Prev Page--General Discussion--Therapeutics || Next Page--General Discussion--Solutions
Part I--General Discussion of Mineral Waters, continued
Chapter IV--Brines and Their Industrial Use
Development of the Salt Industry in America
About the middle of the seventeenth century the Jesuit missionaries, in making journeys among the Indians, in what is now part of the state of New York, heard of certain springs which were regarded with superstition and said to contain demons. Several of these springs were pointed out to the missionaries, and salt was manufactured from the waters by the Indians and traders.
In 1788 the systematic manufacture of salt was begun in the vicinity of Syracuse, and the following year the output of this region was about 200 barrels. Afterward, a premium was offered by the state for any salt produced on the New York reservation. After rock salt was discovered beneath the surface, in 1878, the manufacture of salt from brines became a great industry in central New York. At the present time salt is produced in large quantities in Michigan, Pennsylvania, Ohio, West Virginia, Louisiana, Nevada, Utah, California, and Kansas.
Salt in Kansas
Large areas of the state of Kansas contain salt on the surface or within drilling distance. The principal region, however, is near the middle of the state, extending entirely across from north to south.
The salt is found: First--as brines in salt marshes, which leave salt on the surface by evaporation in the dry season, producing the so-called salt plains; second--rock salt, which is found at varying distances beneath the surface; third--the greater part of the Permian and Coal Measure shales, in the eastern part of the state, have so much salt in them that the water obtained from deep wells is quite strongly saturated with salt and other mineral substances.
Salt Marshes
The salt marshes are found in a zone trending a little east of north and west of south from Republic county to Barber county, and to the Cimarron in Oklahoma.
In Republic county there are two interesting marshes, Tuthill and Jamestown. There are two salt marshes in Mitchell county, near the southern border, and two near the northern border of Lincoln county. Stafford county likewise has two marshes; while south of Harper county, in Oklahoma, there are several salt marshes that were well known to the Indians and earlier settlers of the territory.
Rock salt exists beneath a large area in Ellsworth, Barton, Rice, McPherson, Stafford, Reno, Pratt, Kingman, Sedgwick, Harper and Sumner counties.
The extent of the distribution of salt in the underlying strata of the state may be gathered by reference to the following analyses of brines from various localities:
| Parts of salt per 1000 | |
|---|---|
| Well, Greenwood county, contains | 6.9 |
| Geuda springs, Cowley county | 7.4 |
| Well, Allen county | 16.7 |
| Well, Wyandotte county | 25.7 |
| Lake, Meade county | 42.8 |
| Well, Douglas county, 1300 feet deep | 49.7 |
| Well, Barton county | 54.5 |
| Well, Pawnee county, 755 feet deep | 57.8 |
| Well, Montgomery county, 1091 feet deep | 59.4 |
| Well, McPherson county | 187.0 |
The pioneer salt manufacturer was a Mr. Tuthill. The scene of his operations was in the marsh previously referred to, in Republic county. In the fall of the year the water is generally nearly all evaporated, and the edges of the marsh are dry, and covered with a hard, thin scale of impure salt. Towards the center Of the marsh the surface is more moist, and the scale of salt less thick and solid. [Salt in Kansas; its Composition and Methods of Manufacture. E. H. S. Bailey, Reports Kansas State Board of Agriculture, vol. XIII, pp. 168-180] "This deposit is said to cover about 1000 acres. When the sun is bright, and shines upon the encrusted soil in the distance, the appearance is like that of a chain of lakes, and, indeed, a much closer inspection is necessary to destroy the illusion. A stream of fresh water flows in from the east, but after running a short distance it entirely disappears, nor does it dissolve very much saline matter in its course. Except in the rainy seasons, the marsh consists of 'hummocks' and dried-up lake basins, incrusted with mineral salts.
"The saline incrustation is thick after a period of drought, but ordinarily it is thin, and, in some places, plumose, as if brought to the surface by the moisture of the soil. The incrustation found on the Tuthill marsh is quite similar in composition to the alkali waters of the Western plains. The soluble part of this substance has the following composition, as shown by an analysis made in the laboratory of the State University:
| "Iron and aluminum oxids | .13 |
| Calcium sulfate | .99 |
| Magnesium sulfate | 1.29 |
| Sodium carbonate and organic matter (undetermined) | 3.56 |
| Sodium sulfate | 21.98 |
| Sodium chlorid | 71.82 |
| Insoluble residue | .23 |
| 100.00 |
[Mineral Resources of Kansas, 1898, E. Haworth and M. Z. Kirk.] "In the manufacture of salt, Mr. Tuthill would collect the salt scales from over the marsh and dissolve them in water, allow the earthy impurities to subside, and siphon off the clear brine and evaporate it to dryness to recover the salt and other impurities. When the weather was not favorable for the formation of salt scales over the marsh, he would dip or pump the brine from small wells and haul it to his little salt factory. The brine was evaporated from large kettles in much the same way that our fathers evaporated sugar water in Indiana, Ohio, and the Eastern states. At present this seems like a very primitive method, but at that time it was in accordance with the most approved process. Portions of the arch of Mr. Tuthill's kettle salt plant still stand to mark the spot of his primitive factory.
"In the early sixties Mr. Tuthill made salt and hauled it to Manhattan, where he received as high as ten cents per pound for it. Mr. Hazen says he sold over 100 barrels of salt made by Mr. Tuthill and other farmers from 1873 to 1876, while he kept a store in Seapo.
"This marsh and other similar ones of the state were of great value to hunters in early times. They would come here to "jerk" their buffalo meat. In case they were in too great a hurry to wait to evaporate the brine and get the crystallized salt, they would dip the meat and hides into the strongest pool of brine and then dry them in the sunshine or by a fire. When a considerable quantity of meat was to be "jerked," they would cut the meat into long strips, boil the brine in kettles hung over a fire of buffalo-chips, dip the meat into the strong, hot brine, and lay it out to dry in the sunshine or on a lattice-work made of green poles supported on four posts, with a fire under it. In this way 200 or 300 pounds could be cured in five or six hours.
"Previous to the admission of Kansas into the Union the salt marshes were thought to be of great value, and by act of Congress twelve salt springs were donated to the new state, at the time of her admission, the same to be located by her commissioners. These were all located on marshes where there are no flowing springs, and subsequently these reserves became a part of the endowment of the State Normal School."
Some important brine wells are located at Solomon City, in Dickinson county. The attention of prospectors was called to this deposit on account of a salt spring just west of town, and, in 1867, C. W. Davis, of New Bedford, Mass., drilled a well here which produced excellent brine. Several other wells were drilled, striking brine at from 84 to 100 feet from the surface. With varying fortunes different companies have been manufacturing salt at Solomon City up to the present time. The capacity of the Solomon Solar Salt Company is about 7000 barrels a year.
Plate 3--The Solar Process Salt Plant of Solomon City.

Rock Salt
There are several places in the state where the vast deposits of rock salt are mined directly. At Lyons a shaft was sunk in 1890, and a factory was built for preparing the different grades of salt for the market. This shaft is about 1000 feet deep.
At Kanopolis, on the Union Pacific railroad, a salt mine has been in successful operation for more that ten years. At Kingman there were two salt mines which did a thriving business for several years.
Composition of Rock Salt
For purposes of analysis care was taken to get average samples of the stock. The samples were thoroughly heated, to drive off all moisture, before the analysis was made. In commercial salt there is often considerable moisture, as it is so readily absorbed from the air, and this moisture, of course, makes the salt by so much the less valuable. We should not expect to find so much moisture in coarsely ground rock salt as in evaporated or finely ground salts. The probable combinations of bases and acids are given. The Kingman salt has the following composition (analysis by E. H. S. Bailey and E. C. Case):
| I. | II. | III. | |
|---|---|---|---|
| Sodium chlorid | 97.51 | 99.87 | 99.44 |
| Insoluble residue | .20 | .01 | .09 |
| Calcium sulfate | 1.51 | .07 | .07 |
| Sodium sulfate | .57 | .28 | |
| Magnesium chlorid | .10 | .05 | .12 |
| Iron oxid | .11 | ||
| Totals | 100.00 | 100.00 | 100.00 |
That from Lyons has the following composition:
| I. | II. | III. | IV. | V. | |
|---|---|---|---|---|---|
| Sodium chlorid | 96.85 | 97.39 | 98.20 | 99.78 | 97.95 |
| Insoluble residue | .08 | .09 | .02 | .01 | .14 |
| Calcium sulfate | .97 | 2.02 | 1.25 | .08 | 1.70 |
| Sodium sulfate | 2.00 | .46 | .10 | Trace | |
| Magnesium chlorid | .07 | .02 | .02 | .03 | .14 |
| Calcium chlorid | .51 | ||||
| Iron oxid | .03 | .02 | .55 | .07 | |
| Totals | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Here the vein that is worked is more than eight feet thick. It will be understood that numerous other veins of excellent quality are pierced by the shaft before the depth noted above is reached. These veins are separated from one another by soft shale or clay, and might many of them be worked to advantage.
An analysis of Kanopolis salt, given below shows that it also is extremely pure:
| I. | II. | III. | |
|---|---|---|---|
| Sodium chlorid | 97.94 | 97.23 | 96.99 |
| Insoluble residue | .13 | .08 | .29 |
| Calcium sulfate | 1.78 | 2.04 | 2.60 |
| Sodium sulfate | .10 | .41 | |
| Magnesium chlorid | .05 | .24 | .12 |
| Total | 100.00 | 100.00 | 100.00 |
Evaporated Salt
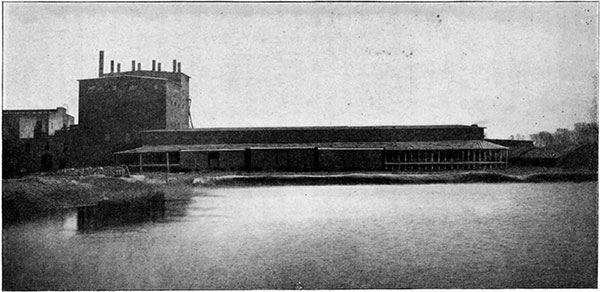
The brine used for making salt is evaporated either by the solar process, as at Solomon City, or by the use of artificial heat, as at Hutchinson and elsewhere.
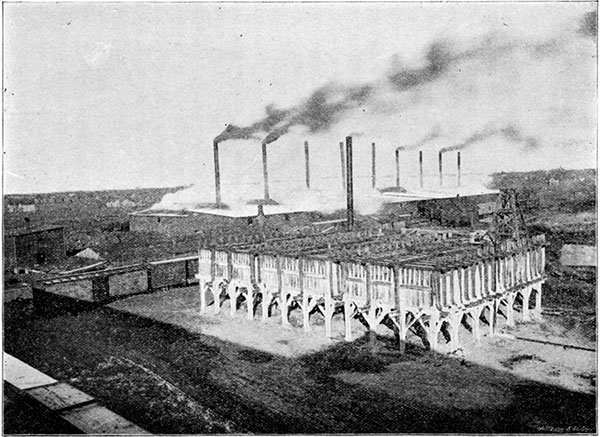
Plate 4--Hutchinson Salt Plant (general view).
The Solar Process
In the solar process the brine is obtained from a well about 100 feet deep, although the chief supply enters the well at a depth of only thirty-five feet.
From "Mineral Resources of Kansas for 1898," we quote the following:
"In making the salt, the brine is pumped from a well by means of a two-and-one-half-inch centrifugal steam-pump, having a capacity of 600 gallons per minute. It is delivered into a reservoir, where it becomes considerably concentrated by evaporation. The sediment pumped from the well subsides, and is shoveled from the bottom of the reservoir from time to time as occasion requires. To effect such a cleaning the pump is stopped, the brine turned into other rooms, the sediment shoveled out, and the reservoir properly cleaned with water. The depth of the brine kept in the reservoir is usually less than twelve inches, but considerable variation is noted from day to day, depending upon the rapidity of evaporation and rapidity of pumping.
"From the reservoir the brine is first carried into the 'water room,' where it is rarely allowed to be more than twelve inches deep. Here, the remainder of the mechanically held impurities subside, leaving an entirely clear brine to be passed on to the 'lime room.' In this second room the evaporation is carried far enough to cause precipitation of the principal impurities held in solution, such as calcium carbonate, calcium sulfate, etc. After sufficient concentration in this room, the brine is next conveyed into the 'pickle room,' or the third one of the smaller rooms. It is left in the 'pickle room' until the concentration becomes so great that salt crystals begin forming. It is then transferred into the last or 'crystal room,' and allowed to remain until concentration causes the precipitation of nearly all the salt.
"By the solar process the evaporation is very gradual. The salt crystals begin forming first on the surface of the brine. If the brine is not agitated too much by the wind, the crystals frequently reach a large size; that is, from one-half to three-fourths of an inch on one side of the cube. This is particularly true where some slender object of support, such as a cord, or splinter from the wall of the vat, or a coarse piece of any kind of material is placed in the brine. Frequently, also, the well-known 'hopper-shaped' crystals are produced instead of the solid cubes.
"After a good bed of salt has been deposited in the' crystal room' it is lifted into large baskets and allowed to drain for a few minutes, after which it is emptied into a horse-car, hauled to the storerooms, and allowed to 'cure,' or thoroughly dry."
At these particular works the brine is sometimes strengthened by adding to it crushed rock salt from some of the Kansas mines. This mixture is said not to yield so good a product as that made from the native brine. The brine, as taken from one of the vats, has been, perhaps, slightly concentrated. It has the following composition:
| Brine | Salt | Salt | |
|---|---|---|---|
| Sodium chlorid | 120.08 | 98.20 | 98.53 |
| Insoluble residue | .03 | .08 | |
| Calcium sulfate | 6.92 | 1.24 | .92 |
| Magnesian sulfate | Trace | .48 | |
| Sodium sulfate | 5.89 | .41 | |
| Magnesium chlorid | .14 | ||
| Water | 867.08 | ||
| 1000.00 | 100.00 | 100.00 | |
| (Specific gravity at 72° F., 1.085 = B° 12.) | |||
Plate 5--The Hutchinson Packing Company's Salt Plant.
Manufacture of Salt by Direct Heat
Most of the salt put upon the market from Kansas is made by evaporation of the brine by direct heat. After the well has been bored it is cased with an iron pipe about five and five-eighths inches in diameter. Inside of this is a smaller pipe which is of sufficient size to allow water to pass between it and the larger pipe. The water that is forced down dissolves the salt, which is forced to the surface through the inner pipe, and is stored in convenient tanks till it can be evaporated. The brine is evaporated either by the "pan process," "grainer process," or by the "vacuum process."
The Pan Process
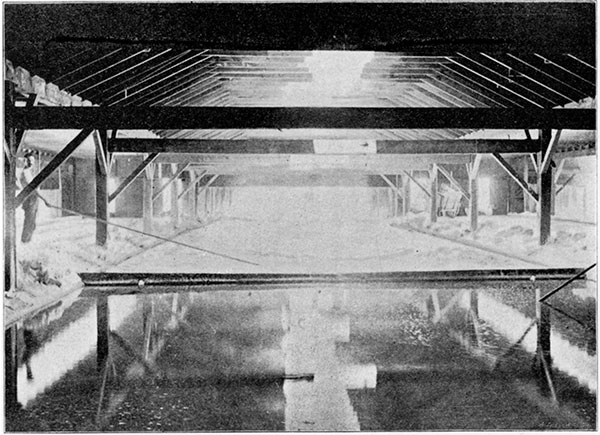
In the pan process the brine is evaporated by direct heat. The pan consists of a wrought-iron vessel about 125 x 25 feet and about 12 inches deep. A coal fire (usually "slack" is used as fuel) is built beneath one end of this pan, and the products of combustion pass under the whole length of the pan. The brine is allowed to trickle into the pan in a slow but constant stream. The first division of the pan, and in some cases several divisions, are so arranged that the brine can be retained there till it had been evaporated sufficiently for it to deposit some of its impurities, especially the calcium sulfate. When this has been accomplished, the brine, which is now saturated with salt, is allowed to flow into the last division of the pan, where the heat is not so intense. Here the salt crystallizes out and falls to the bottom of the pan. It is removed from the pan by being scraped out by workmen with long-handled rakes upon the platform at the side of the tank, where it is left for some time to drain before being shoveled into carts for transfer to the storeroom. Here it stays for several weeks, or until it can be shipped, and it drains still more through the perforated floor provided for this purpose. Each pan is raked once in two hours. Although each pan and furnace form a complete set for making the salt, it is customary in the larger "blocks" to place a number of these pans side by side, and the product is then all dumped into a common storeroom.
As an illustration of the quality of the different brines the following analyses are given. The samples were taken directly from the storage tanks (analysis by E. H. S. Bailey and E. C. Case):
| Wellington | Sterling | Hutchinson | |
|---|---|---|---|
| NaCl (salt) | 247.270 | 293.760 | 286.080 |
| SiO2 | .030 | .025 | .075 |
| MgCl2 | .684 | .966 | 1.190 |
| CaSO4 | 5.464 | 5.185 | 5.404 |
| CaCl2 | .320 | ||
| Na2SO4 | .028 | .706 | |
| H2O (by difference) | 746.524 | 799.358 | 706.931 |
| 1000.000 | 1000.000 | 1000.000 |
The Grainer Process
This is an American system, and was devised for the purpose of producing salt cheaply from comparatively weak brines. By combining the lumber and salt industries the manufacturers were able to utilize the exhaust steam from the sawmills during the day and use direct steam at night for evaporating the brines. In this process the brine is heated in the "settler" and then drawn off into the various evaporating pans, which are heated by steam-pipes running backwards and forwards across the bottom of the pans. The less soluble impurities, especially the gypsum, collect on the hottest part of the steam-pipes. This coating may be removed from time to time, after the mother-liquor has been drawn off, by turning steam into the pipes, thus causing them to expand so that the scales can be broken off.
The process of making salt is a continuous one. New brine is added, and the salt is raked out as often as necessary, and is deposited upon the platform at the side of the pan to drain.
A single example of a commercial brine and of the salt manufactured from it will suffice:
| 1000 parts of brine | Per cent. composition of salt |
|
|---|---|---|
| Sodium chlorid | 264.780 | 98.23 |
| Insoluble residue | .050 | .01 |
| Calcium sulfate | 6.009 | 1.68 |
| Magnesium chlorid | 1.200 | .08 |
| Calcium chlorid | .353 | |
| Iron oxid | .050 | |
| Water | 727.558 | |
| Total | 1000.000 | 100.00 |
Plate 6--Interior of a Hutchinson Salt Plant, Showing Evaporating Pans (end view.)
The Vacuum Process
The third process for making evaporated salt is known as the "vacuum process." This process has not been extensively used, but the apparatus consists essentially of a kettle which is connected with a vacuum pump so that the brine may be boiled at a lower temperature. The salt that is formed is automatically carried away and fresh brine is at the same time supplied to the pan as rapidly as evaporation takes place.
Kansas Salt Compared with Other Brands
Some examples have been given to show the purity of the Kansas salt. The following analyses of other salts that are on the market are quoted for comparison:
| Higgins's | 97.8 |
| Onondaga | 97.7 |
| Ashton's | 97.6 |
| Deckin's | 97.5 |
| Worthington | 97.4 |
With abundant brine, and that of excellent quality, the only obstacle that can stand in the way of economical production of salt in large quantities is cheap fuel. By the use of coal-slack the cost of fuel has been considerably decreased; but still, the expense is large compared with that in some other salt regions where slabs are burned or coal-mines are near at hand. By the use of more economically constructed furnaces much will no doubt be gained, for, by the present methods, much of the coal goes out through the chimney in unburned carbon, under the name of "smoke." This is all lost fuel, of course. Possibly, by some cheap system of compression, the vast quantity of straw and corn-stalks that are produced in the state may yet be utilized as fuel.
In studying economy of production, it will be noted, also, that only a saturated brine should be used for evaporation. Every pound of unnecessary water evaporated adds to the expense; so the rate of pumping of the brine should be carefully watched.
There has been an increasing amount of salt produced in the state since 1888, when the industry began to be fairly established. The latest available report, that for 1899, shows a production of 2,172,000 barrels.
Prev Page--General Discussion--Therapeutics || Next Page--General Discussion--Solutions
Kansas Geological Survey, Geology
Placed on web April 7, 2017; originally published 1902.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/Vol7/06_indus.html