
Kansas Geological Survey, Chemical Quality Series 7, originally published in 1978
Next Page--Appendix

Originally published in 1978 as Kansas Geological Survey Chemical Quality Series 7. This publication is also available as an Acrobat PDF file (2.2 MB).
With increased pressures placed on the groundwater supply of western Kansas by municipalities and irrigators, the need for information on the quality as well as the quantity of the water has become crucial. Both human activities, such as irrigation and oil and gas development, and natural forces, such as the presence of salt beds in the subsurface of much of western Kansas, can have significant effects on the present and future suitability of groundwaters for our use.
In the Great Bend Prairie area, the data collected to date suggest that monitoring groundwater quality in regions overlying Permian-age bedrock,is essential if continued development and usefulness of groundwater resources and productivity of the soil is to be maintained. The presence of salt-bearing bedrock of Permian age in the eastern half of the Great Bend Prairie area south of the Arkansas River coincides with the occurrence of mineralized waters at depth in the aquifer system utilized for irrigation purposes in that area. Increased sodium and chloride levels are encountered in groundwaters from eastern Stafford and northern Reno counties at depths tapped for irrigation. in addition to this natural source of groundwater quality deterioration, local areas exist in the eastern part of this area that appear to reflect subsurface brine contamination.
Other areas covered in this series of studies are Greeley, Wichita, Scott, Lane and southern Wallace counties (K.G.S. Chemical Quality Series 2), Hamilton, Kearny, Finney and northern Gray counties (K.G.S. Chemical Quality Series 4), and Stanton, Grant, Haskell, Morton, Stevens, Seward, Meade, and southern Gray counties (K.G.S. Chemical Quality Series 6). Future sampling programs include northwest Kansas (1978) and the Equus Beds area (1979).
In order to develop data on the quality of groundwater, one hundred-seventy pumping irrigation wells and two flowing artesian wells were sampled in Ford County and the Great Bend Prairie area of central Kansas during July, 1977. Fourteen of the wells are located north of the Arkansas River in Edwards, Pawnee, Barton, Rice and Reno counties. Waters vary from a calcium-bicarbonate type in the western half of the study area to regions of sodium-chloride type waters in eastern Stafford and northern Reno counties. Waters along the Arkansas River valley tend to be of a sulfate type.
The present study represents the fourth year of a program begun in 1974 to establish chemical quality base-line data for irrigation waters of western Kansas. Areas covered by previous studies are west-central Kansas (Hathaway, et al., 1975) and southwestern Kansas (Hathaway, et al., 1976, 1978). The present study includes Ford County and that portion of the Great Bend Prairie area generally occurring within the boundaries of the Arkansas River to the north, U. S. 54 Highway to the south, and the eastern Reno County line (Figure 1). Figure 2 is a general map of the study area.
Figure 1--Index map showing the area covered by this report (red) and areas covered by past reports on the Chemical Quality Program.
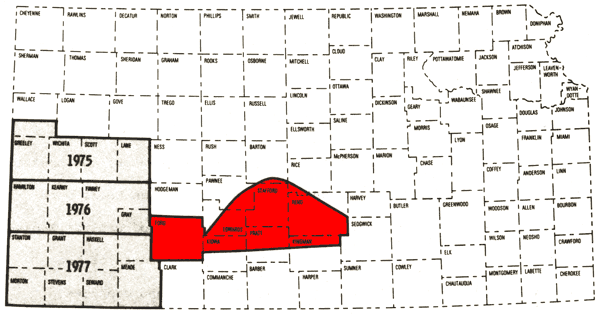
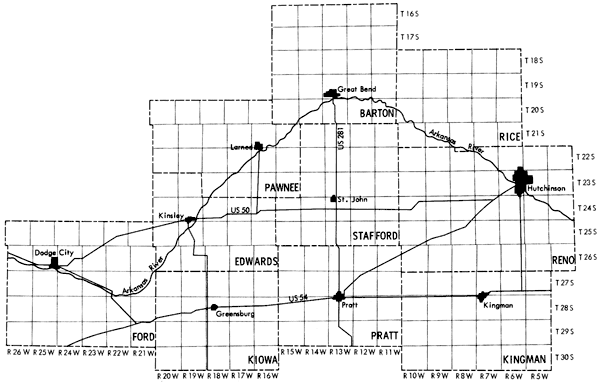
Figure 2--Location of the study area.
Water from wells in the study area is primarily derived from undifferentiated Quaternary-Tertiary age deposits, exceptions being along the Arkansas River valley where recent alluvium serves as an aquifer for wells in the river valley and northeastern Ford County where the Dakota Formation of Cretaceous age is the aquifer unit (Stullken and Fader, 1976; Lobmeyer, personal communication). Historical chemical quality data exist for about six percent of the wells sampled in this study. The historical data suggest that a marked decline in the general quality of the groundwater is to be expected as one proceeds from west to east across the Great Bend Prairie area. This relationship appears to be borne out by the greater density of irrigation wells encountered in the western portion of the Great Bend Prairie area (Stullken and Fader, 1976), and appears to be related to the presence of salt-bearing Permian bedrock units in the eastern sector of the Great Bend Prairie area.
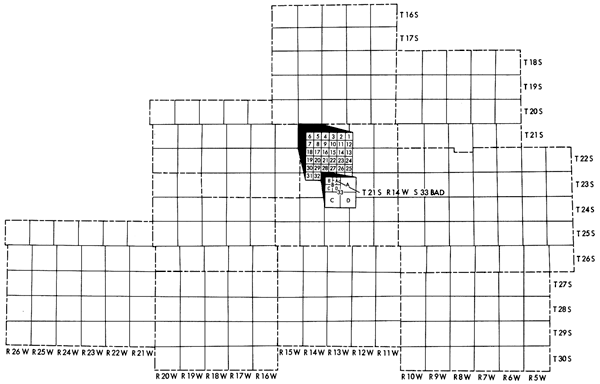
Well locations used in this report are based upon the Bureau of Land Management numbering system. The location is composed of the township, the range, and the section number followed by letters designating the subdivision of the section upon which the well is located (Figure 3).
Figure 3--Illustration of the Bureau of Land Management numbering system used for well locations in this report.
Discussions of the general geologic features of the different counties comprising the study area are found in a number of Kansas Geological Survey Bulletins (Waite, 1942; Latta, 1947; McLaughlin, 1949; Latta, 1950; Fent, 1950a; Bayne, 1956; Lane, 1960; Layton and Berry, 1973; Bayne and Ward, 1973). Adams (1903), Schoewe (1949), Frye and Leonard (1952), and Frye and Schoewe (1953) discuss the physiographic subdivisions of Kansas and provide a general overview of the topographic features of these areas. Discussions of the Pleistocene drainage history of the study area are found in Fent (1950b) and Frye and Leonard (1952). Figure 4 is a general representation of the physiographic divisions of Kansas.
Figure 4--Physiographic provinces of Kansas with study area shown in red.
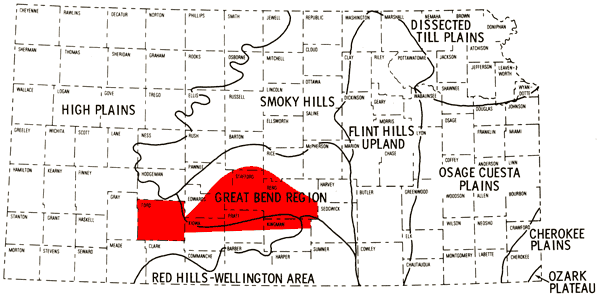
The present study area falls within the Great Plains physiographic province. Ford County, the lower half of the study area in Kiowa and Pratt counties, and the western half of the portion of Kingman County covered by this report belong to the High Plains physiographic region. The High Plains region is comparatively flat and featureless, but locally may exhibit shallow depressions and gentle swells. The general slope of the land surface is from west to east.
The eastern half of the portion of Kingman County covered by this report falls into the Red Hills physiographic region which is a highly dissected portion of the eastern flank of the High Plains region where exposures of Permian bedrock occur. The remainder of the study area falls into the Great Bend physiographic region.
The Great Bend region is on the eastern flank of the High Plains and is a relatively flat alluvial plain whose elevation generally coincides with an eastward projection of the High Plains surface. Formation of the alluvial plain and the northward bend in the Arkansas River in eastern Ford County are related to a northeastward migration of the ancestral Arkansas River. The Great Bend region exists at a level slightly above the present level of the Arkansas River; therefore, a relatively shallow~water table can be anticipated for the northern half of the Great Bend region covered by this report.
Much of the Great Bend region is covered by loamy and sandy soils, and sand dunes are a topographic feature of this region. Limited stream dissection exists over much of the area and high intensity rainfall tends to collect in numerous small basins. The annual rainfall varies from about 20" along the western edge of the study area to about 30" along the eastern boundary.
The Arkansas River is the only major through-flowing stream in the study area. Crooked Creek makes a loop in southwestern Ford County and turns southward to join the Cimarron River in northern Oklahoma. Other streams in the study area, such as Rattlesnake Creek, Peace Creek, Salt Creek, Mulberry Creek, Coon Creek, and the North and South Forks of the Ninnescah River, are tributaries to the Arkansas River and flow in an easterly direction to intercept it. Two salt marshes occur along the course of Rattlesnake Creek in northeastern Stafford County, attesting to the shallow water table in that area. Groundwater flow within the study area for the most part is in a general eastward direction.
The consolidated rocks making up the bedrock in the study area range from Cretaceous age in western Ford County to Permian age in eastern Reno County (Waite, 1942; Fader and Stullken, 1978), with the transition occurring in the vicinity of U. S. 281 Highway which trends north-south through the central part of Pratt and Stafford counties (Figure 5).
Figure 5--Generalized bedrock geology of the study area.
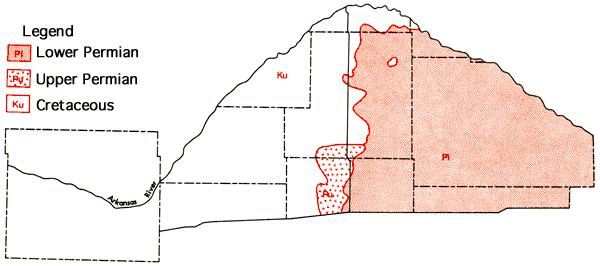
The bedrock topography of the Great Bend area presented by Fader and Stullken (in preparation) suggests the presence of several eastward trending bedrock channels related to the complex Pleistocene drainage history of the area (Fent, 1950b; Frye and Leonard, 1952).
The soils of the study area can be placed into two broad categories-deep silty soils of the upland areas (High Plains region) and loamy and sandy soils of the stream valleys and Great Bend region. Most of the upland soils are well drained. The sandy soils of stream valleys and Great Bend area vary from well drained to poorly drained. The soils tend to be saline and some even exhibit a sodium-rich horizon (sodic soils) in lowland areas where shallow water tables occur and/or drainage is poor. Figure 6 represents a generalized soils association map of the study area which has been produced through a combination of data for individual counties. This has involved some grouping of various soil associations presented in the general county soil maps and some regrouping of soil associations used in those reports in order to better reflect the pattern of soils in the, natural landscapes for the multi-county area (Roth, 1973; Dodge, et al., 1965; Horsch, et al., 1968; Rockers, et al., 1966; Horsch, 1974; Dodge, personal communication; Tomasu, personal communication; Hoffman, personal communication). A brief description of the soil association groups used in this map is provided in Table 1.
Figure 6--Soil association map of the study area (see Table 1 for group descriptions).
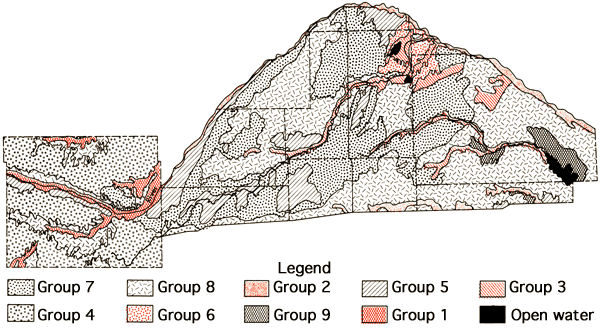
Table 1--Soil Association Groups
| 1. Upper Arkansas Valley and Tributaries | Silty, sandy, and loamy soils are on nearly level and gently undulating landscapes. A few small areas of saline and sodic soils occur. The major soil series are Dale, Leshara, Canadian, and Las Animas. |
| 2. Lower Arkansas Valley and South Fork of Ninnescah Valley | Sandy and loamy soils are on nearly level and gently undulating landscapes. There are a few small areas of saline and sodic soils. The major soil series are Canadian, Zenda, Waldeck, and Platte. |
| 3. Wet Soils in Narrow Valleys | Sandy, loamy, and clayey soils are on nearly level and gently sloping landscapes. Depth to the water table ranges from 1 to 4 feet. There are. numerous areas of saline and sodic soils. The major soils are Plevna, Drummond and Natrustolls. |
| 4. Loess Soils of the High Plains | Silty and clayey soils are on nearly level and gently sloping landscapes. The amount of stream dissection is limited in the western part and increases to the east. The major soil series are Harney, Spearville, and Ulysses. |
| 5. Sandy Soils on Uplands | Sandy soils are on undulating to dune landscapes. There is little runoff from these areas. The major soil series are Pratt and Tivoli. |
| 6. Wet, Sandy Soils on Uplands | Sandy soils are on nearly level to dune landscapes. Depths to the water table range from 1 to 4 feet in many of these soils. There is little run- off from these areas. Areas of saline and sodic soils are common. The major soil series are Dillwyn, Tivoli, and Elsmere. |
| 7. Sandy and Loamy Soils of the Great Bend Region | These sandy and loamy soils are on nearly level to undulating landscapes. There is little runoff from these areas. A few areas of saline and sodic soils occur, usually associated with depressional relief. The major soil series are Attica, Naron, Pratt, and Cariwile. |
| 8. Loamy and Clayey Soils of the Great Bend Region and the High Plains | These loamy and clayey soils are on nearly level to strongly sloping landscapes. Limited amount of stream dissection occurs in areas of these soils in the northern part of the study area. There are a few small areas of saline and sodic soils in the. eastern part. The major soil series are Farnum, Naron, Shellabarger Blanket, Bethany, Cariwile, and Mansic. |
| 9. Red Soils Formed on Upper Permian Formations | These loamy and clayey soils are on nearly level to strongly sloping landscapes. The major soil series are Renfrow, Quinlan, and Nash. |
Groundwater samples were collected from 170 pumping irrigation wells and two flowing artesian wells over a five day period, July 25-29, 1977. Heavy rains in the western portion of the Great Bend Prairie during the collection period made sampling difficult and greatly reduced the number of wells available for sampling during this period. As a result, the ratio of samples actually collected to samples anticipated for this year's program was lower than that experienced in previous years. In particular, the sampling efficiency was notably lower than anticipated in Kiowa, Edwards, and Pawnee counties. Another factor which contributed to the sampling problems in the Great Bend Prairie portion of the study area was the fact that no network of groundwater level observation wells existed at the time, and there was no prior knowledge of which wells were suitable for sampling.
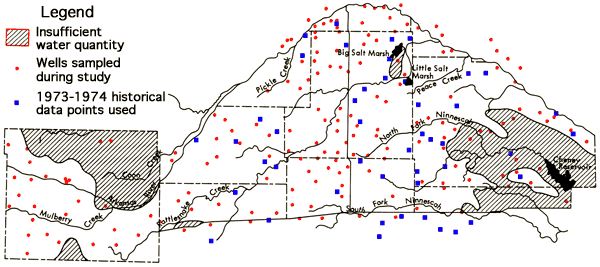
Of the wells sampled, only about six percent have historical chemical quality data. At 19 of the wells a duplicate set of samples was collected by individual field personnel (Duplicate sets). Three wells were sampled by two different people to generate duplicate sets in which the time interval between samplings was up to one day (Overlap sets), one of these wells also coincided with a duplicate set site. The sampling problems mentioned above served to greatly reduce the number of overlap data sets obtained. In addition, 14 wells were sampled north of the Arkansas River between Kinsley, Edwards County, and Hutchinson, Reno County, in order to evaluate the uniformity of chemical quality data in the alluvial valley system. The locations of wells sampled during the collection period, as well as principal drainage systems of the study area, are shown in Figure 7.
Figure 7--Location of wells in the study area, saturated thickness, and principal drainage systems.
Sample handling and analytical procedures employed were similar to those described earlier (Hathaway, et al., 1975, 1976, 1978). one variation employed was to refrigerate samples for nitrate (NO3) and phosphate (PO4) analyses at 4°C rather than freezing them. Bicarbonate (HCO3) and fluoride (F) analyses were performed at a field laboratory established in St. John. Determinations of the concentrations of silica (SiO2), calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), strontium (Sr), chloride (Cl), sulfate (SO4), nitrate (NO3), phosphate (PO4), and the trace elements iron (Fe), manganese (Mn), copper (Cu), and nickel (Ni) were made in the Kansas Geological Survey laboratories in Lawrence.
Self-adhering plastic labels were used at the field laboratory to affix laboratory identification numbers to the sample bottles of the various sets of samples. This served to eliminate the contamination problem encountered when identification tags with wire twists were used at the Liberal field laboratory in 1976. Trace element contamination resulting from the inclusion of deposits that have built up around infrequently used taps is minimized by flushing the sampling outlet several minutes before a sample is collected. Standard deviations for the major constituents, based upon the 19 duplicate sets and three overlap sets, are presented in Table 2. The number of sets from overlap sites is minimal; however, the standard deviations noted for both types of sets are similar to those of the 1976 study (Hathaway, et al., 1978).
A general compilation of the chemical quality data for all irrigation and flowing artesian wells sampled is given in Appendix A by county and location.
Areal chemical quality maps presented in this report were plotted manually using the forty foot saturated thickness contour of map M-5 (Bayne and Ward, 1969) as a general minimum thickness cut-off for mapping purposes. Northern and southern boundaries in the Great Bend Prairie area were taken as the Arkansas River and Highway U. S. 54, respectively. For wells existing in regions with less than 40 feet of saturated unconsolidated sediments, the chemical quality is indicated only for the area immediately surrounding the well. 1973 and 1974 historical data from the Great Bend Prairie area (Stullken and Fader, 1976) have been included to improve the data density in this area. However, only data from irrigation wells or observation wells with depths similar to those of irrigation wells in same area were used since deterioration of water quality with increasing depth is readily apparent from the historical data for areas overlying Permian bedrock. Wells with pH values listed as being greater than 8.2 were also excluded from the historical data used.
Table 2--Standard Deviations of Data for Duplicate Sets
| Determination | Individual Duplicate Sets ±σ |
Overlap Duplicate Sets ±σ |
|---|---|---|
| SiO2 | 0.7 ppm* | 2.4 ppm |
| Ca | 0.9 ppm | 1.7 ppm |
| Mg | 0.3 ppm | 0 ppm |
| Na | 0.3 ppm | 0.8 ppm |
| K | 0.08 ppm | 0.06 ppm |
| Sr | 0.03 ppm | 0.1 ppm |
| HCO3 | 0.9 ppm | 0.9 ppm |
| SO4 | 4.0 ppm | 4.0 ppm |
| Cl | 1.0 ppm | 2.2 ppm |
| F | 0.03 ppm | 0.04 ppm |
| NO3 | 0.3 ppm | 0.6 ppm |
| Total Dissolved Solids | 5.6 ppm | 13 ppm |
| Specific Conductance | 26 µmho** | |
| *parts per million or milligrams per liter **micro-mhos at 25°C 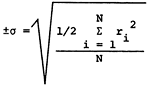 ri = range of analysis of sample N = number of sample pairs |
||
A fairly wide spectrum of water chemistries--based not only upon the amounts of dissolved solids, but also upon the chemical nature of the dissolved solids--is found in the study area. Figure 8 presents the areal distribution of specific conductance values for groundwaters of the study area. Groundwaters from the alluvium of the Arkansas River valley exhibit a higher specific conductance, and dissolved solids load, than waters from the upland areas of the western half of the study area. Specific conductances for waters.from eastern Stafford and northern Reno counties again are higher than those found for groundwaters from the western half of the study area, reflecting increased dissolved loads which appear to be related to the occurrence of salt-bearing bedrock formations of Permian age.
Figure 8--Specific conductance (in µmhos) for the study area. Levels correspond to medium, high, and very high salinity hazard ranges.
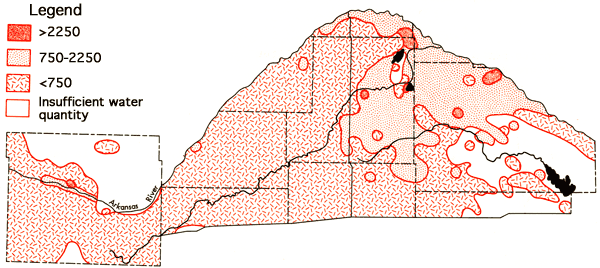
A comparison of the specific conductance data (Figure 8) and the occurrence of Permian bedrock formations (Figure 5) indicates that waters with specific conductance values below 750 micromho, and lower dissolved solid levels, extend eastward into regions overlying bedrock of Permian age in Pratt and Stafford counties. Similar situations are noted in Pratt County for the areal distribution of sodium values (Figure 9) and chloride values (Figure 10). The existence of these better quality waters in regions overlying bedrock of Permian age seems to coincide with inferred channels in the bedrock surface, based upon the topographic map of the bedrock surface by Fader and Stullken (1978), which could serve to direct the movement of the eastward flowing groundwater. The data for some areas on the specific conductance, sodium, and chloride areal maps representing higher level values come from the 1973-1974 historical data for the area and are primarily derived from test holes. The quality of data for water samples from the test holes may not be of equal quality to that obtained for the irrigation wells owing to inadequate pumping before collection of samples and differences in sample handling procedures, but these data still serve to indicate in a general fashion the apparent nature of waters in areas where data from irrigation wells are scarce.
Figure 9--Concentration levels of sodium (ppm).
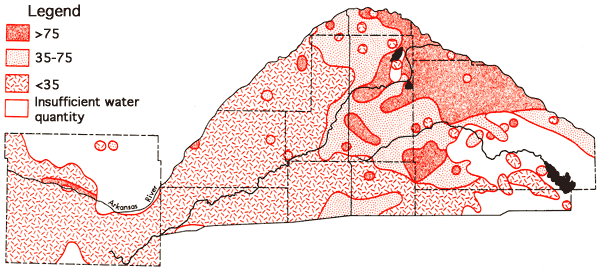
Figure 10--Concentration levels of chloride (ppm).
Data derived from water samples taken at varying depths in the test hole program indicate a general deterioration of water quality with depth exists in the eastern half of the study area (east of U. S. 281, approximately). This deterioration results from an increase in the sodium and chloride contents of the waters with depth. The fact that an increase, relative to western Stafford County, is noted in the sodium and chloride concentrations of groundwaters from the shallower aquifer levels tapped for irrigation in the eastern Stafford and northern Reno counties suggests that some degree of hydraulic connection exists between the lower and upper portions of the aquifer system in this area. Confinement of portions of the aquifer must also exist in some regions as is attested to by the presence of the two flowing artesian wells in the area between Big Salt Marsh and Little Salt Marsh in northeastern Stafford County. The well at T22-R11W-9BBB is 42 feet deep and flows at about three gallons per minute. The well at T22-R11W-27A is 85 feet deep and appears to have a lesser flow rate. The waters from both of these wells are low in chloride (about 8 ppm), suggesting the source of these waters is isolated from, and different than, waters derived from test holes in the immediate area. Taken together, the above observations indicate. the complex nature of the aquifer system, especially in the eastern portion of the Great Bend Prairie area where variations in chemical quality are most evident.
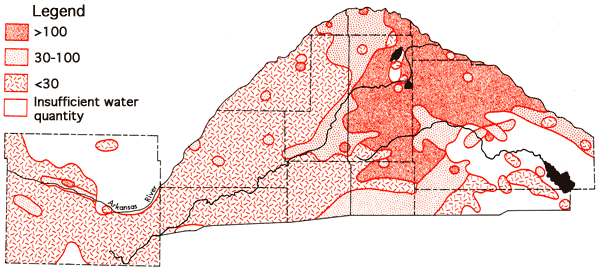
Figure 11 depicts the areal distribution of sulfate values for the study area. Generally speaking, extensive areas with higher sulfate Values tend to be restricted to the Arkansas River valley and regions adjacent to the river valley in southeastern Barton and north-central Reno counties. Magnesium levels show a marked variation between different portions of the study area. in Ford County the average magnesium content of waters north of the Arkansas River valley is 23 ppm, whereas the. average south of the river valley is 12 ppm. The average value for the river valley throughout the study area is 42 ppm. In the Great Bend Prairie portion of the study area many of the samples collected had magnesium concentrations equal to or less than 5 ppm (Figure 12).
Figure 11--Areal distribution of sulfate values (ppm).
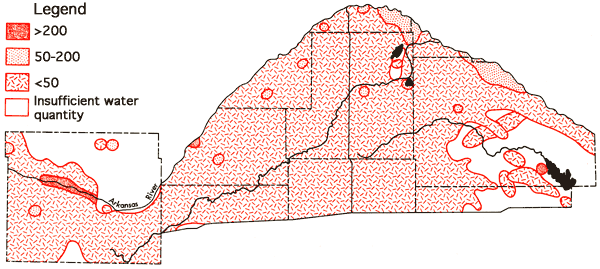
Figure 12--Wells with high concentration levels of magnesium.
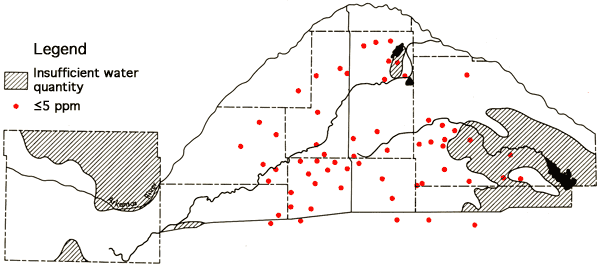
Fluoride levels in the study area in excess of 1.0 ppm are generally restricted to the area north of the Arkansas River in Ford County. The two wells in northeastern Ford County which,produce water from the Dakota Formation, T25-R23W-14ADD and T25-R22-17CBB, exhibit the highest fluoride values in the study area--2.8 and 2.5 ppm, respectively. Nitrate concentrations in excess of 40 ppm were most prominent along the Arkansas River valley and the southeastern corner of the study area, i.e., northeastern Kingman and south-central Reno counties. In regions of shallow water tables and sandy soils the nitrate values might be expected to fluctuate considerably during the course of the growing year and from year to year. Subsequent sampling would be necessary to determine if these higher nitrate values are only temporal effects from recent applications of fertilizer. The ranges and mean values for selected major and minor chemical species, specific conductance, and total dissolved solids are summarized in Table 3.
Table 3--Summary of ranges and means of chemical quality data.
| Variable | Min/Max (ppm) |
Mean (ppm) |
|---|---|---|
| Ca | 21/294 | 71 |
| Mg | 3.1/127 | 11 |
| Na | 8.0/365 | 54 |
| K | 0.9/9.4 | 3.2 |
| Sr | 0.1/5.5 | 0.5 |
| SiO2 | 17/64 | 27 |
| HCO3 | 42/443 | 225 |
| SO4 | 0.2/816 | 56 |
| Cl | 3.1/606 | 61 |
| F | 0.2/2.8 | 0.6 |
| NO3 | 0.4/168 | 24 |
| Total Dissolved Solids | 143/1973 | 413 |
| Specific Conductance | 220/2620 | 660 |
Figures 13 and 14 represent areal groundwater classification by cation and anion types, respectively. Water-type classifications used in this report are based upon percent milliequivalent contributions of the various. chemical species to the total number of milliequivalents per liter of cations or anions. The milliequivalent per liter values reflect the combining capacities of the various chemical species in the waters. Table 4 provides a set of constants which can be used to convert chemical quality data from parts per million to milliequivalents per liter.
Figure 13--Groundwater classification by cation type (waters must have ≥ 50% of the cation milliequivalent abundance to qualify as Na or Ca types).
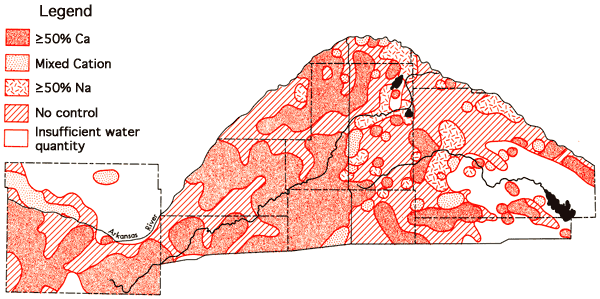
Figure 14--Groundwater classification by anion type (waters must have ≥ 50% of the anion milliequivalent abundance to quality as SO4, HCO3, or Cl types).
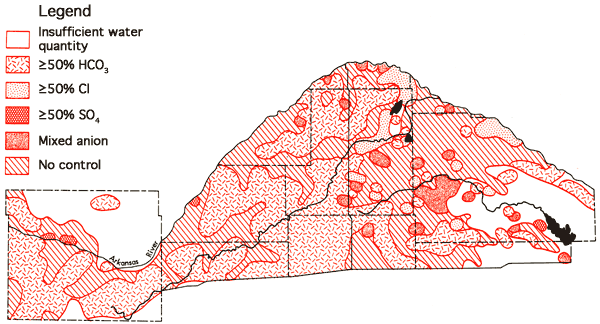
Table 4--Factors for conversion from parts per million to milliequivalents per liter.
| Species | Multiply By |
|---|---|
| Calcium (Ca) | 0.04990 |
| Magnesium (Mg) | 0.08226 |
| Sodium (Na) | 0.04350 |
| Potassium (K) | 0.02557 |
| Strontium (Sr) | 0.02283 |
| Bicarbonate (HCO3) | 0.01639 |
| Sulfate (SO4) | 0.02082 |
| Chloride (Cl) | 0.02821 |
| Fluoride (F) | 0.05264 |
| Nitrate (NO3) | 0.01613 |
Areas of no control in Figures 13 and 14 reflect regions where the sample density is too low to allow extention of water-type boundaries. It is evident from these figures that a Ca-HCO3 water type can be predicted with a greater degree of certainty for the no control areas of Ford County south of the Arkansas River and the western half of the Great Bend Prairie area than predictions of the water types associated with no control areas of the eastern half of the Great Bend Prairie area where transitions toward Na-Cl type waters occur. The association of Ca-HCO3, transition, and Na-Cl type waters in close proximity in the eastern portion of the study area again points up the need for additional data collection in the no control regions to support the geochemical and hydrologic interpretation of the character of the aquifer system in this area.
Stiff diagrams (Stiff, 1951) for groundwater samples from the 36 square mile area of T24-R13W are shown in Figure 15 (the location of this region in the study area is shown in Figure 16). Groundwaters are a Ca-HCO3 type in the western half of this area and undergo transition toward a Na-Cl type in the eastern half of the study area. The pattern displayed by the well at T24-R13W-15BBD is of interest because it suggests a chloride source other than Na-Cl from Permian bedrock formations is contributing to the chloride load of the groundwater at this location. A possible source of the excess chloride in this sample is a subsurface brine containing calcium chloride. The calcium content of this water also appears to be elevated relative to the amounts of bicarbonate and sulfate present--anionic species with which calcium is usually associated in groundwaters of central and western Kansas.
Figure 15-Stiff diagrams for selected wells from T24-R31W. (Location of this region is shown on Figure 16.).
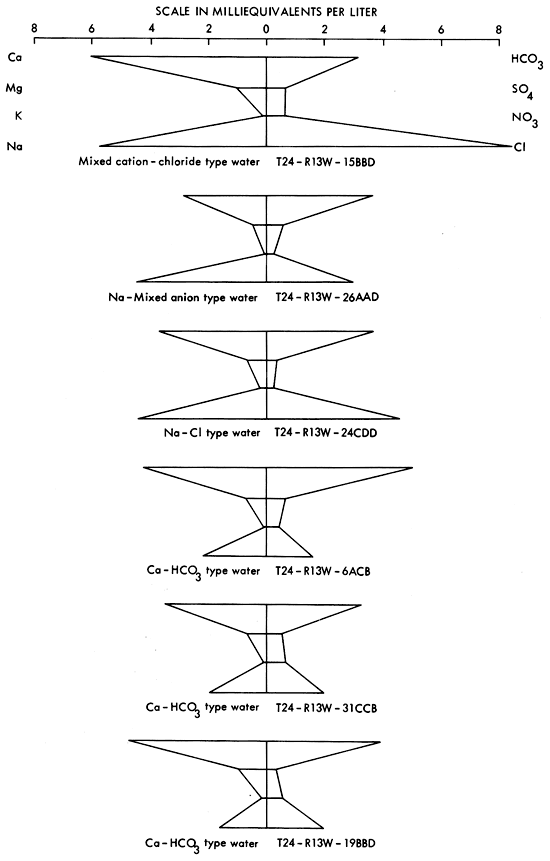
Figure 16--Na-Cl ratios for wells with chloride values equal to or greater than 100 ppm. The section for which the Stiff diagrams in Figure 15 were prepared is also shown.
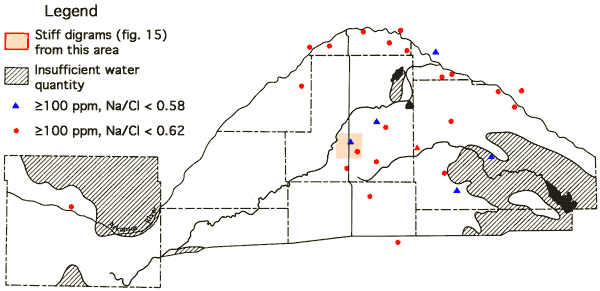
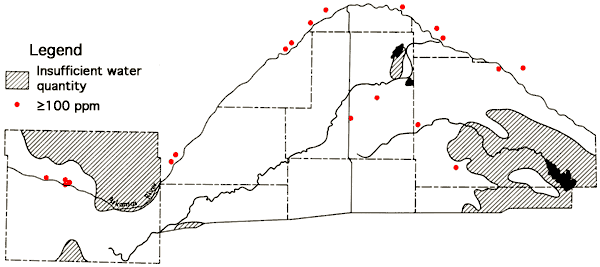
A determination of Na-Cl ratios for wells with chloride values equal to or greater than 100 ppm is summarized in Figure 16. In situations where the source of chloride is sodium chloride from Permian bedrock sources, a ratio of 0.65 is expected. Ratios above 0.65 suggest an auxiliary source of sodium, such as sodium sulfate or sodium bicarbonate salts in the soils. Ratios below 0.65 indicate an auxiliary source of chloride, such as a calcium chloride containing subsurface brine. The Na-Cl ratio for oil brines from Pratt County is reported to be ≤0.59 (Layton and Berry, 1973). Locations of wells having chloride values in excess of 100 ppm and Na-Cl ratios ≤0.58 are: Reno County--T24-R7W-31CBA, T24-R10W-19D, T26-R9W-10DDB; Rice County--T20-R10W-36ACD; and Stafford County--T23-R12W-22BCC, T24-R13W-15BBD. Figure 17 indicates locations of irrigation wells whose waters have calcium contents exceeding a 100 ppm level. The higher levels noted along the Arkansas River valley reflect the presence of calcium sulfate in the soils of the river valley system. However, the wells having higher calcium levels in the Great Bend Prairie portion of the study area are seen to correspond to wells with Na-Cl ratios ≤0.58. Three sets of samples collected by two different individuals at well T24-R10W-19D all exhibited Na-Cl ratios of 0.44 and Ca levels of 109-110 ppm.
Figure 17--Irrigation wells with calcium content ≥100 ppm.
In view of these observations, additional sampling at the above sites seems warranted, not only to confirm the data obtained from the present sampling program for the other five wells apparently enriched with calcium chloride, but also to monitor any variation in water chemistry which might occur during the course of the pumping season.
The areal distribution of sodium adsorption ratio (SAR) values is shown in Figure 18. SAR values are computed using the equation
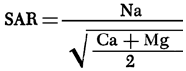
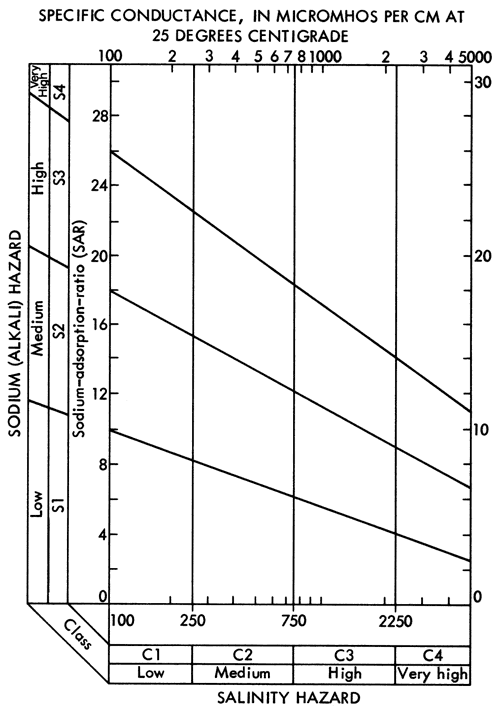
where values in parentheses are milliequivalents per liter of the specified ions. Major areas with SAR values in excess of 3.0 are found in eastern Stafford and northern Reno counties, the areas showing a transition to a Na-Cl water type. SAR values less than 1.5 are common west of U. S. 281 and in a zone extending eastward along the southern boundary of the study area from Pratt County into southern Reno and Kingman counties. The areal distribution of SAR values in Pratt and Stafford counties exhibits similar relationships to the bedrock geology and topography as noted for the areal distribution of specific conductance (Figure 8), sodium (Figure 9), and chloride (Figure 10) values. Specific conductance data from Figure 8 and SAR data from Figure 18 can be used, with the aid of Figure 19, to evaluate the general suitability of groundwaters for irrigation purposes (U. S. Salinity Laboratory Staff, 1954). The alkali and salinity hazard classification of the water must be coupled with a consideration of the soil characteristics and depth to the water table in the overall compatibility evaluation. In general, it should be anticipated that a degree of caution should be exersised in prolonged use of groundwater from areas where the SAR values exceed 3.0.
Figure 18--Values for sodium adsorption ratio (SAR) for the study area.
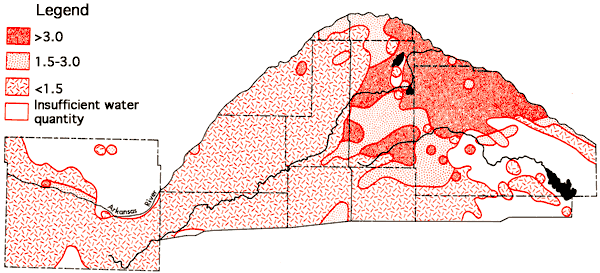
Figure 19--Diagram showing the relationship between specific conductance and the sodium adsorption ratio in evaluation of salinity and alkali hazards for irrigation waters.
The modified Piper diagram (Piper, 1944) in Figure 20 serves to summarize the chemical quality data for irrigation and artesian wells located in the study area that were sampled in July 1977. Wells with a Ca-HCO3 water type, typical of most wells in the western half of the area, are located in the lower right part of the diagram. A gradation to SO4-type waters of the Arkansas River valley is observed in moving vertically to the upper right portion of the diagram. The Na-Cl type waters of eastern Stafford and northern Reno counties are located in the upper left part of the diagram, and a belt of transition waters lies along the diagonal from the CA-HCO3 waters of the lower right to the NaCl waters of the upper left. In general, waters from the transition belt represent wells in the. eastern half of the study area. Here lower mineralized water containing salt from the Permian age bedrock formations appears to be mixing with better quality shallow groundwater derived from the western portion of the study area. This interaction produces waters in which the salt makes some contribution to the dissolved solids load of the waters, but has not reached a point at which it dominates the water chemistries. The water chemistries of the two flowing artesian wells (bottom of Figure 18) suggest a base-softening process in the isolated or confined aquifer systems, i.e., an exchange of calcium in solution for sodium in minerals of the aquifer formation which leads to a reduction of calcium in solution and an increase in sodium in solution. The wells with chloride values equal to or exceeding 100 ppm and having Na-Cl ratios less than 0.58 are also designated. These waters are found along the upper central portion of the CA-HCO3 to Na-Cl transition belt. These waters are seen to contain a higher relative calcium and magnesium content than the more "normal" chloride-type waters of the upper left area of the figure.
It should be emphasized that the chemical quality data collected in the present study represents the status of the study area at a specific point in time under set conditions of well density, pumpage, and well depths. This is particularly important with regard to discussions concerning the quality of waters in the eastern half of the study areas. Increased rates of withdrawal of water in this area may lead to alteration of the chemical quality areal distribution patterns, and completion of wells at deeper levels within the aquifer system most assuredly will change the interpretation of the overall water quality. For these reasons, monitoring of the groundwater quality in regions overlying Permian age bed,rock-appears essential to continued development of the groundwater resources of the area.
The services of personnel from the U.S.G.S. offices in Lawrence and Garden City, Kansas, and T. McClain, K.G.S., in the collection of water samples; M. Flanagan, C. Miller, M. Cortese and H..N. Le in the operation of laboratory facilities; and P. Kubica, K.G.S., in map preparation are gratefully acknowledged. The authors are also indebted to Mr. J. Hood and Mr. G. Highfill, Superintendent of Schools, St. John, Kansas, for providing field laboratory facilities. The cooperation of Mr. R. Wendelburg, President of Groundwater Management District #5, and Mr. R. Sloan, Manager of Groundwater Management District #5, and his staff greatly facilitated the.undertaking of this study.
Adams, G.I., 1903, Physiographic Divisions of Kansas: Transactions of the Kansas Academy of Science, Vol. 18, pp. 109-123.
Bayne, C.K., 1956, Geology and Groundwater Resources of Reno County, Kansas: Kansas Geological Survey, Bulletin 120, 94 p. [available online]
Bayne, C.K., and Ward, J.R., 1969, Saturated Thickness and Specific Yield of Cenozoic Deposits in Kansas: Kansas Geological Survey Map M-5.
Bayne, C.K., and Ward, J.R., 1973, Geology and Hydrology of Rice County, Central Kansas: Kansas Geological Survey, Bulletin 206, Part 3, 17 p. [available online]
Dodge, D.A., Tomasu, B.I., Haberman, R.L., Roth, W.E., and Baumann, J.B., 1965, Soil Survey of Ford County, Kansas: U.S. Department of Agriculture, Series 1958, No. 32, 84 p.
Fader, S.W., and Stullken, L.E., 1978, Geohydrology of the Great Bend Prairie, South-Central Kansas: Kansas Geological Survey, Irrigation Series 4 (in press). [available online]
Fent, O.S., 1950, Geology and Ground-water Resources of Rice County, Kansas: Kansas Geological Survey, Bulletin 85, 142 p. [available online]
Fent, O.S., 1950b, Pleistocene Drainage History of Central Kansas: Transactions of the Kansas Academy of Science, Vol. 53, No. 1, pp. 81-90.
Frye, J.C., and Leonard, A.B., 1952, Pleistocene Geology of Kansas: Kansas Geological Survey, Bulletin 99, 230 p. [available online]
Frye, J.C., and Schoewe, W.H., 1953, The Basis for Physiographic Subdivision of Kansas: Transactions of the Kansas Academy of Science, Vol. 56, No. 2, pp. 246-252.
Hathaway, L.R., Magnuson, M.L., Carr, B.L., Galle, O.K., and Waugh, T.C., 1975, Chemical Quality of Irrigation Waters in West-Central Kansas: Kansas Geological Survey, Chemical Quality Series 2, 45 p. [available online]
Hathaway, L.R., Carr, B.L., Galle, O.K., Magnuson,, M.L., Waugh, T.C., and Dickey, H.P., 1977, Chemical Quality of Irrigation Waters in Hamilton, Kearny, Finney, and Northern Gray Counties: Kansas Geological Survey, Chemical Quality Series 4, 33 p [available online]
Hathaway, L.R., Carr, B,L., Flanagan, M.A., Galle, O.K., Waugh, T.C., and Dickey, H.P., 1978, Chemical Quality of Irrigation Waters in Southwestern Kansas; Kansas Geological Survey, Chemical Quality Series 6, 35 p . [available online]
Horsch, M.L., 1974, Soil Survey of Rice County, Kansas,: U.S. Department of Agriculture, 63 p.
Horsch, M.L., Hoffman, B.R., and Gier, D.A., 1968, Soil Survey of Pratt County, Kansas: U.S. Department of Agriculture, 56 p.
Lane, C.W., 1960, Geology and Groundwater Resources of Kingman County, Kansas: Kansas Geological Survey, Bulletin 144, 174 p. [available online]
Latta, B.F., 1947, Geology and Groundwater Resources of Kiowa County, Kansas: Kansas Geological Survey, Bulletin 65, 151 p. [available online]
Latta, B.F., 1950, Geology and Groundwater Resources of Barton and Stafford Counties, Kansas: Kansas Geological Survey, Bulletin 88, 228 p. [available online]
Layton, D.W. and Berry, D.W., 1973, Geology and Groundwater Resources of Pratt County, South-Central Kansas: Kansas Geological Survey, Bulletin 205, 33 p. [available online]
McLaughlin, T.G., 1949, Geology and Groundwater Resources of Pawnee and Edwards Counties, Kansas: Kansas Geological Survey, Bulletin 80, 189 p. [available online]
Piper, A.M., 1944, A Graphic Procedure in Geochemical Interpretation of Water Analyses: Transactions American Geophysical Union, Vol. 25, pp. 914-923.
Rockers, J.J., Ratcliff, I., Dowd, L.W., and Bouse, E.F., 1966, Soil Survey of Reno County, Kansas: U.S. Department of Agriculture, Series 1960, No. 29, 72 p.
Roth, W.E., 1973, Soil Survey of Edwards County, Kansas: U.S. Department of Agriculture, 64 p.
Schoewe, W.H., 1949, The Geography of Kansas, Part II, Physical Geography: Transactions of the Kansas Academy of Science, Vol. 52, No. 3, pp. 261-333.
Stiff, H.A., 1951, The Interpretation of Chemical Water Analysis by Means of Patterns: Journal of Petroleum Technology, Vol. 3, No. 10, pp. 15-17.
Stullken, L.E, and Fader, S.W., 1976, Hydrologic Data From the Great Bend Prairie, South-Central Kansas: Kansas Geological Survey, Basic Data Series, Groundwater Release No. 5, 50 p.
U.S. Salinity Laboratory Staff, 1954, Diagnosis and Improvement of Saline and Alkali Soils: U.S. Department of Agriculture Handbook, 60, 160 p.
Waite, H.A., 1942, Geology and Groundwater Resources of Ford County, Kansas: Kansas Geological Survey, Bulletin 43, 250 p. [available online]
Kansas Geological Survey
Placed on web Nov. 22, 2010; originally published in August 1978.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS7/index.html