian age which occur in Kansas include evenly stratified predominantly marine deposits in the lower part of the section and irregularly bedded, mainly nonmarine deposits in the upper part. Light ash-gray to cream-colored limestone beds, many of which are distinguished by abundance of flinty chert form persistent benches or escarpments, among which the so-called Flint Hills are most prominent. The Flint Hills extend across Kansas from Nemaha County on the Nebraska border to western Chautauqua County adjoining the Oklahoma boundary. The escarpments are east-facing because the gentle regional dip of the Permian strata is westward. Between the limestone formations and members of the Lower Permian are gray, green, and red shale units, in part containing marine fossils and in part representing nonmarine sedimentation. Sandstone is virtually absent in this part of the section.
"Middle and upper parts of the Permian succession consist mainly of shale and sandstone, many of them red in color. Thick deposits of salt which occur do not crop out, because the Kansas climate is not arid enough to allow such soluble rock to be exposed at the surface. Gypsum beds, some of minable thickness, may be seen, however, and a few thin but persistent dolomites occur in the redbeds part of the Permian section.
"The outcrops of Permian rocks in Kansas form a belt extending from Washington, Marshall, Nemaha, and Brown Counties on the northern boundary to Meade, Clark, Comanche, Barber, Harper, Sumner, and Cowley Counties on the Kansas-Oklahoma line. The total outcrop thickness is about 3,000 feet."
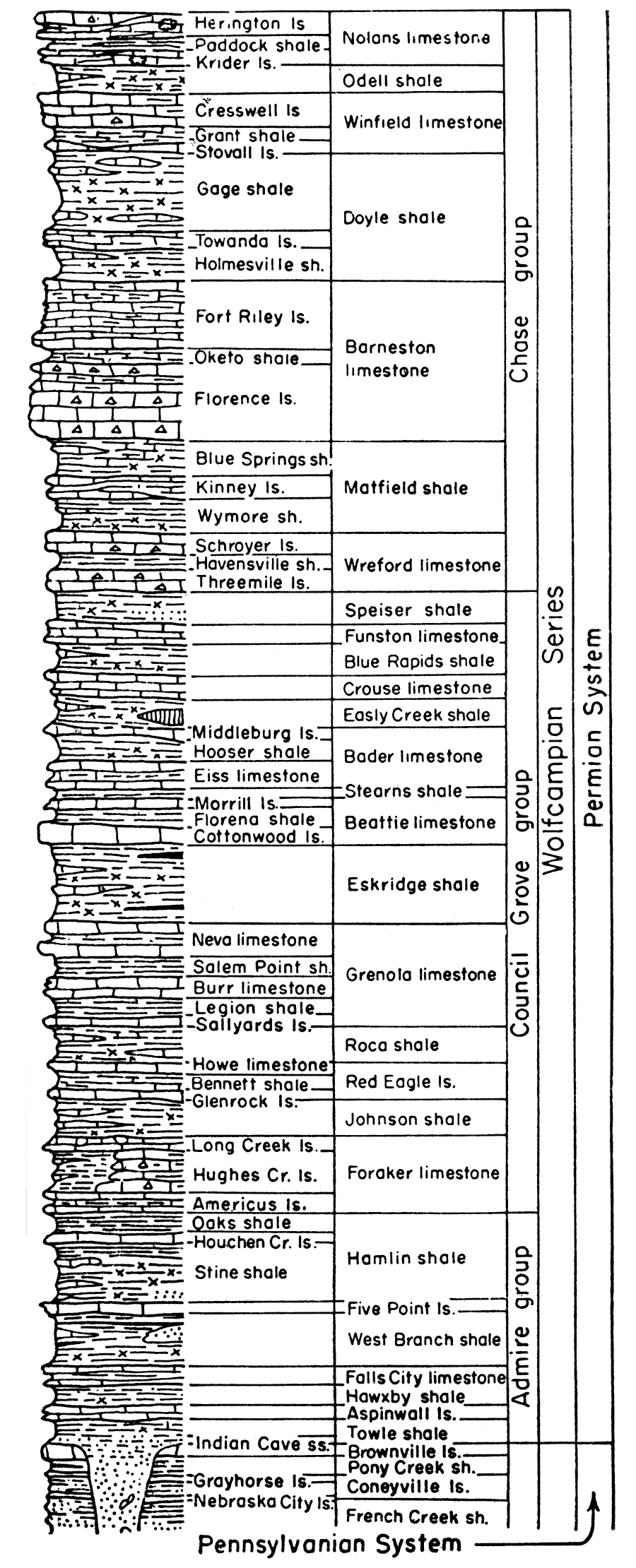
Permian rocks in the Midcontinent region are divided into four series from oldest to youngest as follows: Wolfcampian, Leonardian, Guadalupian, and Ochoan. Wolfcampian and Leonardian rocks are known in Kansas and Guadalupian may be present; at present the division between Leonardian and Guadalupian rocks in Kansas is not established (Swineford, 1955), because of difficulty in correlating the redbed facies in Kansas with marine rocks of the type section. There are no known Ochoan rocks in Kansas. A graphic column of the Permian rocks in Kansas (Fig. 3) shows the stratigraphic relationship of the different beds.
Figure 3—Generalized stratigraphic section of Permian rocks in Kansas. Note that the Wellington rocks are represented as subsurface rocks.
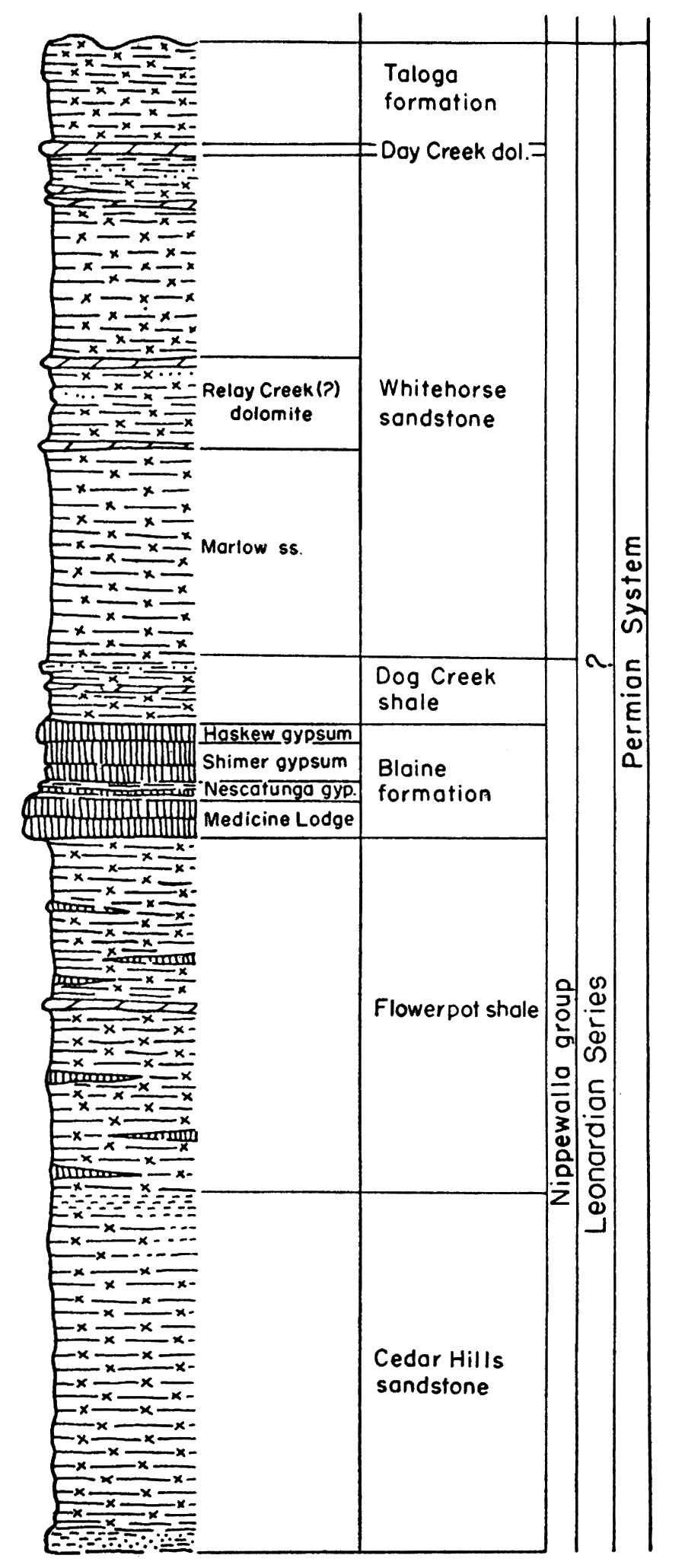
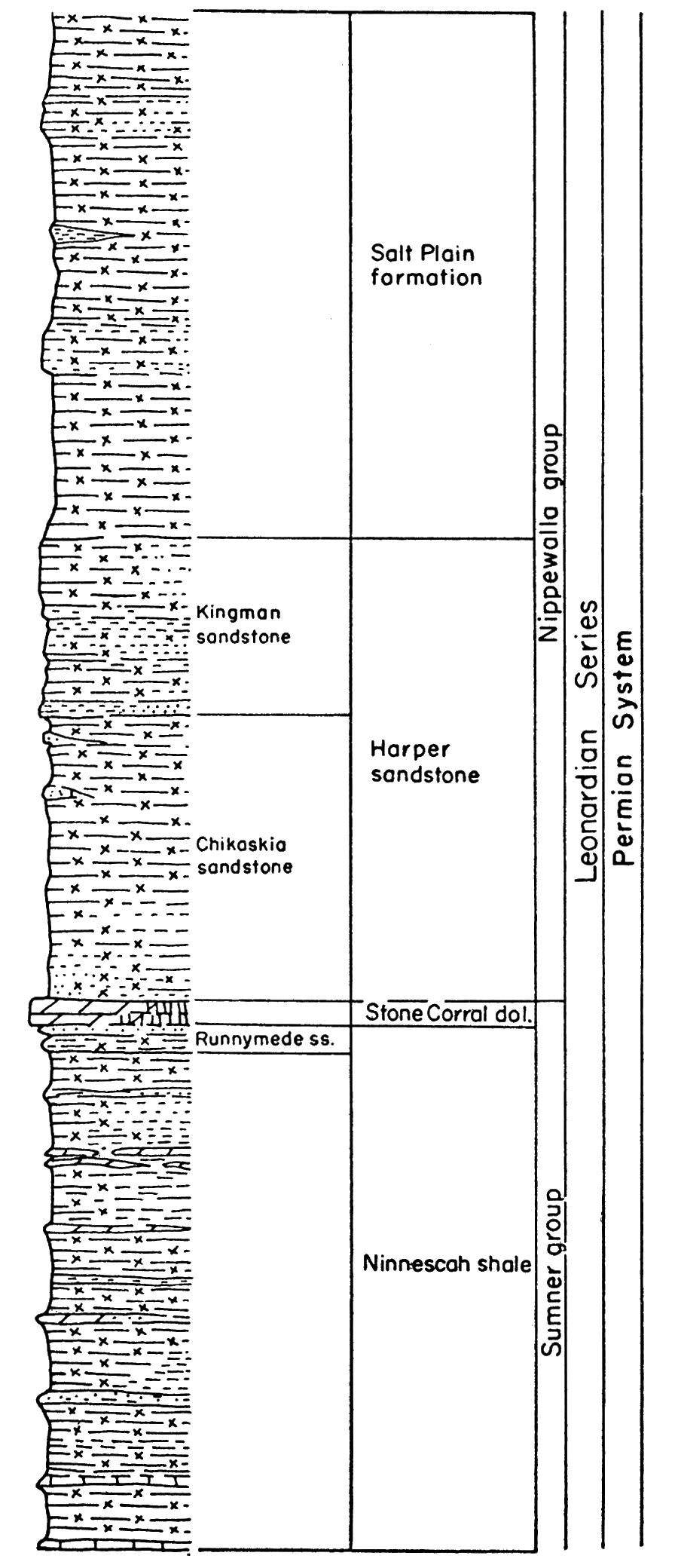
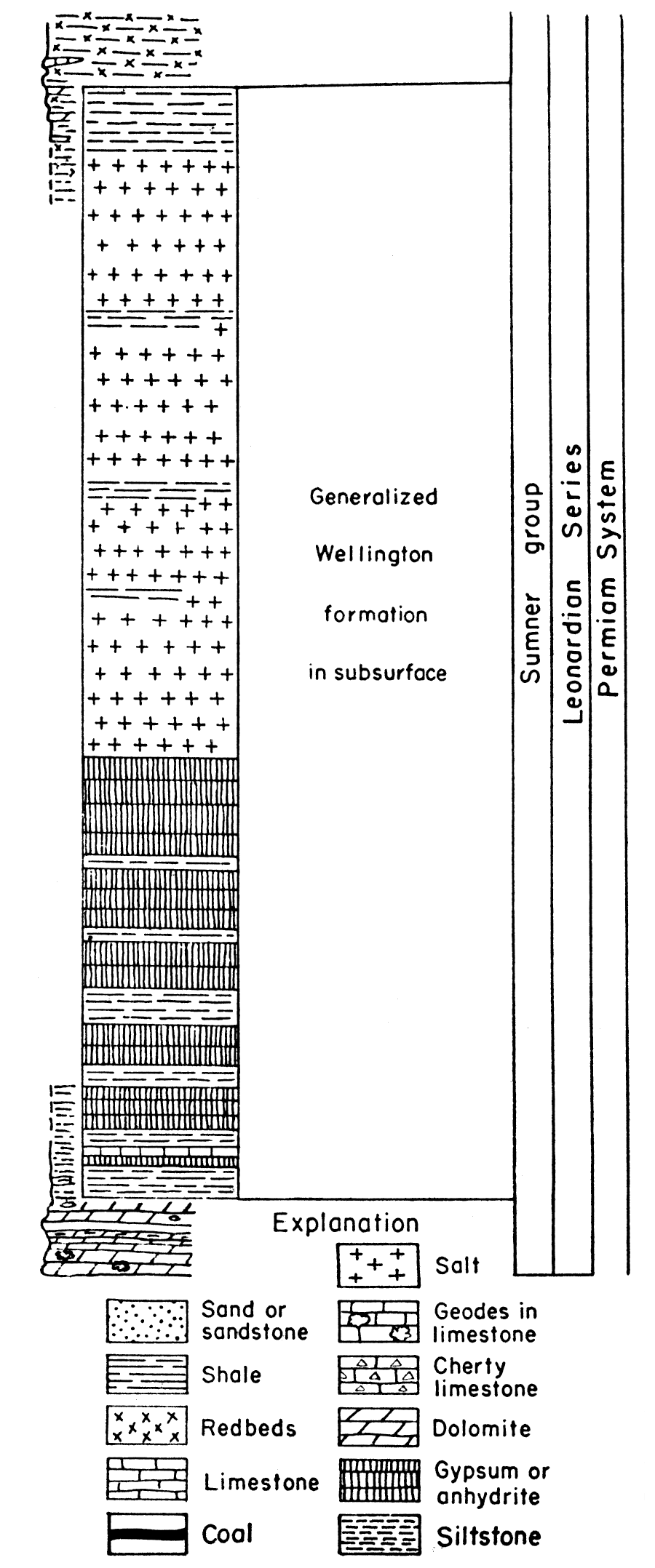
The very extensive gypsum and anhydrite deposits have been exploited in relatively few places. Economic factors, rather than quality of the deposits, may delay or prohibit actual mining operations. Even the unworked and unworkable deposits are beneficial in other ways, however. Some of these deposits underlie broad areas in the subsurface, and are easily recognized. Stratigraphic correlation and determination of geologic structure are thereby greatly facilitated, contributing significantly to petroleum exploration. Moreover, in certain areas the erosion of the gypsum-anhydrite beds by subsurface solution has allowed weathered rock to accumulate on the surface rather than to be washed away. The deep soil thus produced is well suited to agriculture.
Prev Page--Introduction || Next Page--Blaine Formation
Kansas Geological Survey, Geology
Placed on web Feb. 20, 2018; originally published March 21, 1956.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/133/03_geology.html