Prev Page—Development || Next Page—Groundwater Conditions
Ground Water, continued
Chemical Character of Ground Water
The chemical character of the ground waters in Barton and Stafford Counties is shown by the analyses of water from 62 representative wells in Barton County (Table 8) and 27 representative wells in Stafford County (Table 9). The amounts of chloride in samples of water from three wells in Stafford County are given in Table 9. In addition, the chemical analyses of 11 samples of water collected from test holes in Stafford County and the amounts of chloride in 2 samples collected from test holes in Barton County are given in Table 10. The analyses, which were made by Howard Stoltenberg in the Water and Sewage Laboratory of the Kansas State Board of Health, show only the dissolved mineral content of the waters and do not in general indicate the sanitary condition of the waters. The constituents given were determined by the methods used by the U.S. Geological Survey.
Chemical Constituents in Relation to Use
The following discussion of the chemical constituents of ground water has been adapted from publications of the United States Geological Survey.
Dissolved solids—The residue left after a natural water has evaporated consists of rock materials which may include some organic material and some water of crystallization. Waters containing less than 500 parts per million of dissolved solids generally are entirely satisfactory for domestic use, except for the difficulties resulting from their hardness and, in some areas, excessive iron content or corrosiveness. Waters having more than 1,000 parts per million are as a rule not satisfactory, for they are likely to contain enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respects.
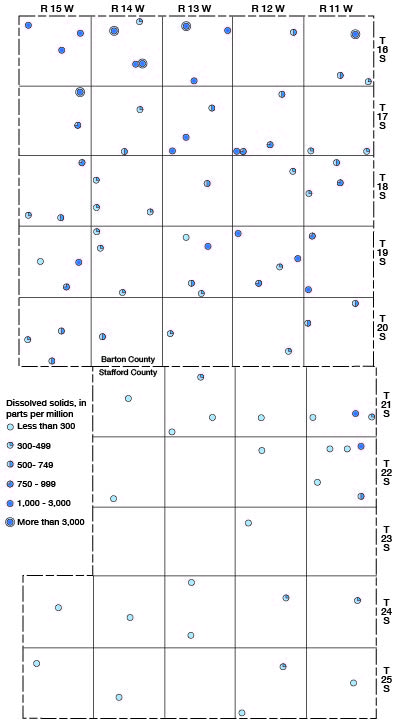
The ground waters from about half the wells sampled in Barton and Stafford Counties contained less than 500 parts per million of dissolved solids and are entirely satisfactory for most ordinary purposes. The waters from 23 of the wells sampled contained between 500 and 1,000 parts per million of dissolved solids, the waters from 16 wells contained between 1,000 and 3,000 parts, and the water from 5 wells (16-11-11cc, 16-13-8aa, 16-14-9bc, 16-14-26bb, and 17-15-2ad) contained more than 3,000 parts. The highest concentration of dissolved solids, 6,323 parts per million, was in the sample of water from well 17-15-2ad.
The areal distribution of dissolved solids in the well waters of Barton and Stafford Counties is shown in Figure 15.
Figure 15—Content of dissolved solids of well waters in Barton and Stafford Counties.
Hardness—The hardness of water, which is the property that generally receives the most attention, is most commonly recognized by its effects when soap is used with the water. Hard water is objectionable because it forms with soap a sticky insoluble curd difficult to remove from containers and fabrics; requires greater quantities of soap to produce lather; and forms scale in boilers and pipes with resultant loss in heat transfer and boiler failure. Calcium and magnesium cause virtually all the hardness of ordinary waters. These constituents are also the active agents in the formation of the greater part of the scale in steam boilers and in other vessels in which water is heated or evaporated.
In addition to the total hardness the table of analyses shows the carbonate hardness and the noncarbonate hardness. The carbonate hardness is that due to the presence of calcium and magnesium bicarbonates. It is almost completely removed by boiling. In some reports this type of hardness is called temporary hardness. The noncarbonate hardness is due to the presence of sulfates or chlorides of calcium and magnesium, but it cannot be removed by boiling and has sometimes been called permanent hardness. With reference to use with soap there is no difference between the carbonate and noncarbonate hardness. In general the noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness of less than 50 parts per million is generally rated as soft, and its treatment for removal of hardness under ordinary circumstances is not necessary. Hardness between 50 and 150 parts per million does not seriously interfere with the use of water for most purposes, but it does increase somewhat the consumption of soap, and its removal by a softening process is profitable for laundries or other industries using large quantities of soap. Waters in the upper part of this range of hardness will cause considerable scale in steam boilers. Hardness of more than 150 parts per million can be noticed by anyone, and if the hardness is 200 or 300 parts per million, it is common practice to soften water for household use or to install cisterns to collect soft rainwater. Where municipal water supplies are softened, an attempt is generally made to reduce the hardness to 50 to 80 parts per million. The additional improvement from further softening of a whole public supply is not deemed worth the increase in cost.
Samples of water collected from wells in the Barton-Stafford area ranged in hardness from 29 to 1,564 parts per million. Of the samples of water, 2 (wells 16-12-12cb and 18-15-1bc) had less than 50 parts per million of hardness, 9 had between 50 and 150 parts, 14 had between 151 and 200 parts, 55 had between 201 and 500 parts, 8 had between 501 and 1,000 parts, and the water from well 16-14-26bb had 1,564 parts. Of the public water supplies in the area only that of Hoisington is treated, but some of the industrial supplies are softened.
Iron—Next to hardness, iron is the constituent of natural waters that in general receives the most attention. The quantity of iron in ground waters may differ greatly from place to place, even though the waters are derived from the same formation. If a water contains much more than 0.1 part per million of iron the excess may separate out and settle as a reddish sediment. Iron, which may be present in sufficient quantity to give a disagreeable taste and to stain cooking utensils, fixtures, and fabrics, may be removed from most waters by simple aeration and settling or filtration, but a few waters require the addition of lime or some other substance
Of the 89 samples of water collected from wells in Barton and Stafford Counties, 25 contained less than 0.1 part per million of iron, 31 contained between 0.1 and 1.0 part, 19 contained between 1.1 and 3.0 parts, 13 contained between 3.1 and 10 parts, and 1 (well 25-11-15dd) contained 11 parts.
Chloride—Chloride is an abundant constituent of seawater. It is dissolved in small quantities from rock materials and in some localities comes from sewage. The sources of chloride are many, however, and its presence in large quantities cannot be taken as a definite indication of pollution. Chloride has little effect on the suitability of water for ordinary use unless there is enough to give the taste of salt. Waters high in chloride may be corrosive when used in steam boilers.
The quantity of chloride was determined for 92 samples of water collected from wells in the Barton-Stafford area. Of these 92 samples, 62 contained less than 50 parts per million of chloride, 15 contained between 150 and 500 parts, 6 contained between 501 and 1,000 parts, and 9 contained more than 1,000 parts. The greatest concentrations of chloride were found in waters from the Dakota and Meade formations. A further discussion on this subject is given on pages 131-134.
Fluoride—Although determinable quantities of fluoride are not so common as are fairly large quantities of the other constituents of natural waters, it is desirable to know the amount of fluoride present in waters that are likely to be used by children. Fluoride in water has been shown to be associated with the dental defect known as mottled enamel, a permanent condition which may appear on the teeth of children who drink water containing fluoride during the period when their permanent teeth are formed. It has been said that waters containing 1.5 part per million or more of fluoride are likely to produce mottled enamel, although the effect of 1.5 part per million is not usually very serious (Dean, 1936, pp. 1269-1272). If the water contains as much as 4 parts per million of fluoride, 90 percent of the children who drink it are likely to have teeth with mottled enamel, and 35 percent or more of these cases will be classified as moderate or worse. Small quantities of fluoride, not sufficient to cause mottled enamel, are likely to be beneficial by inhibiting dental caries (tooth decay) (Dean, Arnold, and Elvove, 1942, pp. 1155-1179).
The fluoride content of most of the water samples from wells in Barton and Stafford Counties was low. Of the 89 samples analyzed, 69 contained less than 1 part per million of fluoride, 9 contained between 1 and 1.9 parts, 8 contained between 2 and 3 parts, and those from wells 16-13-12ad, 16-15-6dd, and 17-15-23ad contained 3.8, 3.8, and 3.4 parts, respectively.
Water for Irrigation
The suitability of water for use in irrigation is commonly considered to depend mainly on the total quantity of soluble salts and on the ratio of the quantity of sodium to the total quantity of sodium, potassium, calcium, and magnesium together. The quantity of chloride may be large enough to affect the use of the water, and in some areas other constituents, such as boron, may be present in sufficient quantity to cause difficulty. In a discussion of the interpretation of analyses with reference to irrigation in southern California, Scofield (1933) states that if the total concentration of dissolved salts is less than 700 parts per million there is not much probability of harmful effects in irrigation use. If it exceeds 2,100 parts per million there is a strong probability of damage to either the crops or the land or both. Water containing less than 50 percent sodium (the percent-sodium factor is the ratio of the quantity of sodium to the total quantity of sodium, calcium, potassium, and magnesium) is not likely to be injurious, but if it contains more than 60 percent its use is inadvisable. Similarly, a chloride content of less than 142 parts per million is not objectionable, but more than 355 parts per million is undesirable. Later writers, notably Magistad and Christiansen (1944), have modified the percent-sodium standards to show a class 1 water as having below 60 percent sodium; a class 2 from 60 to 75 percent; and a class 3 over 75 percent. It is recognized that the harmfulness of irrigation water is so dependent on the nature of the land, the crops, the manner of use, and the drainage that no hard-and-fast limits can be adopted.
At the time of this investigation all irrigation wells obtained their water supply from the Meade formation. Ground water in the Meade formation is everywhere moderately hard but in most places it is suitable for irrigation use. In northeastern Stafford County the ground water locally is highly mineralized. Because of the uneven topography and sandy soil, irrigation is not feasible in this part of Stafford County except perhaps in very local areas. In other parts of Stafford County, the quality of the water taken from wells in the Meade formation was suitable for irrigation use. However, some of the samples taken from test holes that penetrated the lower part of the Meade formation were highly mineralized and were not suitable for irrigation (Table 10). Before a well is constructed for irrigation anywhere in Stafford County, it is recommended that a sample from a test hole be analyzed first to determine the chemical suitability of the water.
Chemical Character in Relation to Stratigraphy
Ground waters in the various water-bearing formations in Barton and Stafford Counties have a wide range in quality. There is also a wide difference in the quality of waters from different places and from different depths in the same formation. Although no wells derive water from Permian rocks or the Cheyenne sandstone in this area, scanty data indicate that the waters contained in them are highly mineralized and probably unfit for ordinary uses. The same is true for the Kiowa shale except possibly in its area of outcrop.
The quality of water found in the seven principal water-bearing formations of Barton and Stafford Counties is discussed below. The typical quality of water in the four major water-bearing formations is shown graphically in Figure 16.
Figure 16—Quality of water from the four major water-bearing formations in Barton and Stafford Counties, Kansas.
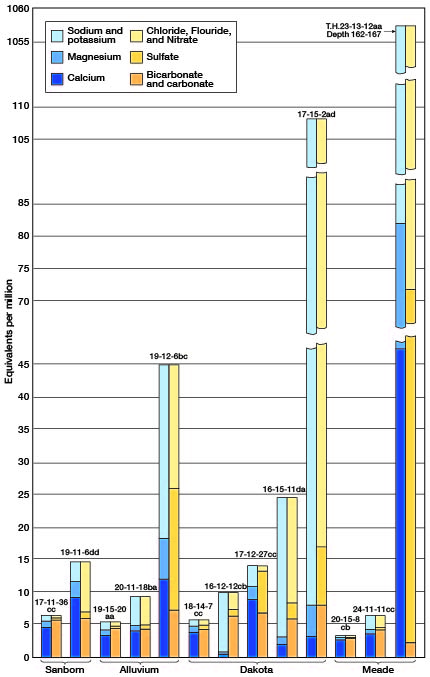
Dakota formation—Waters from wells tapping sandstones of the Dakota formation have a wide range in chemical composition; a few are low in mineral content and comparatively soft, whereas others are highly mineralized and hard. Thirty samples of water from the Dakota formation were collected and analyzed. The samples of water from wells 16-12-12cb and 18-15-1bc in sandstones of the Dakota formation were the softest waters of any of those analyzed from the Barton-Stafford County area, having hardnesses of 29 and 45 parts per million, respectively. Both of these are soft sodium bicarbonate waters that probably have resulted from a natural softening process in which calcium bicarbonate water has exchanged part of its calcium and magnesium for sodium by a base-exchange process. This is the same process used in the common zeolite-type home water softeners. Hardnesses of the other 28 samples from the Dakota ranged from 101 to 626 parts per million. More than half of these samples had less than 300 parts of hardness, 7 had between 301 and 500 parts, and 4 had more than 500 parts.
The dissolved solids in samples collected from wells tapping the Dakota ranged from 305 parts per million in the sample from well 18-14-7cc to 6,323 parts in the sample from well 17-15-2ad. Only 7 of the samples contained less than 500 parts per million of dissolved solids, 10 contained between 500 and 1,000 parts, and 13 contained more than 1,000 parts. Chloride is both the most variable and most objectionable constituent of many waters from the Dakota. The chloride concentration in the samples ranged from 15 parts per million in well 17-15-23ad to 3,220 parts in well 17-15-2ad. Seventeen of the 30 samples contained less than 150 parts per million of chloride, 4 contained between 150 and 500 parts, 2 contained between 501 and 1,000 parts and 7 contained more than 1,000 parts.
The fluoride content of 14 of the 30 samples from the Dakota formation was greater than 1 part per million, and the samples from wells 16-13-12ad and 16-15-6dd had the highest fluoride contents of any of the samples analyzed-3.8 parts per million. Many of the samples analyzed had excessive concentrations of iron. Only 3 of the 30 samples had less than 0.1 part per million of iron, 7 had between 0.1 and 1.0 part, 11 had between 1.1 and 3.0 parts, and 11 had between 3.1 and 6.9 parts.
Greenhorn limestone—A few domestic and stock wells in the northern half of Barton County derive water from the Greenhorn limestone. Only one sample of water from the Greenhorn limestone was analyzed (well 16-14-2cb); it contained 356 parts per million of dissolved solids and had a hardness of 306 parts. Both the chloride and fluoride contents were low and the iron concentration was 1.2 parts per million.
Carlile shale—The Fairport chalky shale member of the Carlile shale yields small quantities of water to a few dug wells on the upland in northern Barton County. Samples of water from wells 16-11-27cd and 16-14-26bb, which tap these rocks, differed considerably in quality; they contained, respectively, 673 and 2,277 parts per million of dissolved solids and had 442 and 1,564 parts of hardness.
Undifferentiated Pleistocene—A few domestic and stock wells in the immediate vicinity of Galatia in northwestern Barton County obtain small supplies of water from sands and gravels of the undifferentiated Pleistocene deposits. Only one sample of water from these deposits was analyzed (well 16-15-15dc); it was a relatively soft sodium chloride water that contained 1,203 parts per million of dissolved solids and had a hardness of 73 parts. The chloride content of this sample was 460 parts per million.
Meade formation—Analyses of 42 samples of water from the Meade formation were made, 32 of which were collected from wells (Tables 8 and 9) and 10 from test holes (Table 10). In addition, three samples were collected from wells and analyzed for their chloride content only. Most of the waters analyzed were moderately hard to hard calcium bicarbonate waters. Of the 32 samples of water collected from wells, 4 had less than 200 parts per million of dissolved solids, 14 had between 201 and 300 parts, 11 had between 301 and 676 parts, 1 (well 22-11-2cd1) had 1,959 parts, and 1 (well 21-11-27aa) had 2,150 parts. The hardness of the 32 samples from wells ranged from 129 to 368 parts per million. The iron content of these samples was relatively low - 18 of the 32 samples contained 0.1 part per million or less of iron, 13 contained between 0.11 and 1.1 parts, and 1 (well 25-11-15dd) contained 11 parts. Locally, waters in the Meade formation in the immediate vicinity of Big Marsh and Little Marsh in northeastern Stafford County are high in chloride. Samples of water from wells 21-11-27aa, 22-11-2cd1, 22-11-35ab, and 22-12-1ad contained, respectively, 895, 835, 272, and 1,145 parts per million of chloride. The chloride content of the other samples from wells in the Meade formation in Barton and Stafford Counties ranged from 6 to 121 parts per million.
In the buried lowland areas of Stafford County, waters near the base of the Meade formation are highly mineralized. Wells in these areas do not extend to the base of the Meade formation; therefore, analyses of water samples from them do not show the quality of the water at the base of the formation. Analyses of samples of water collected from the lower part of the Meade formation from nine test holes are given in Table 10. The mineral content of waters from test holes 22-11-5dC 22-11-28bc, and 23-13-22bb, which encountered bedrock at shallow depths, was relatively low. Samples of water from test holes 21-11-24CC, 21-12-25bb, 21-13-24bb, 22-12-23CC, 23-13-12aa, and 24-11-28dd, which are located in areas of greater depth to bedrock (buried lowlands), were highly mineralized. The dissolved solids in these samples ranged from 1,584 to 62,178 parts per million and the chloride ranged from 760 to 34,950 parts. The distribution of chloride in the Meade formation from place to place and with depth in Stafford County is shown in Figure 17.
Figure 17—Chloride content of waters in the Meade formation in Stafford County, Kansas.
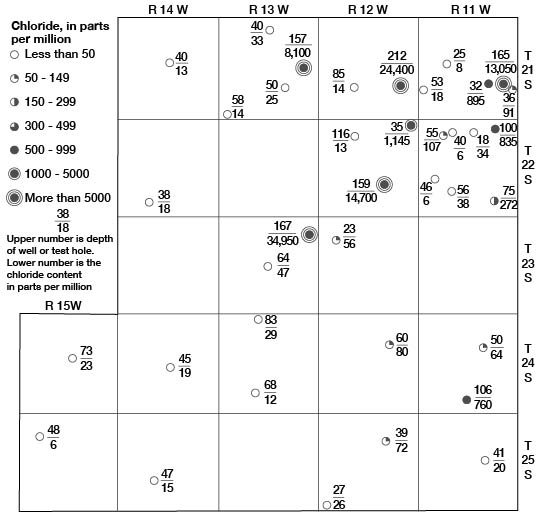
The highly mineralized waters in the Meade formation probably have their source in the underlying rocks-the Permian rocks, Cheyenne sandstone, and Kiowa shale. These rocks are known to contain highly mineralized waters. Where the highly mineralized waters in these bedrock formations is under greater head than the water in the overlying Meade formation, it moves upward into the Meade formation. The highly mineralized water tends to concentrate in the basal part of the Meade formation and in the lowest places on the bedrock floor because of its greater specific gravity. At the same time the upper part of the Meade formation is receiving fresh water from infiltration of local rainfall. The resultant mineralization of the water of the Meade varies with depth and with position above the bedrock floor. A high bedrock ridge trending approximately perpendicular to the direction of movement of ground water (Fig. 11) has caused the highly mineralized waters at the base of the Meade formation to be forced upward in northeastern Stafford County. At Big and Little Marshes the waters are discharged at the surface, causing high salinity in the waters in the marshes, which is further concentrated by evaporation.
Sanborn formation—The quality of water in the Sanborn formation is indicated by the analyses of seven samples of water from wells (17-11-31dc, 17-11-36CC, 18-11-15bc, 18-14-25cb, 18-14-30cb, 19-11-6dd, and 19-12-13ad) tapping these deposits. These samples contained 317 to 1,502 parts per million of dissolved solids and had 242 to 606 parts of hardness. Samples from wells 18-11-15bc, 18-14 25cb, and 18-14-30cb contained excessive iron, having 5.0, 10, and 7.7 parts per million, respectively. One sample (19-12-13ad) contained 560 parts per million of chloride; the chloride content of the other six samples ranged from 16 to 174 parts per million. The fluoride was low in all of the samples.
Alluvium—Sixteen samples from wells tapping alluvium in Barton County were analyzed. One (well 19-13-4cc) of these represents treated water and, therefore, will not be considered in this discussion. The other 15 samples were collected from widely scattered wells in Arkansas, Dry Walnut, and Walnut Valleys, and Cheyenne Bottoms. As would be expected, there is considerable variation in the chemical character of the waters.
The hardness of samples of water from wells in alluvium ranged from 202 to 904 parts per million, and the dissolved solids ranged from 271 to 2,728 parts per million. In all but one sample (19-12-6bc) analyzed, the fluoride was less than 1 part per million, and in this sample it was only 1.1 parts. Four (17-12-31dc1, 19-12-6bc, 19-12-28cc, and 19-15-24bc) of the fifteen samples had more than 200 parts per million of chloride and eleven had between 17 and 168 parts of chloride.
Chemical Character of Surface Water in Big Marsh Vicinity
The chloride analyses of five samples of water collected from Rattlesnake Creek on October 2, 1942, indicate that highly mineralized waters enter this stream in the lower part of its course in Stafford County. The results of the analyses are given in Table 11. According to these analyses, the chloride content increases downstream and reaches the greatest concentration below Big Marsh at the Stafford-Rice County line. A sample of water collected from Little Marsh on the same day had 1,440 parts per million of chloride. Two samples were collected from Big Marsh on October 10, 1942, one sample being collected at the south side of sec. 6, T. 22 S., R. 11 W., from the small stream entering Big Marsh from the southwest and had 1,690 parts per million of chloride. The other sample, which was taken from the drainage ditch at the north side of sec. 27, T. 21 S., R. 11 W., contained 4,060 parts of chloride. A sample of water from Big Marsh collected from the same spot on July 10, 1944, contained 10,870 parts per million of dissolved solids, 5,900 parts of chloride, 1.3 parts of iron, and had a hardness of 439 parts. The above analytical results reported for creek waters represent conditions at the time of sampling only and are not necessarily average conditions that would be obtained if the sampling period extended over a long period of time.
Table 11—Concentration of chloride in five samples of water collected October 2, 1942, from Rattlesnake Creek in Stafford County, Kansas.
| Sampling Point | Chloride (parts per million) |
|---|---|
| NE sec. 13, T. 24 S., R. 14 W., about 3 miles above St. John | 14 |
| West side sec. 1, T. 23 S., R. 13 W., about 3 miles above Hudson | 400 |
| West side sec. 1, T. 23 S., R. 12 W., about 6 miles above Little Marsh | 1220 |
| West side sec. 26, T. 22 S., R. 11 W., opposite Little Marsh | 1355 |
| Stafford-Rice County line | 1810 |
Prev Page—Development || Next Page—Groundwater Conditions
Kansas Geological Survey, Barton and Stafford Geohydrology
Web version Dec. 2001. Original publication date Dec. 1950.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Barton/07_gw5.html