
Kansas Geological Survey, Chemical Quality Series 6, originally published in 1977
Next Page--Appendix

Originally published in 1977 as Kansas Geological Survey Chemical Quality Series 6.
The utilization of irrigation waters in Kansas has increased dramatically since the drought period of the 1950's. With this development has come the need for information which can be used in the determination of compatibility of groundwaters with soils and crops and in the management of the groundwater systems tapped for agricultural purposes. An important component of this information is the chemical character of the waters under consideration.
Both human activity in the area of oil and gas development and natural sources such as mineralized waters from bedrock formations of Permian Age serve as potential agents for the alteration and deterioration in quality of groundwaters tapped for agricultural purposes in southwestern Kansas. The scarcity of historical groundwater data for portions of Morton, Stevens, and Seward counties and the general unavailability of data regarding subsurface brine chemistries of the area make an evaluation of the impact of past fuel resource development upon groundwater quality extremely difficult in these counties. Similarly, the historical data for Meade County appears inadequate to define clearly the area in which deterioration of groundwater quality results from upward movement of mineralized waters. The current study points to the need for additional sample data from these four counties in order to adequately monitor future changes in chemical quality with increased development of groundwater resources.
Other areas covered in this series of studies are Greeley, Wichita, Scott, Lane, and southern Wallace counties (Kansas Geological Survey Chemical Quality Series 2) and Hamilton, Kearny, Finney, and northern Gray counties (Kansas Geological Survey Chemical Quality Series 4). In addition, samples were collected in the Great Bend Prairie area and Ford County in 1977, and preparation is underway for the sampling of northwest Kansas during 1978.
Two-hundred-sixty-four pumping irrigation wells were sampled in Stanton, Grant, Haskell, Morton, Stevens, Seward, Meade, and southern Gray counties and adjoining portions of Hamilton, Kearny, Finney and, northern Gray counties during July 1976. The general quality of these waters, with the exception of an area west of the Crooked Creek-Fowler fault complex in Meade County, places them in the medium to high salinity hazard and low alkali hazard ranges. Waters in the area west of the fault complex in Meade County are of a Na-Cl type and may fall in the medium to high alkali hazard range.
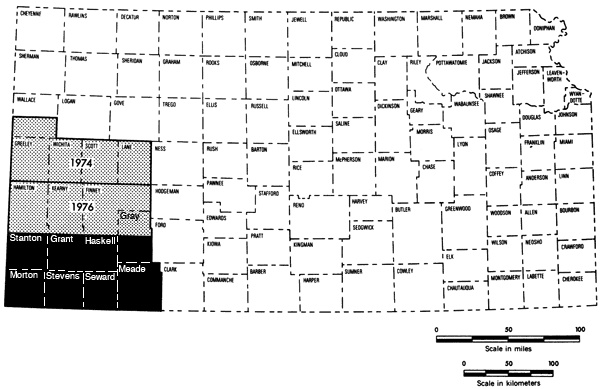
This study represents the third part of a program begun in 1974 to establish chemical quality base-line data for irrigation waters of western Kansas. Previous studies have covered the west-central Kansas area (1974) and the Hamilton-Kearny-Finney-northern Gray county area (1975). The present 7-1/2-county area represents the final, lower portion of the southwestern Kansas region of groundwater studies (Figure 1).
Figure 1--Index map showing the area covered by this report (dark area) and areas covered by past studies in the Chemical Quality Program.
Irrigation waters in the present study are derived mainly from undifferentiated Quaternary-Tertiary sequences; however, in the western third of the study area undifferentiated Mesozoic or undifferentiated Quaternary-Tertiary-Mesozoic sequences are important sources of groundwater. Alluvium associated with the Cimarron River system in the southern portion of the study area also serves as an aquifer locally for wells located along that system.
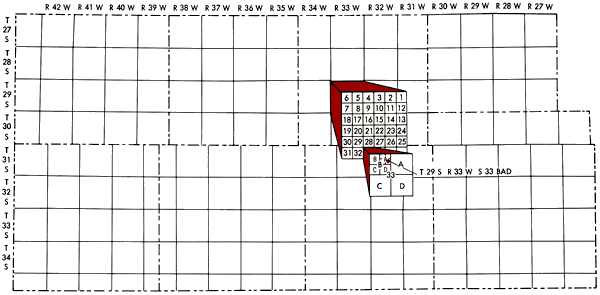
Well locations used in this report are based upon the Bureau of Land Management numbering system. The location is composed of the township, the range, and the section number followed by letters designating the subdivision of the section on which the well is located (Figure 2).
Figure 2--Map of study area illustrating Bureau of Land Management numbering system used for well locations.
Smith (1940) has described the general topographic features of southwestern Kansas. The study area is a part of the central High Plains region and exhibits a general west to east slope of the land surface.
The Cimarron River, located in the southern portion of the study area, is the major stream in this region. The river enters the state in Morton County trending to the northeast; but bends in southern Grant County, assumes a southeasterly trend in Seward County and leaves the state in Meade County. The Cimarron Bend area south of the river is dotted with low uneven sandy hills and has no major outside drainage.
The North Fork of the Cimarron is the main tributary to the river in the study area; however, Crooked Creek in the eastern part of the study area joins the Cimarron River below the study area in Oklahoma. Bear Creek trends in a northeasterly direction through Stanton and Grant counties before terminating in a basin in Grant and Kearny counties.
Consolidated rocks of undifferentiated Lower Cretaceous-Jurassic age form the bedrock in Stanton, Grant, and Haskell counties, but give way to rocks of Permian age in Morton, Stevens, Seward, and Meade counties (Figure 3). The extent to which Permian-age rocks contribute to the bedrock formation increases from west to east (i.e., from Morton County to Meade County) (Gutentag, Lobmeyer and Slagle, in prep.).
Figure 3--Bedrock geology of the study area.
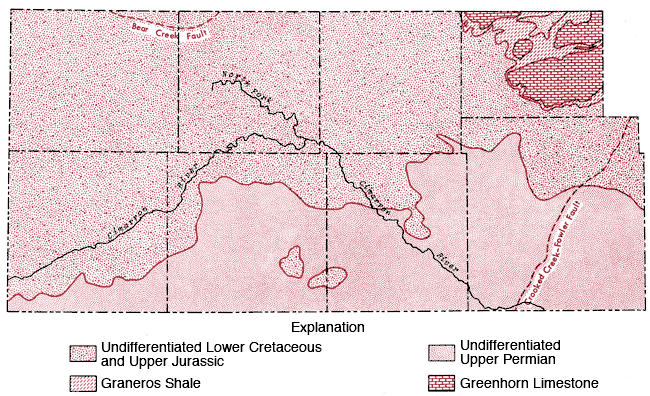
This general trend is somewhat altered in southern Gray County, where a regional elevation of the bedrock surface exists and upper Cretaceous (Graneros Shale and Greenhorn Limestone) and undifferentiated lower Cretaceous rocks form the bedrock surface (McGovern and Long, 1974).
Two fault systems (Bear Creek and Crooked Creek-Fowler) occur in the study area and tend to restrict the availability of groundwater on the up-throw side of the faults, i. e. in northeastern Stanton and eastern Meade counties, respectively, in the area covered by this report.
Figure 4 is a generalized soil map of the study area. This map has been produced by combining data for individual counties. Some grouping of the various soil associations presented on general county soil maps and some regrouping of mapping units to better reflect the proportional pattern of soils in natural landscapes for a multi-county area was necessary (Dickey, Eeds and Hecht, 1961; Fleming, McBee, Hamilton and Sallee, 1961; Dickey, Swafford and Markley, 1965; Dickey, Swafford and Markley, 1963; Hamilton, Jantz, Lobmeyer, Markley and Swafford, 1968; Hamilton, Markely, Swafford and Dickey, 1969; Tomasu, 1977; Tomasu and Roth, 1968). A brief description of the soil associations used in this regional map is given in Table 1.
Figure 4--Soil association map of the study area.
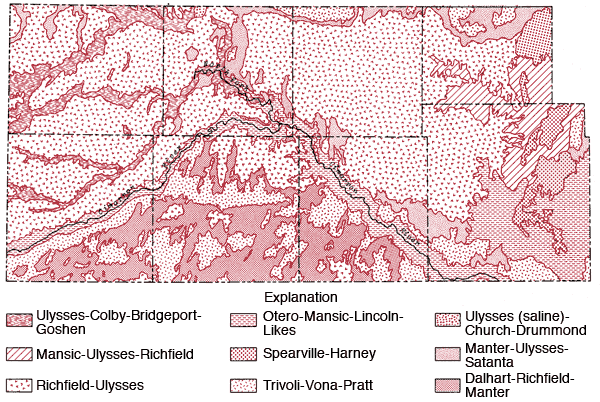
Table 1--Soil Association Groups
| 1. Richfield-Ulysses: | These silty soils are on broad, nearly level landscapes. This is the dominant soil association of the uplands. Little or no runoff occurs from these soils to stream systems. |
| 2. Ulysses-Colby-Bridgeport-Goshen: | Most of this association consists of silty and loamy soils on nearly level landscapes. These soils are associated with upland drainage ways. |
| 3. Spearville-Harney: | These clayey and silty soils are located on broad, nearly level landscapes along the eastern edge of the study area. |
| 4. Mansic-Ulysses-Richfield: | Loamy and silty soils occurring on nearly level to strongly sloping landscapes. Found along the eastern edge of the study area along upland drainage ways. |
| 5. Tivoli-Vona-Pratt: | These sandy soils are found on undulating and hilly landscapes. The main areas of occurrence are south of the Cimarron River and north of the river in the eastern fourth of the study area. Little or no runoff occurs from these areas to stream systems. |
| 6. Manter-Ulysses-Satanta: | Loamy soils occurring on nearly level and gently undulating landscapes. Generally occur in transitional zones between sandy soils and silty soils on uplands. |
| 7. Ulysses (saline)-Church-Drummond: | Silty and clayey soils found on nearly level landscapes. They are slightly to highly saline and are found in the terminal portion of the Bear Creek. |
| 8. Otero-Mansic-Lincoln-Likes: | These loamy and sandy soils occur on nearly level to rolling landscapes. They occupy the flood plains and sloping areas along the Cimarron River. Some local spots of saline soils may occur in the flood plain. |
| 9. Dalhart-Richfield-Manter: | Loamy and silty soils occurring on nearly level to gently undulating landscapes. They occur south of the Cimarron River. There is little or no runoff from these areas to stream systems. |
A notable feature of the regional map is the general transition from the silty upland Richfield-Ulysses soil association north of the Cimarron River to the more sandy Tivoli-Vona-Pratt and Dalhart-Richfield associations south of the Cimarron River.
Groundwater samples were collected from 264 pumping irrigation wells over a four-day period, July 26-29, 1976. Sampling procedures and field determinations were the same as described earlier (Hathaway, et al., 1975, 1976). Historical chemical quality data, other than that gathered in the present series of studies, exist for about 20% of the wells sampled, with about half of those historical records existing for wells in Seward and Stevens counties (13 each). Duplicate sets of samples were collected at 50 sites. Twenty duplicate sets of samples were generated by individual field personnel collecting duplicate sets of samples from wells within their sampling area (Duplicate Sets). Thirty-two sets of duplicate samples were collected from wells located along the boundaries of different sampling areas by two individuals sampling the same well (overlap Sets). In two cases wells used for an individual's duplicate set also corresponded to an overlap set. The time interval between collection of the pairs of samples from the overlap sites ranges from the same day to three days.
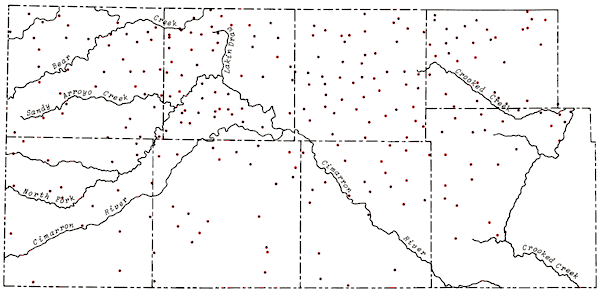
The locations of wells sampled in the study area as well as principal drainage systems of the area are shown in Figure 5. Wells were sampled north of the study area to provide greater control in mapping the chemical quality data and to provide greater interfacing of the data from this study with that collected the previous year. Analytical data for the control wells north of the study area are included in the compilation of chemical quality data in Appendix A. The distribution of wells sampled during the study follows closely the regions shown to have pumping potentials of 500 gal/min or greater on Map M-4A, General Availability of Ground Water and Normal Annual Precipitation in Kansas (C.K. Bayne et al., 1975). The density of wells sampled decreased somewhat in the southern third of the study area, particularly in parts of those regions overlying bedrock of Permian age.
Figure 5--Location of wells in study area and principal drainage systems.
Analytical procedures employed were the same as those described earlier (Hathaway, et al., 1975, 1976). Determinations of specific conductance and pH were made at the time of collection. Bicarbonate (HCO3), chloride (Cl), and fluoride (F) analyses were performed at a field laboratory established in Liberal. Analyses of silica (SiO2), calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), sulfate (SO4), nitrate (NO3), phosphate (PO4), strontium (Sr), and the trace elements were made in the Kansas Geological Survey laboratories in Lawrence.
Standard deviations for the major constituents in the 20 duplicate sets and 32 overlap sets are presented in Table 2. The standard deviations for the overlap sets are about twice that for the individual sets, indicating some time dependent variation in the sampling. The variations in the analytical data for the overlap samples average about 3.5% and as such do not appear to present a problem in grouping the sample data for the purpose of regional mapping of chemical quality data. Variation in the specific conductance for the overlap sets is 3.8%.
Table 2--Standard Deviations of Data for Duplicate Sets
| Determination | Individual Duplicate Sets ±σ |
Overlap Duplicate Sets ±σ |
|---|---|---|
| SiO2 | 1.5 ppm* | 3.2 ppm |
| Ca | 0.6 ppm | 1.2 ppm |
| Mg | 0.2 ppm | 0.5 ppm |
| Na | 0.4 ppm | 1.0 ppm |
| K | 0.05 ppm | 0.1 ppm |
| Sr | 0.02 ppm | 0.05 ppm |
| HCO3 | 1.4 ppm | 2.6 ppm |
| SO4 | 1.8 ppm | 3.8 ppm |
| Cl | 0.5 ppm | 1.8 ppm |
| F | 0.02 ppm | 0.1 ppm |
| NO3 | 0.2 ppm | 0.5 ppm |
| Total Dissolved Solids | 6 ppm | 11 ppm |
| Specific Conductance | 21 µmho** | |
| *parts per million or milligrams per liter **micro-mhos at 25°C 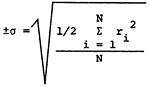 ri = range of analysis of sample N = number of sample pairs |
||
In the case of the trace elements iron (Fe), manganese (Mn), copper (Cu), nickel (Ni), and zinc (Zn), where concentration levels are usually in the parts per billion ranges, a more complicated situation exists as there is greater chance for contamination. First, incomplete flushing of the sampling outlet at the well can result in higher trace element levels due to inclusion of deposits which have built up around infrequently used taps. In this respect, direct sampling of the main flow of the well would be best, but this is not always possible under actual field conditions. Secondly, identification tags were attached to the trace element sample bottles in the field laboratory by means of galvanized wire twists, in several cases there was visual evidence to suggest that these wires had produced Fe contamination and possibly Zn and some Mn contamination as well. Where Fe contamination was apparent, Fe values have been omitted in the reported data.
A general compilation of chemical quality data for groundwater samples collected during this study is presented in Appendix A by county and location.
Chemical quality maps presented in this report were plotted manually using the <100 gal/min boundary from water quantity map M-4A as a cut-off for mapping. [Note: The diagonal pattern on the maps represents areas of insufficient water quantity.] Historical data back to 1970, and data for surface samples collected during July 1976 from the Cimarron River and Crooked Creek in Meade County, were also used to establish relative chemical quality boundaries in areas where NaCl begins to make an important contribution to the dissolved salt load of the groundwater.
Lack of comparable recent historical data for the remainder of the southern portion of the study area makes it impossible to increase the sample density in this region and reduce the uncertainty associated with the interpretation of the chemical quality data for this portion of the study area.
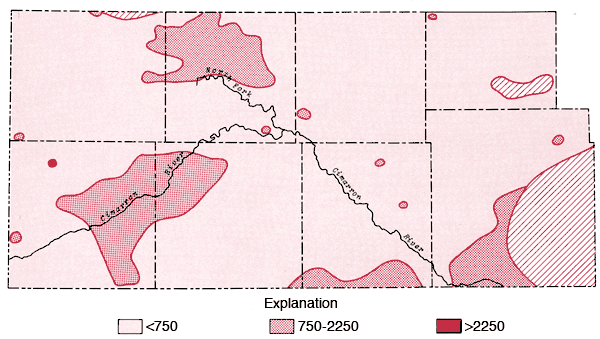
The areal distribution of the specific conductance values for groundwater samples from the study area is displayed in Figure 6. From this figure it is apparent that areas of higher dissolved solids content exist either side of Lakin Draw in the area between Bear Creek and the North Fork of the Cimarron River, along the Cimarron River in eastern Morton and western Stevens counties, south of Liberal in Seward County, and in the eastern portion of the mapped area of Morton County. Higher values in the Lakin Draw-Bear Creek area may be related to accumulation of dissolved solids in shallow depressions in this region.
Figure 6--Map of specific conductance (in µmhos) for the study area. Levels correspond to medium, high, and very high salinity hazard ranges.
Areal distributions of SO4, F, Na, and Cl are shown in Figures 7, 8, 9, and 10, respectively. Using the physiographic divisions of Smith (1940), one relatively common feature of these maps is the higher values found for waters associated with the Stanton and Cimarron Valley areas compared to waters from the Finney sand plain, Haskell, and south-central Cimarron Bend areas. Mg, Sr, and K all exhibit distribution patterns similar to that of SO4. Both Na and Cl reach the highest levels of the study area in the Odee and southern Meade (below the City of Meade) areas. The higher Na-Cl levels of this area have been attributed to an upward movement of highly mineralized water from the Permian bedrock of this area (Gutentag, Lobmeyer and Slagle, in prep.). Measurements made near the Cimarron River in this area indicate the hydrostatic head in the Permian bedrock is greater than that for the overlying unconsolidated sediments (Gutentag, personal communication). Nitrate values in excess of 30 ppm are found in the area of the Bear Creek depression (McLaughlin, 1946) in northeastern Stanton and northwestern Grant counties. Minimum, maximum, and mean concentration levels of the major constituents, total dissolved solids, and specific conductance values are listed in Table 3.
Figure 7--Map of concentration levels of sulphate (in ppm) for the study area.
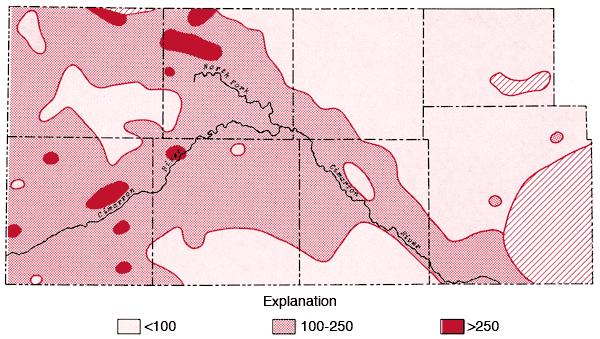
Figure 8--Map of concentration levels of fluoride (ppm).
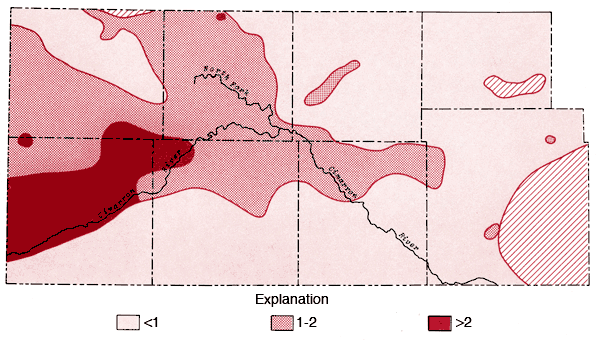
Figure 9--Map of concentration levels of sodium (ppm).
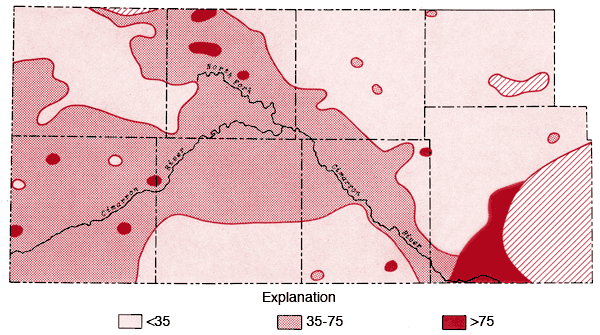
Figure 10--Map of concentration levels of chloride (ppm).
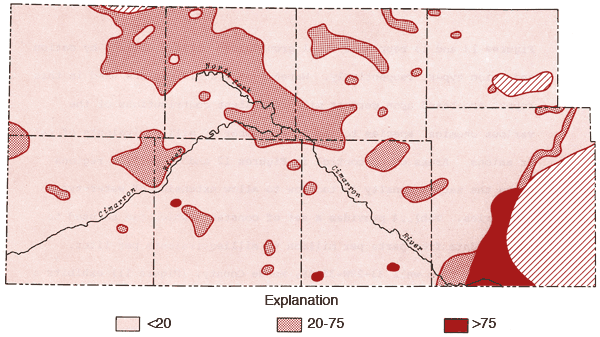
Figure 11 is an areal display of Stiff diagrams (Stiff, 1951) for selected wells from the study area. This figure serves to illustrate the diversity in chemical character of groundwaters from this region as well as indicating the relative amounts of dissolved solids in these waters. These diagrams reflect the milliequivalents per liter levels, or relative combining capacities, of selected chemical species in these waters.
Table 3--Summary of ranges and means of chemical quality data.
| Variable | Min/Max (ppm) |
Mean (ppm) |
|---|---|---|
| Ca | 31/387 | 62 |
| Mg | 5.6/77 | 20 |
| Na | 7.9/317 | 36 |
| K | 2.1/9.4 | 4.1 |
| Sr | .4/10 | 1.2 |
| HCO3 | 151/323 | 204 |
| SO4 | 13/1344 | 109 |
| Cl | <1.0/445 | 22 |
| F | 0.3/3.5 | 1.0 |
| NO3 | 0.4/84 | 14 |
| SiO2 | 12/43 | 23 |
| Total Dissolved Solids | 164/2112 | 380 |
| Specific Conductance | 280/2400 | 609 |
Figure 11--Areal display of Stiff diagrams for selected wells from the study area. Diagrams are centered on their well locations.
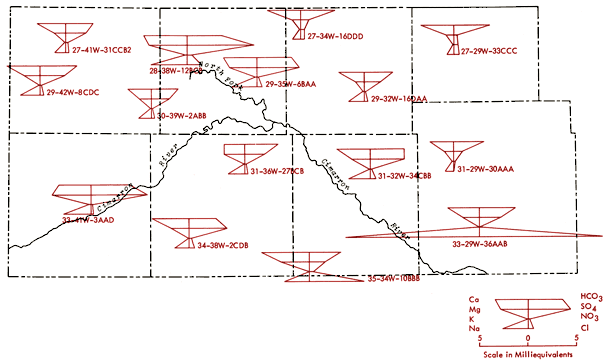
Figures 12 and 13 represent areal groundwater classifications by cation and anion types, respectively. Water-type classifications used in this report are based upon percent milliequivalent contributions of the various chemical species to the total number of milliequivalents of cations or anions. Areas of no control in Figures 12 and 13 reflect regions where the sample density is too low to allow extension of water-type boundaries. Table 4 provides a set of constants which can be used to convert data from parts per million to milliequivalents per liter.
Figure 12--Groundwater classification by cation type (waters must have > 50% of the cation milliequivalent abundance to qualify as Na or Ca types).
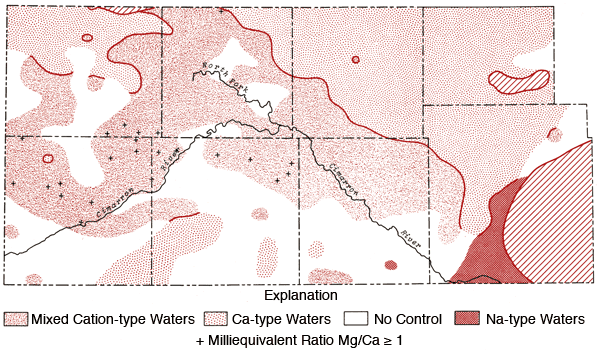
Figure 13--Groundwater classification by anion type (waters must have > 50% of the anion milliequivalent abundance to qualify as SO4, HCO3, or Cl types).
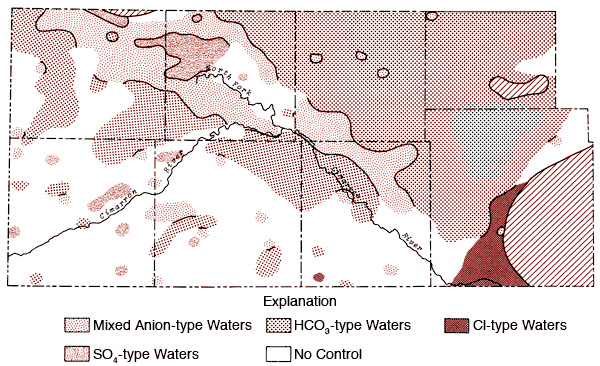
Table 4--Factors for conversion from parts per million to milliequivalents per liter.
| Species | Multiply By |
|---|---|
| Calcium (Ca) | 0.04990 |
| Magnesium (Mg) | 0.08226 |
| Sodium (Na) | 0.04350 |
| Potassium (K) | 0.02557 |
| Strontium (Sr) | 0.02283 |
| Bicarbonate (HCO3) | 0.01639 |
| Sulfate (SO4) | 0.02082 |
| Chloride (Cl) | 0.02821 |
| Fluoride (F) | 0.05264 |
| Nitrate (NO3) | 0.01613 |
Water from well 33-29W-36AAB, Meade County, (Figure 11) exhibits a strong NaCl component and lies within the domain of Na-Cl type waters of Figures 12 and 13. The water chemistry of this well is consistent with the notion of an upward movement of mineralized water from the Permian bedrock and resultant deterioration of the water quality of groundwater in the unconsolidated sediments.
Well 35-34W-10BBB, Seward County, (Figure 11) produces water which approaches a Ca-Cl type. This water type is atypical when compared to other irrigation waters in the study area and suggests the possibility of intermixing of a Ca-Cl type brine with groundwater from the unconsolidated sediments in this region. Historical data for this well indicate a Ca-Mg-HCO3 water type for a sample collected in 1966, but approaching the Ca-Cl water type for a sample collected in 1974. The 1974 data are also in good general agreement with data from the 1976 collections covered by this report.
The Stiff diagrams for wells 31-29W-30AAA (Meade County), 27-29W-33CCC (Gray County), 27-34W-16DDD (Haskell County), and 29-32W-16DAA (Haskell County) are typical patterns for the Ca-HCO3 type waters found in the northeast quadrant of the study area.
The pattern of the cation portion of the Stiff diagram for well 33-41W-3ADD (Morton County) typifies the general pattern found for a band extending from northern Morton and southern Stanton counties into northern Stevens County (see Figure 12) and a single well in north-central Grant County where the milliequivalent ratio Mg/Ca > 1. Waters of this type could arise from loss of Ca by CaCO3 precipitation from saturated solutions or from differential leaching of Mg and Ca from calcareous deposits in the subsurface. Preliminary calculations suggest all of these waters are saturated with respect to calcite. About 70% of the wells in this band have HCO3-type waters and a number of these waters also exhibit F levels in excess of 2 ppm.
Figure 12 also shows that the area surrounding the Cimarron River and its tributaries in the western half of the study area is dominated by mixed cation type waters. Sulfate waters are found to be restricted to the western half of the study area (Figure 13), with the largest continuous area of occurrence being in the region between Bear Creek, the North Fork of the Cimarron and Lakin draw. An area of mixed anion type waters extends southeastward from south of the Bear Creek area in northern Stanton County to north of the Cimarron River in Seward County. The variability of anion type and low sample density in the southwestern quadrant and related areas of Figure 13 testify to the need for additional sampling in this area in order to better understand the water chemistry of groundwaters from the region.
A map of values of the sodium adsorption ratio (SAR) for groundwaters from the study area is presented in Figure 14. SAR values are computed using the equation
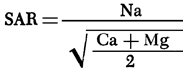
where values in parentheses are milliequivalents per liter of the specified ions. SAR values in excess of 3.0 are prominent in the region of Na-Cl type waters in Meade County. Water from well 35-29W-36ABB (Figure 11) has a SAR value of 8.7 and surface water samples from the Cimarron River and Crooked Creek within this part of Meade County exhibit values on the order of 11 and 18, respectively.
Figure 14--Map of values for sodium adsorption ratio for the study area.
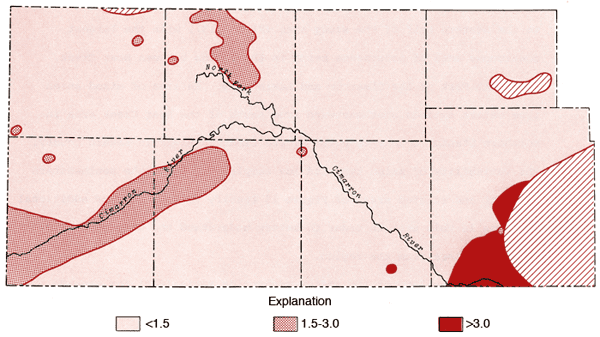
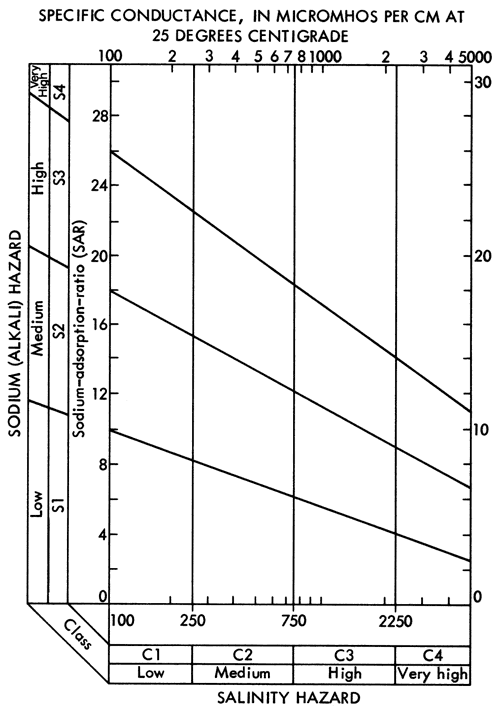
Specific conductance data (Figure 6) and SAR data (Figure 14) can be used with the aid of Figure 15 to evaluate the general suitability of waters for irrigation purposes. Groundwaters in the study area, with the exception of the Na-Cl type zone in Meade County, generally fall into the medium to high salinity hazard ranges and low alkali hazard range. Groundwaters from well 33-29W-36AAB in the Na-Cl type zone in Meade County falls in the high salinity-medium alkali hazard category. Therefore, it is extremely important to monitor the chemical quality of irrigation waters derived from and around the Na-Cl type zone in Meade County. Considering the relatively few data points used here to locate the general boundary of the Na-Cl type waters and the apparent artesian character of Permian mineralized waters in this area, a program to better define the limits of the zone for Na-Cl type waters in the unconsolidated sediments and potential effects upon the chemical quality by the mining of groundwater in this area seems warranted.
Figure 15--Diagram showing the relationship between specific conductance and the sodium adsorption ratio in evaluation of salinity and alkali hazards for irrigation waters.
In conclusion, it should be noted that correlations of water quality with parameters such as surface and bedrock topography, depth to water, and soil type have not been treated in any great detail in the foregoing discussion. For the most part, these relationships in the present study area are generally more subtle than those noted in the two previous studies (Hathaway, et al., 1975, 1977). Computer analysis may aid in the evaluation of these and other aspects of the system. Another largely unknown quantity is the long-term effect of the extensive oil and gas development in the study area upon groundwater quality. Lack of a satisfactory uniform historical data base for groundwater in the area and the general unavailability of data related to chemistries of subsurface brines from the producing horizons make assessment of this last consideration extremely difficult for the southwestern portion of the state.
The services of personnel from the U.S.G.S. office in Garden City, Kansas, in the collection of the water samples; and A. M. Diaz of the U.S.G.S. office in Lawrence, Kansas, in the operation of the field laboratory are gratefully acknowledged. A special note of appreciation is also due Dr. W. Kirk, President, and Mr. W. G. Bolton, Natural Science Division, of Seward County Community College for making laboratory facilities available during the sampling program.
Dickey, H.P., Eeds, A.A. and Hecht, L.A., 1961, Soil Survey of Stevens County, Kansas: U.S. Department of Agriculture, Series 1958, no. 13, 33 p.
Dickey, H.P., Swafford, W.R. and Markley, Q.L., 1963, Soil Survey of Morton County, Kansas: U.S. Department of Agriculture, Series 1960, no. 8, 51 p.
Dickey, H.P., Swafford, W.R. and Markley, Q.L., 1965, Soil Survey of Seward County, Kansas: U.S. Department of Agriculture, Series 1961, no. 28, 51 p.
Fleming, E.L., McBee, C.W., Hamilton, V.L. and Sallee, K.H., 1961, Soil Survey of Stanton County, Kansas: U.S. Department of Agriculture, Series 1958, no. 15, 41 p.
Gutentag, E.D., Lobmeyer, D.H. and Slagle, S.E., 1981, Geohydrology of Southwestern Kansas: Kansas Geological Survey, Irrigation Series 7, 73 p. [available online]
Gutentag, E.D. and Stullken, L.E., 1974, Ground Water in Haskell County, Kansas: U.S. Geological Survey, Hydrologic Investigations Atlas HA-515. [available online]
Hamilton, V.L., Jantz, D.R., Lobmeyer, M.A., Markley, Q.L. and Swafford, W.R., 1968, Soil Survey of Haskell County,, Kansas: U.S. Department of Agriculture, 49 p.
Hamilton, V.L., Markley, Q.L., Swafford, W. R., and Dickey, H.P., 1969, Soil Survey of Grant County, Kansas: U.S. Department of Agriculture, 52 p.
Hathaway, L.R., Carr, B.L., Galle, O.K., Magnuson, M.L., Waugh, T.C. and Dickey, H.P., 1977, Chemical Quality of Irrigation Waters in Hamilton, Kearny, Finney and northern Gray Counties: Kansas Geological Survey, Chemical Quality Series 4, 33 p. [available online]
Hathaway, L.R., Magnuson, M.L., Carr, B.L., Galle, O.K. and Waugh, T.C., 1975, Chemical Quality of Irrigation Waters in West-Central Kansas: Kansas Geological Survey, Chemical Quality Series 2, 46 p. [available online]
McGovern, H.E. and Long, W.A., 1974, Ground Water in Gray County, Kansas: U.S. Geological Survey, Hydrologic Investigations Atlas HA-517. [available online]
McLaughlin, T.G., 1946, Geology and Ground-water Resources of Grant, Haskell and Stevens Counties, Kansas: Kansas Geological Survey Bulletin 61, 221 p. [available online]
Smith, H.T.U., 1940, Geological Studies in Southwestern Kansas: Kansas Geological Survey, Bulletin 34, 212 p. [available online]
Stiff, H.A., 1951, The Interpretation of Chemical Water Analysis by Means of Patterns: Journal of Petroleum Technology, vol. 3, no. 10, p. 15-17.
Tomasu, B.I., 1977, Soil Survey of Meade County, Kansas: U.S. Department of Agriculture, 63 p.
Tomasu, B.I. and Roth, W.E., 1968, Soil Survey of Gray County, Kansas: U.S. Department of Agriculture, 59 p.
U.S. Salinity Laboratory Staff, 1954, Diagnosis and Improvement of Saline and Alkali Soils: U.S. Department of Agriculture Handbook, 60, 160 p.
Kansas Geological Survey
Placed on web Jan. 14, 2011; originally published in January 1978.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS6/index.html