Prev Page--Ground Water || Next Page--Mineral Resources
Chemical Quality of Water
Water samples for this report were obtained from 31 test holes, 35 domestic-supply wells, and 14 stream sites. Complete standard chemical analyses were performed by the Kansas State Department of Health. In addition, chemical-quality data were available from earlier reports, and from records for six public supplies (pl. 2).
Most water samples were collected from test holes and from domestic wells open to the entire thickness penetrated, which may encompass more than one aquifer. At a few locations, samples were obtained from sandstone aquifers isolated from shallow unconsolidated deposits. Samples from isolated Kiowa Formation and Stone Corral Formation aquifers were obtained at three locations north of Lyons (pl. 2).
Arbuckle Group
Of all the deep subsurface formations underlying Rice County, the Arbuckle Group generally contains the least concentrated brine; concentrations of chloride range from 10,000 to 20,000 mg/l (milligrams per liter). Within the Arbuckle Group, brines with the least concentration of mineral constituents generally occur in areas of structural high, which may indicate that the water is of meteoric origin. The water probably entered these rocks during pre-Mississippian and again in Early Pennsylvanian time when the Ellis arch was elevated, and the overlying rocks were eroded and beveled. Since that time, the chemical quality of this water has been degraded owing to intermixing with water in adjacent formations. The natural concentration of the brine probably has been increased significantly in local areas because of the large amount of oil-field brines of higher concentration that is injected into the Arbuckle Group through disposal wells.
Stone Corral Formation
Five water samples were obtained from the Stone Corral Formation; three were obtained while the formation was isolated from other aquifers by inflatable packers. Analysis of the samples indicates that the water is of poor quality. Four of the water samples taken downdip from the outcrop ranged from 4,980 to 6,430 mg/l in dissolved-solids concentration (pl. 2). The fifth sample, taken from well 19-6W-36cbc near the outcrop area, had a dissolved-solids concentration of 3,870 mg/l. The water from this well is known to have had a relatively low dissolved-solids concentration at one time, but pollution from oil-field brine has caused the quality to deteriorate to its present level.
Water from the Stone Corral Formation in Rice County is of the sodium chloride type; however, concentration of sulfate also is very high. The high sulfate content may be explained by the solution of gypsum, which is abundant in the formation. The high chloride concentration west of the outcrop area may have resulted from slow leaching of salt from deposits within the Stone Corral.
Kiowa and Dakota Formations
Waters from the Kiowa and Dakota Formations commonly are very hard and of the calcium bicarbonate type; they are very similar in quality to water in the unconsolidated deposits. The similarity of chemical type may indicate that the waters have comparative freedom of movement both laterally and vertically between the three aquifers. Where the Kiowa and Dakota aquifers are separated from the unconsolidated aquifer by an appreciable thickness of shale, the water may be of the sodium bicarbonate type. The calcium ion probably has been exchanged for the sodium ion through a natural softening process. The concentration of dissolved solids in waters from these formations generally ranges from 290 to 690 mg/l. In a few wells, however, the water has been contaminated by industrial brines, resulting in concentrations as high as 54,940 mg/l.
An indication of local hydraulic connection between the Kiowa and Stone Corral Formations was found during hydraulic tests of well 19-8W-27abb. An inflatable packer was used to isolate the aquifers during these tests. Pattern diagrams (fig. 11) showing quality of samples of water from the Kiowa and Stone Corral aquifers indicate the similarity of the waters. The water sample collected from the Kiowa Formation (fig. 11 B) was of the sodium chloride type, which is typical of water from the Stone Corral Formation (fig. 11 A). However, the proportion of the bicarbonate ion to other ions is higher in the Kiowa water than in the Stone Corral water, and the total concentration of mineral constituents in the Kiowa water is lower.
Figure 11--Mixing of water in a well.
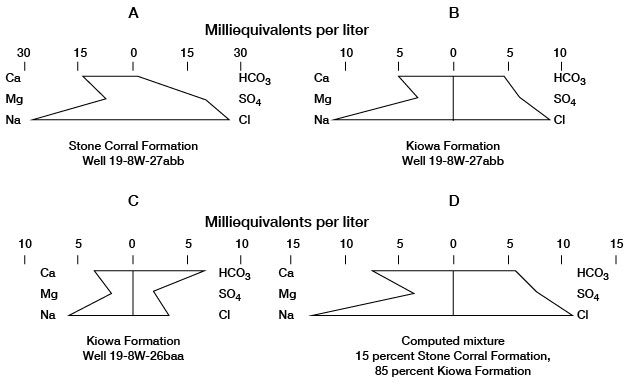
The diagram for the analysis of water from well 19-8W-26baa (fig. 11 C) is typical for sodium bicarbonate water from the Kiowa Formation. The diagram of a computed mix (fig. 11 D) of 85 percent Kiowa water from this well and 15 percent Stone Corral water from well 19-8W-27abb is almost identical in shape and concentration with the diagram of Kiowa water from well 19-8W-27abb (fig. 11 B), which indicates probable mixing of waters from the two formations at this well. The potentiometric head in the Stone Corral aquifer is above the base of the Kiowa aquifer in well 19-8W-27abb, although it is below the potentiometric head in the Kiowa aquifer. If the head in the Kiowa aquifer is lowered sufficiently while the well is pumped and the Harper Sandstone separating the two aquifers is fractured or permeable, water from the Stone Corral could migrate into the Kiowa. The authors believe that such a condition existed at well 19-8W-27abb and that there was mixing of water from the two aquifers.
Pleistocene Deposits
Water collected from shallow unconsolidated deposits commonly is very hard and of the calcium bicarbonate type (pattern diagrams, pl. 2). A few samples were a softer water of the sodium bicarbonate type. Water of the sodium chloride type is present locally as a result of pollution from oil-field brine and solution of salt from surface operations of salt mines. Analyses of water from these deposits generally range from 200 to 480 mg/l dissolved solids. In a few wells, however, concentrations were much higher; the greatest known concentration of dissolved solids is 5,430 mg/l. Water with the least dissolved-solids concentration generally occurs in the dune sand and alluvium in the southern part of the county.
Streams
The major streams in the county were sampled at low-flow stage and all differ somewhat in the chemical quality of their water. Although the dissolved-solids concentration varies with stream discharge, the chemical type of the water for each stream seems to be consistent over a period of time (pl. 2). Analyses of water from Plum Creek and Little Arkansas River indicate a calcium bicarbonate type water of relatively low dissolved-solids concentration. Water in Cow Creek is of the sodium chloride type and appears to decrease in dissolved-solids concentration downstream. Locally, however, chloride concentration increases considerably as a result of industrial waste entering the stream. The Arkansas River upstream from Rattlesnake Creek contains water of the sodium sulfate type. Because water in Rattlesnake Creek is extremely high in sodium chloride concentration, water in the Arkansas River below the mouth of Rattlesnake Creek becomes a sodium chloride type water that also contains high concentrations of sulfate and dissolved solids. The quality of water of all the streams except Cow Creek has resulted largely from naturally occurring conditions. The quality of water in Cow Creek is to a large extent determined by industrial waste.
In Rice County ground water moves from the aquifers toward the streams. Generally the ground water, which is of better quality than the water in the streams during low-flow periods, tends to dilute the mineral concentration of the surface water. However, in areas where there has been industrial pollution, water of poorer quality may enter the stream from the aquifer.
Prev Page--Ground Water || Next Page--Mineral Resources
Kansas Geological Survey, Geology
Placed on web Aug. 19, 2008; originally published April 1974.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Rice/05_qual.html