Prev Page--Distribution and Use of Water || Next Page--Surface Water
Ground Water
More than 100 observation wells with 1 1/4- or 2-inch (3.2- to 5.1-cm) diameter casings were installed in and adjacent to the irrigation district by the U.S. Bureau of Reclamation, the Kansas Department of Health and Environment, the Kansas Geological Survey, and the U.S. Geological Survey from 1947 to 1971. Water levels were measured and water samples for chemical analysis were collected periodically at selected wells.
Pertinent data for each well, depths to water below land surface datum, and results of chemical analyses of water samples collected from 1964 to 1971 are included in a separate basic data report by Leonard and Stoltenberg (1972). Only the most significant information is portrayed herein.
Areal Changes in Water Levels
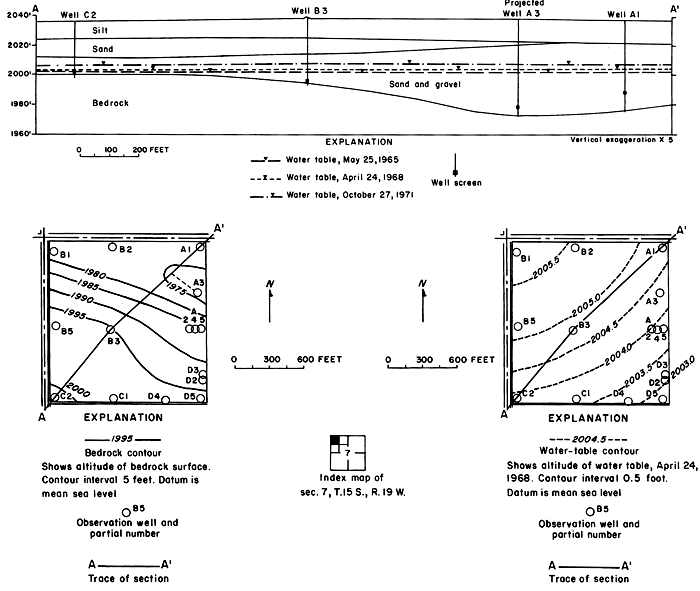
As shown in plate 1, the water table generally sloped gently toward the south and east and was interrupted by erosional remnants of relatively impermeable bedrock. Maximum and minimum recorded depths to water between 1964 to 1971 show that the water level in most of the wells in and adjacent to the irrigated land fluctuated within a range of from 4 to 10 feet (1.2 to 3.0 m), but generally remained more than 20 feet (6.1 m) below the land surface under the high terrace. The lowest levels generally were recorded near the beginning of irrigation in 1964-65; the highest in 1969 or 1970. Some areas of seepage developed in topographically low areas along natural drains, and where the bedrock was near the surface between the irrigated acreage and the river. However, the water table remained well below the root zone under most of the irrigated land.
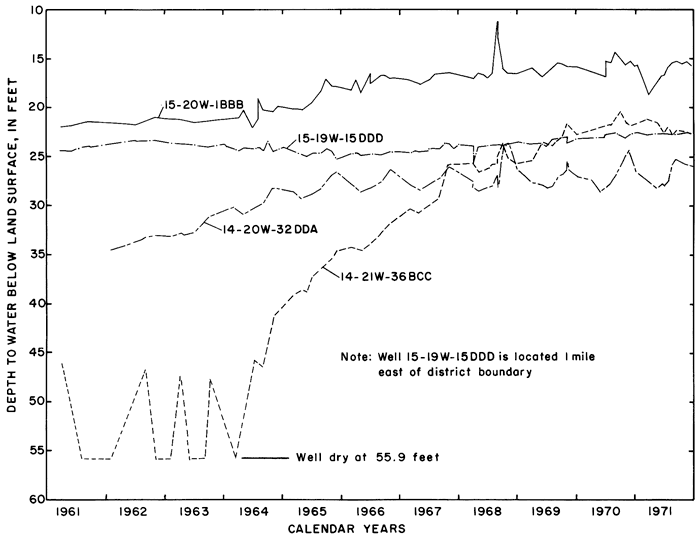
Hydrographs of selected observation wells in and adjacent to the district (fig. 5) illustrate the progressive rise of the water table in or down the groundwater gradient from the irrigated area in response to irrigation. Water levels generally were highest in the late summer and early fall as excess rainfall and irrigation water accumulated in the aquifers. As a result of drainage from the aquifer and the paucity of recharge during the winter, water levels normally declined to a minimum in early spring.
Figure 5--Fluctuations of water levels in selected observation wells, 1961-71.
Areal Changes in Chemical Quality
Although most of the wells were screened in lithologically similar sediments, the chemical quality of water differed widely from well to well and changed with time. The concentrations of the ions in successive samples from many of the wells remained constant only after about 1 gallon (3.8 l) for each 10 feet (3.0 m) of standing water was withdrawn and discarded. After 1967, a sampler designed specifically for use in the 1 1/4- or 2-inch (3.2- or 5.1-cm) diameter casings was used instead of conventional bailers. "Mean" or "typical" analyses were inadequate to describe the chemical quality or ground water in the area.
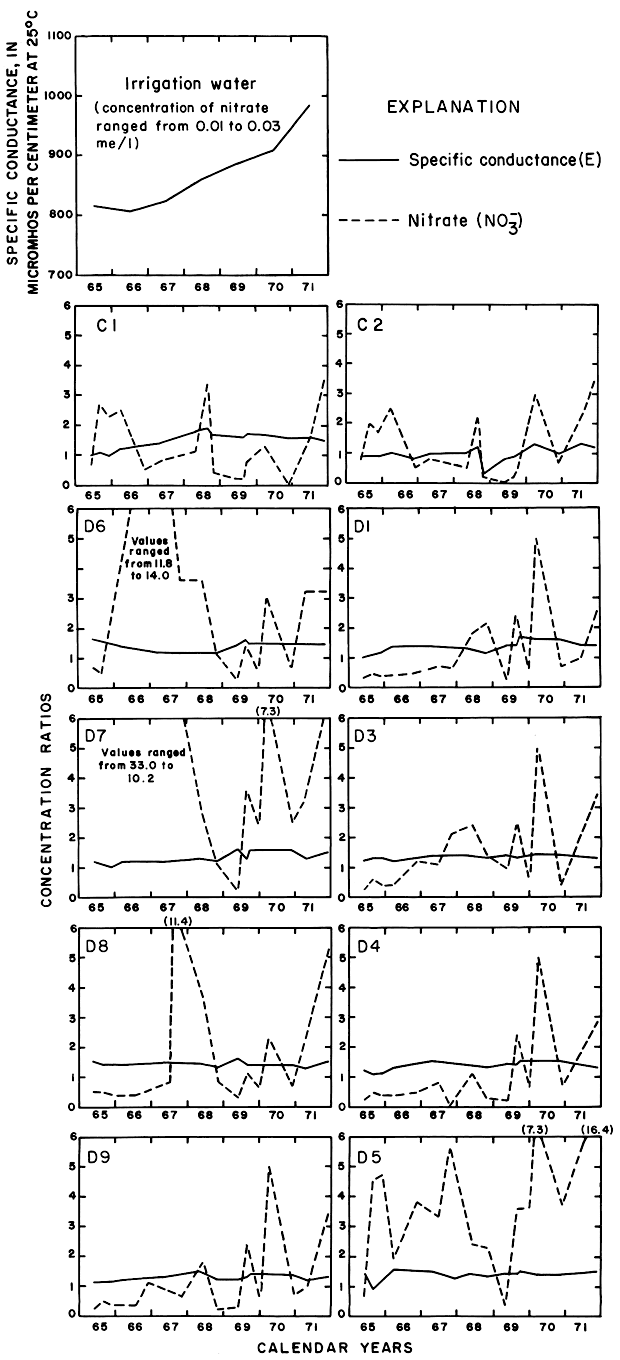
The rising water table in and adjacent to the irrigated land reflected infiltration of excess water. If all of the thousands of tons of dissolved solids delivered to the district in net input (N) were dissolved in the excess water remaining after evapotranspiration (Xn) (table 2), the concentration of a given ion in that water theoretically would be equal to N/Xn times the concentration of the ion in the irrigation water. For example, the concentration ratios (CR) shown in table 2 range from about 1.1 to 1.9. If Ei represents the specific conductance of the irrigation water, the corresponding values of the specific conductance of the excess water (Xn) would range from 905 micromhos per centimeter at 25°C in 1965 to 1848 micromhos per centimeter at 25°C in 1971. The estimated specific conductance (EiI/Xi) of soil water (Xi) in the irrigated fields would be higher.
If the specific conductance of the excess water were determined by mixing the excess water for each year with the excess from preceding years, the specific conductance of the cumulative mixture (Ex) would range from about 966 micromhos per centimeter at 25°C in 1965 to 1,220 micromhos per centimeter at 25°C in 1971. The latter alternative more closely approximates the natural situation and the results of analyses of ground water in the area.
Mixing with natural ground water, selective precipitation of the least soluble salts on the surface and in the soil profile, ion-exchange reactions, and leaching of residual salts from the soil profile can alter the ionic composition of the ground water from the predicted values. To relate variations in the composition of sampled waters to the irrigation water in parts of this report, the specific conductance and concentrations of the ions are expressed as "concentration ratios" to the specific conductance and concentrations of the corresponding ions in the irrigation water. Abnormally high or low ratios for individual ions reveal that processes other than dilution and evapotranspiration affected the composition of the water.
The locally variable chemical composition of natural waters in the area was caused chiefly by soluble minerals in the soil and rock. Calcium, derived chiefly from limestone, caliche, and gypsum is normally the predominant cation. Bicarbonate from limestone and sulfate from gypsum or the oxidation of pyrite in shale are predominant anions. Most of the sodium and chloride seems to have been derived from the soil, however, locally high concentrations of both ions are associated with oil-field or livestock operations. Water in the Dakota Formation, which underlies the area at depth, contains high concentrations of sodium and chloride ions. Although the water generally is confined by overlying shale, there are unconfirmed reports that a few abandoned wells in the Dakota flowed at the surface near the river. The plant nutrients, potassium, nitrogen, and phosphorus, are constituents of the rocks, particularly organic shale, as well as of fertilizers that are used extensively in the irrigated area.
Between 1965 and 1971, the specific conductance in most of the well waters increased and the water type changed as shown in plate 3. In 1965, calcium-bicarbonate type water underlaid most of the eastern part of the area. Sodium-sulfate and sodium-bicarbonate types generally were associated with buried channels in the bedrock (pl. 2). By 1971, nearly all of the well waters were of the calcium-sulfate type, evidently as a result of infiltration of calcium-sulfate type irrigation water. Most of the changes in concentrations were progressive, but the rates of change were most pronounced during 1967-69.
The increases in specific conductance (proportional to the concentration of dissolved solids) in many of the well waters were larger than would be predicted from the estimated consumptive use of irrigation water, and the concentration ratios for the ions varied widely. Evidently, residual salts that had accumulated in the soils and aquifers under semiarid conditions before irrigation began bad been leached to the ground-water reservoir. However, the changes in the distribution of the ions from well to well and in the configuration of the water table as irrigation continued indicate that lateral movement of ground water had significantly affected the chemistry of the well waters. Lack of uniformity in the chemistry of the ground water is related to lack of homogeneity of the subsurface hydrogeologic and chemical environment.
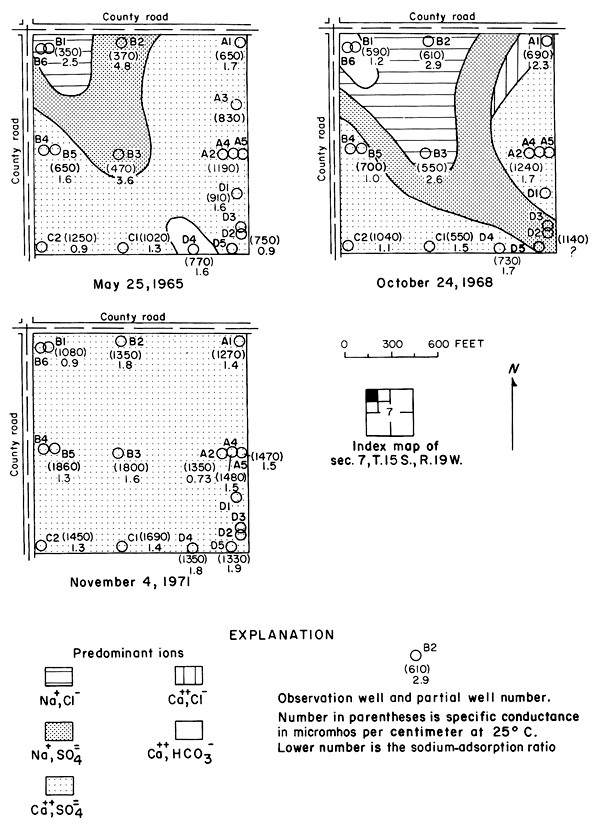
The specific conductance of water is a measure of the total concentration of all of the ionic constituents, however, each of the constituents can behave differently in the hydrologic environment. In the Cedar Bluff area, changes in the distribution and concentration of the chloride ion are more readily related to changes in the hydrologic system than are the changes in the concentrations of the other ions or in the specific conductance.
Under the conditions prevailing in the study area the chloride ion is virtually unaffected by precipitation or ion exchange; therefore, it is a relatively effective tracer. Chloride comprised only about 7 percent of the anions in the irrigation water at a maximum concentration of about 26 mg/l (table 3). The concentrations of chloride in many well waters and in groundwater discharge from the district to tributaries, as shown in figure 6, far exceeded the concentration of chloride in the irrigation water and the concentrations that were predicted from consumptive use.
Figure 6--Map showing concentration of chloride in water from wells and tributaries, Fall 1971, and changes in the concentration of chloride from Fall 1965 to Fall 1971. [A larger version of this figure is available.]
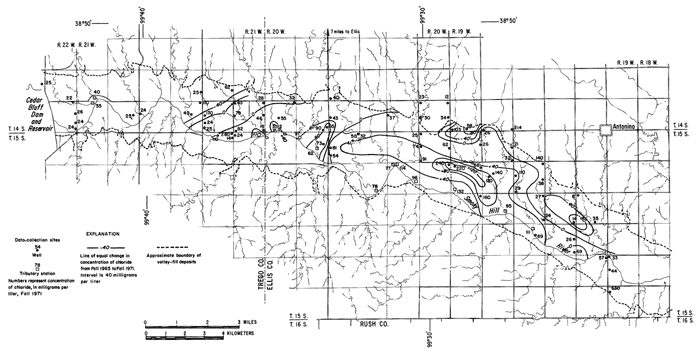
In general, the concentration of chloride increased under the irrigated land except where high concentrations prevailed in 1965. In the western part of the area, locally high concentrations are believed to have been associated with pollution of the shallow aquifers by oil-field brine. For example, a decrease of more than 200 mg/l in the domestic well serving the Cedar Bluff Irrigation District office (15-20W-5AD) in the central part of the area is attributed to dilution and displacement to the south of waters containing brine from improper oil-field operations in the past. Similar changes were noted elsewhere.
The wide distribution of chloride in transient storage suggests a more extensive source than the limited disposal of oil-field brine in the area. The greatest increase in concentration over an appreciable area was on, and north of, an elongated erosional remnant of bedrock that separates the water table under the high terrace from the water table in the alluvium along the river (pl. 2). In part of the area, high concentrations of chloride appear to have been caused by evapotranspiration of water from a shallow water table over the bedrock. The barrier is breached by tributaries that are sustained mainly by chloride-rich drainage from the irrigated land. The nature and configuration of the changes shown in plate 3 and in figure 6 seem to indicate that infiltrated water in excess of crop requirements leached chloride and other accumulated salts from the soil, then mixed with and partially displaced natural water down gradient. More detailed data from three experimental plots support this hypothesis.
Experimental Plots
In 1964, nine or more observation wells with 2-inch (5.1-cm) galvanized steel casing were installed in each of three experimental plots (pl. 1) that are underlain by differing types and thicknesses of unconsolidated sediment. Brass well-point screens in most of the wells are set near the undulating base of the saturated section at depths ranging from 20 to 70 feet (6 to 21 m). Additional wells were installed in 1967 and 1971 to test for vertical stratification of the ions in the groundwater reservoir. To minimize interference with farming operations the wells were located along the edges of the fields and on berms separating graded benches. Despite the inconvenience, the owners, Mr. Vernon Moore (plot 1) and Mr. Philip Nicholson (plots 2 and 3) and their renters graciously made their fields available and contributed valuable assistance and information.
Plot 1 includes about 10 acres (4.0 ha) of a larger field in the northeastern part of the district, south of and adjacent to the irrigation canal near the upper boundary of the high terrace. Plot 3, about 40 acres 16.2 ha) in area, is about 1 1/2 miles (2.4 km) to the south, down the regional ground-water gradient. Both are located near and east of geologic section C-C' in plate 2. Both were primarily cornfields during the study, although a minor amount of grain sorghum was planted in each during some years. Irrigation water was transferred by siphon to the individual furrows from lateral ditches along the western boundaries. Plot 2 is located near the western edge of the district on a low alluvial terrace near the river. Alfalfa was grown in plot 2 until 1970; grain sorghum was planted in 1971.
Inflow to the fields consists mainly of applied irrigation water and direct precipitation on the land surface, but some ground-water inflow probably migrated laterally from adjacent land. Rainfall was recorded daily for varying periods at stations near the fields. The distribution of water during 1966-71 is summarized in table 4. Annual applications of irrigation water exceeded the irrigation requirement for all three fields.
Table 4--Distribution of rainfall and applied water on experimental plots, 1966-71.
| Water | Plot | 1965 | 1966 | 1967 | 1968 | 1969 | 1970 | 1971 |
|---|---|---|---|---|---|---|---|---|
| Annual rainfall (Pa), in inches1 | 1,3 | 23.8 | 19.4 | 24.3 | 18.6 | 29.3 | 20.6 | 24.6 |
| 2 | 23.82 | 14.4 | 20.8 | 14.8 | 20.0 | 15 .92 | 23 .02 | |
| Effective rainfall (Pe), in inches3 | 1,3 | 16.1 | 15.6 | 21.3 | 14.5 | 20.8 | 11.1 | 14.0 |
| 2 | 18.0 | 11.4 | 17.6 | 12.1 | 16.4 | 12.8 | 11.5 | |
| Consumptive use (C), in inches: corn |
1,3 | 22.1 | 25.3 | 23.4 | 26.3 | 24.4 | 25.2 | 26.7 |
| Consumptive use (C), in inches: alfalfa (1965-70), sorghum (1971) |
2 | 28.0 | 29.1 | 28.5 | 33.1 | 31.9 | 32.3 | 22.8 |
| Irrigation requirement, in inches (R = C - Pe) |
1,3 | 6.0 | 9.7 | 2.1 | 11.8 | 3.6 | 14.1 | 12.7 |
| 2 | 10.0 | 17.7 | 10.9 | 21.0 | 15.5 | 19.5 | 11.3 | |
| Farm deliveries (I), in inches4 | 1 | 18.8 | 13.8 | 4.3 | 21.6 | 8.6 | 16.1 | 14.0 |
| 3 | 24.1 | 16.0 | 12.5 | 17.8 | 13.8 | 19.5 | 15.8 | |
| 2 | 17.4 | 28.7 | 24.5 | 38.5 | 20.6 | 28.0 | 28.0 | |
| Excess farm deliveries (Xi = I - R), in inches |
1 | 12.8 | 4.1 | 2.2 | 9.8 | 5.0 | 2.9 | 1.3 |
| 3 | 18.1 | 6.3 | 10.4 | 6.0 | 10.2 | 5.4 | 3.1 | |
| 2 | 7.4 | 19.0 | 13.6 | 17.5 | 5.1 | 8.5 | 16.7 | |
| Seasonal concentration ratio (CRi -- I/Xi) |
1 | 1.5 | 3.4 | 1.9 | 2.2 | 1.7 | 8.1 | 10.8 |
| 3 | 1.3 | 2.6 | 1.2 | 3.0 | 1.4 | 3.6 | 5.1 | |
| 2 | 2.4 | 1.5 | 1.8 | 2.2 | 4.1 | 3.3 | 5.5 | |
| Annual concentration ratio [CRa -- 1/(Pa + I - C)] |
1 | 0.9 | 1.8 | 0.8 | 1.6 | 0.6 | 1.4 | 1.2 |
| 3 | .9 | 1.6 | .9 | 1.8 | .7 | 1.3 | 1.2 | |
| 2 | 1.3 | 2.0 | 1.5 | 1.9 | 2.4 | 2.4 | 1.0 | |
| 1 Inches X 2.54 equal centimeters. 2 Based on rainfall at Cedar Bluff Dam. 3 Based on rainfall during growing season for each crop in experimental plots. 4 Written communications, R. Schamel, Manager, Cedar Bluff Irrigation District. |
||||||||
Samples were collected for chemical analysis more frequently in the experimental plots than in the remainder of the study area. Soil samples from the fields and from adjacent nonirrigated areas were analyzed to determine if the natural salt content of the soil in the irrigated areas has been sufficiently depleted by leaching to account for the observed changes in the irrigation water. Plots 1 and 3 on the high terrace typify most of the irrigated land in the district; therefore far more data were collected from them than from plot 2.
PLOT 1
Unconsolidated fluvial deposits and colluvium overlain by loess increase in thickness from about 10 feet (3 m) at the northwestern corner of the plot to about 80 feet (24 m) near the southeastern corner (fig. 7). Drillers' and gamma-ray logs, as well as particle size analyses of cores and auger cuttings reveal wide vertical and lateral variation in lithology. Silt and clay predominate, but the proportion of sand and gravel in most of the wells increases with depth.
Figure 7--Configuration of bedrock surface and water table in plot 1.
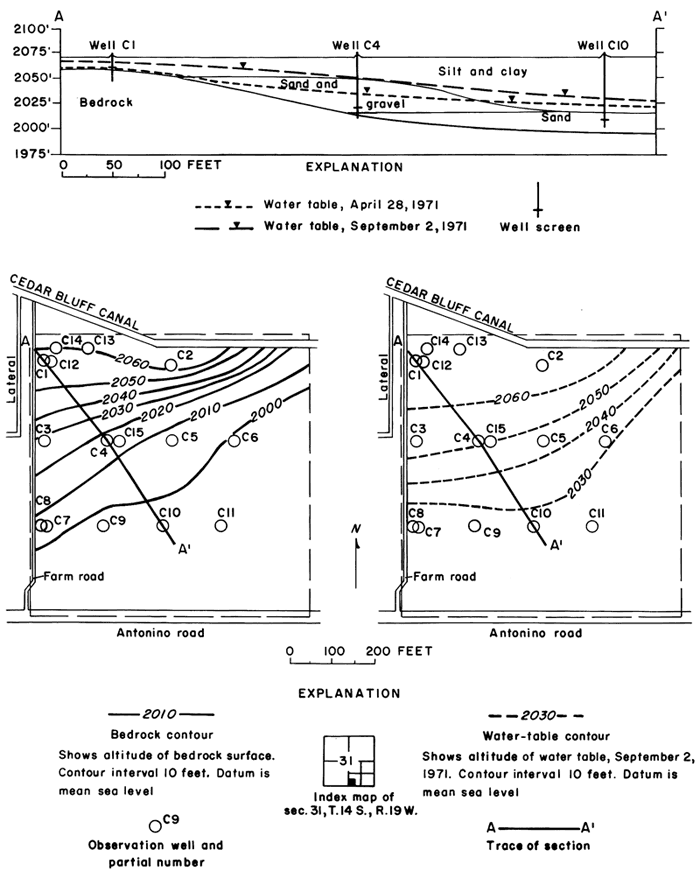
The sampled water entered the observation wells through brass screens set near the bedrock surface in discontinuous lenses of permeable sand and gravel that seem to be the main conduits for lateral migration of ground water. Many of the water samples were obtained by personnel of the Entomology Department of Kansas State University in conjunction with a study of pesticides in the soil (Knutsen and others, 1971). Results of that study show that the movement of chlorinated hydrocarbons was confined to the upper foot of soil.
Inflow to the field consisted mainly of applied irrigation water and direct precipitation on the land surface. Overland runoff from highland areas to the north is intercepted by the main irrigation canal that forms the northern boundary of the field. Seepage from the canal and from a lateral west of the field seemed to have a negligible effect on the configuration of the water table, which rose rapidly in response to infiltration of excess water applied during the irrigation season (fig. 8).
Figure 8--Fluctuations of water levels in observation wells in plot 1, May 1965 to November 1971.
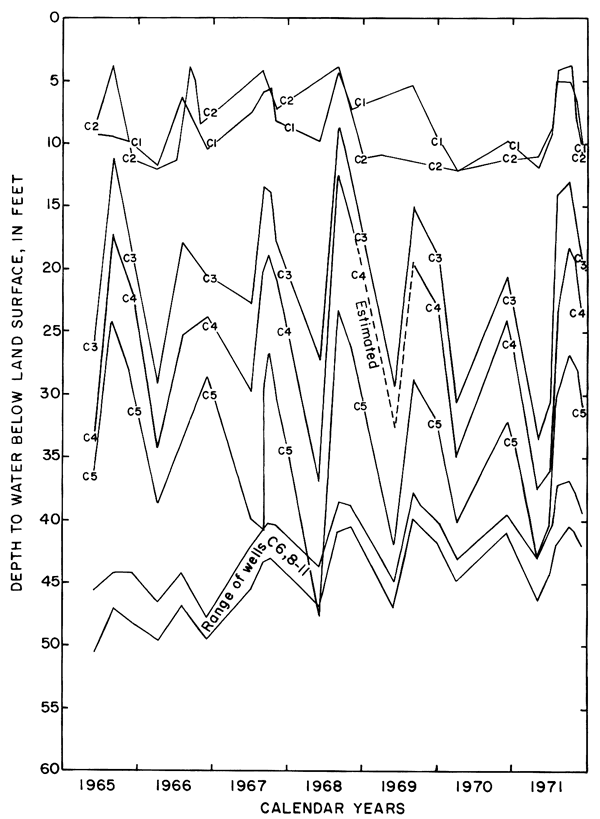
Mainly as a result of the inhomogeneity of the aquifer and the wide variation in depth of well screens, changes in water levels in the wells were neither simultaneous nor of equal magnitude. The southeasterly slope of the water table was maintained during the irrigation season, but water levels fluctuated over a wider range in the central wells than elsewhere. Excess water from irrigation evidently infiltrated the sand and gravel penetrated by wells C3, C4, and C5 more rapidly than the less permeable fine-grained parts of the aquifer penetrated by the other wells. The fluctuations in wells C6, and C8 to C11 show a more subdued seasonal response and a general rise as water moved down gradient under the extensive irrigated area to the south.
Although the wells in plot 1 were only about 200 feet (61 m) apart, the chemical quality of waters differed from well to well. The variations and fluctuations with time, as shown in figure 9, indicate that calcium-sulfate type irrigation water mixed with and displaced other types of ground water to the south and east down gradient. In August 1968, 70 cubic centimeters of 40 percent Rhodamine BA dye were injected to a depth of 18 inches (46 cm) in the soil 10 feet (3.0 m) north of well C1. The first appearance of dye in wells C3, C4, and C5 one year later, and in wells C10 and C11 four months after that confirmed the direction of movement of the infiltrated water.
Figure 9--Specific conductance, sodium-adsorption ratio, and predominant ions in water from wells in plot 1.
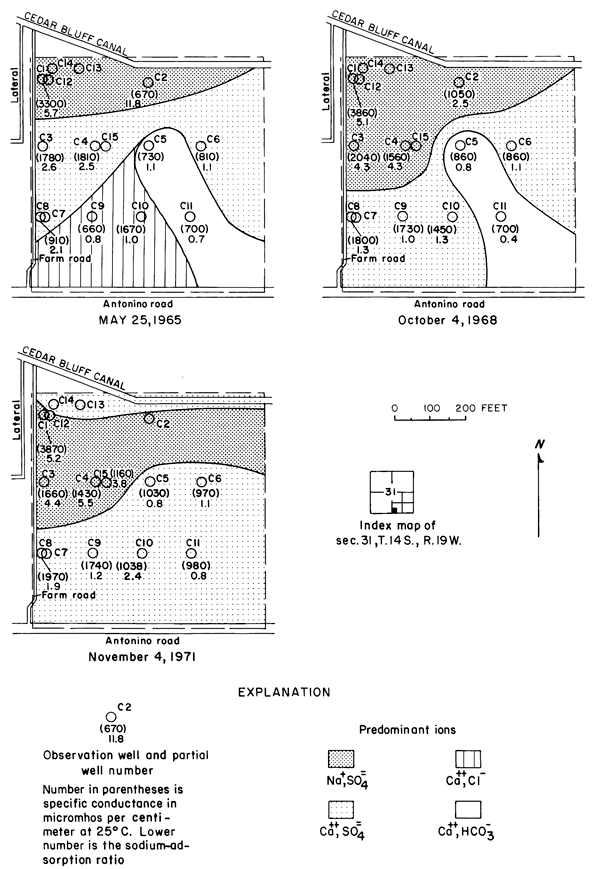
In May 1965, sodium was the predominant cation in waters from the relatively shallow wells C1 and C2 in the northwestern part of the field. Sodium evidently had accumulated in the fine-grained sediments penetrated by the two wells where the water table lay near the surface before irrigation began. Salts of sodium are relatively soluble but sodium ions are readily adsorbed by some clay minerals. By 1968, significant quantities of sodium had been leached and displaced by infiltrated water into the permeable sand and gravel penetrated by wells C3 and C4. The persistence of sodium in wells C1 to C4 in 1971 suggests that abundant calcium in the percolating water continued to displace sodium from the fine-grained sediments by ion exchange.
Progressive changes in the specific conductance and concentrations of the major ions in the well waters are shown in terms of concentration ratios in figures 10, 11, and 12. The graphs representing each well are located in the illustrations in the same relative positions as are the wells in the experimental plots. Theoretically the concentration ratios should approach the ratios used for predicting the specific conductance of soil water and recharge (tables 3 and 4) after accumulated residual salts are flushed from the soil by excess water from irrigation. Figure 12 shows that the ratios for the specific conductance of most of the well waters remained relatively constant or decreased to values between one and two after an initial rise during the first 2 years of irrigation. The ratios for most of the ions seem to be approaching that range.
Figure 10--Concentration ratios for cations in water from wells in plot 1, 1965-71.
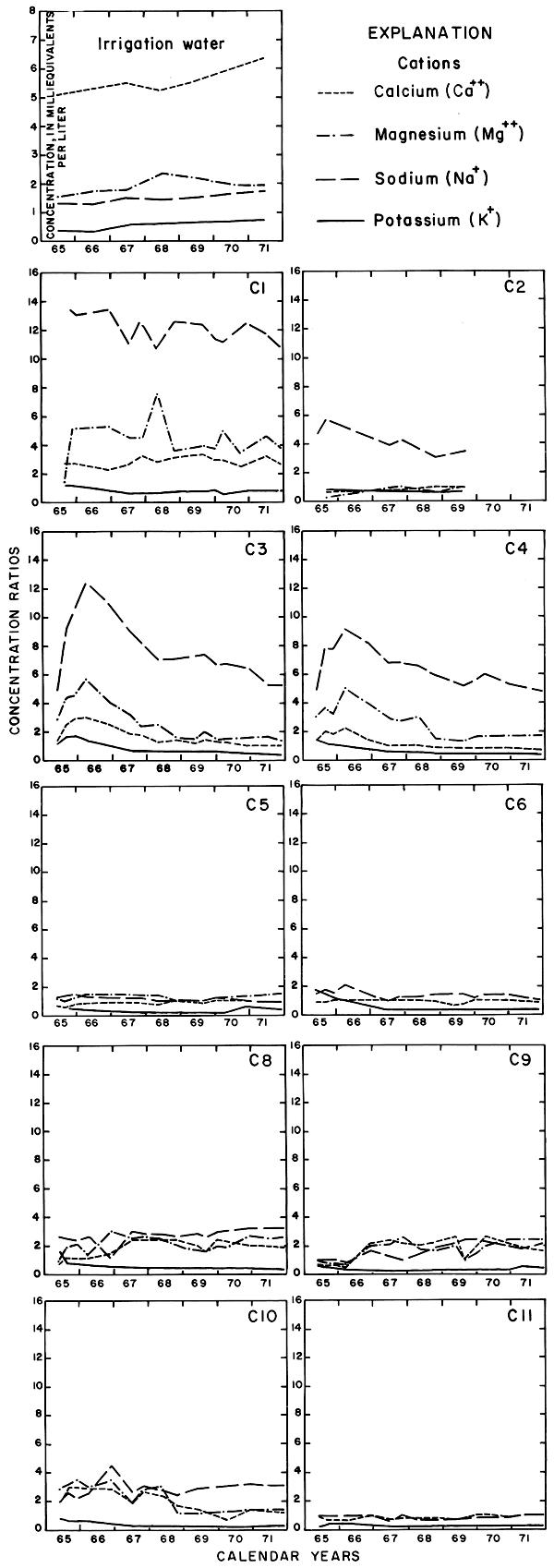
Figure 11--Concentration ratios for selected anions in water from wells in plot 1, 1965-71.
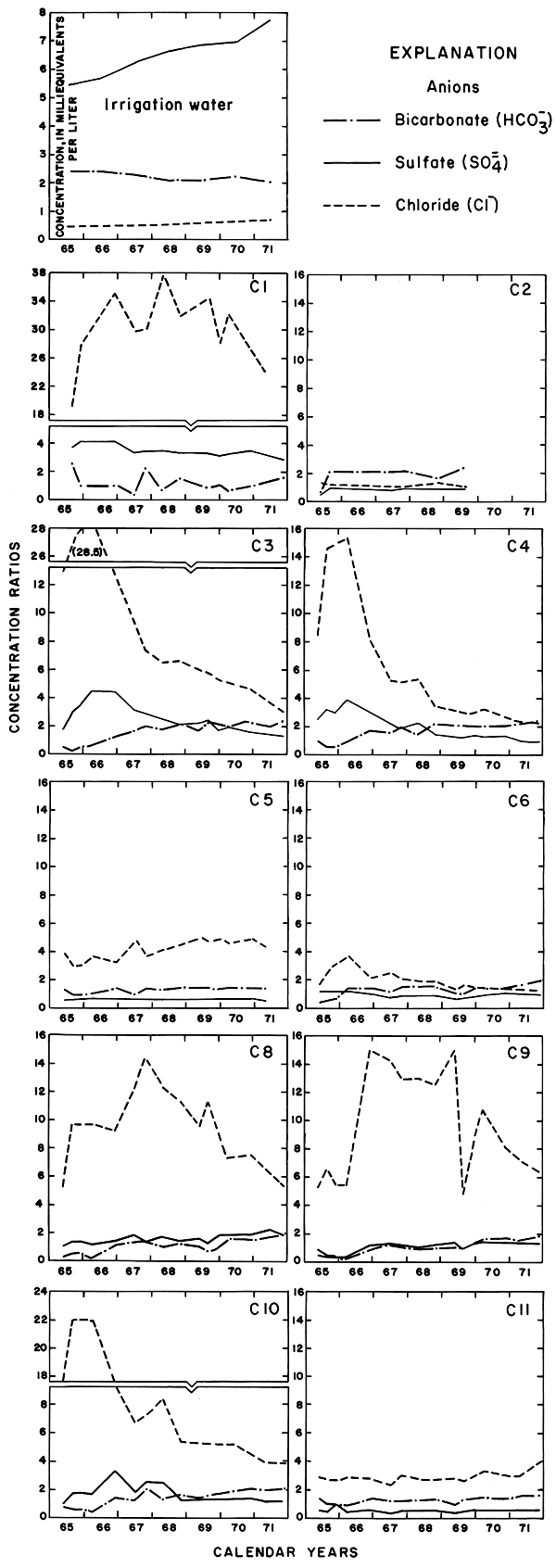
Figure 12--Concentration ratios for the specific conductance and nitrate in water from wells in plot 1, 1965-71.
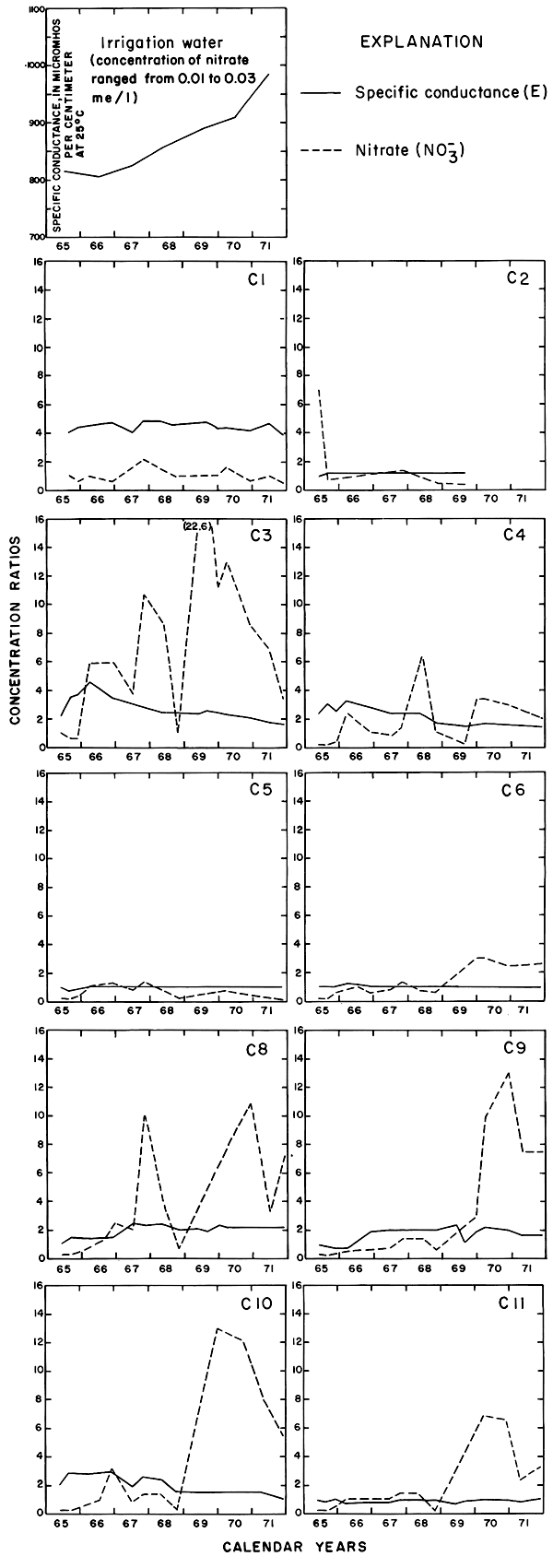
For most wells, the configurations of the curves for calcium, sulfate, and specific conductance were somewhat similar. Convergence of the curves indicates infiltration of excess water from irrigation, but the concentration ratios for calcium and sulfate were generally equal to or less than the ratios for the other constituents, which were evidently derived from another source. In 1971, for example, the ratio for sulfate in most of the wells was equal to or less than the ratio for specific conductance, but the ratio for bicarbonate increased to two or more. In part, the changes reflect the inverse effect on the ratios of the progressive increase in sulfate at the expense of bicarbonate in the irrigation water. However, the changes are mainly dependent on the availability to percolating water of bicarbonate from caliche and fragments of limestone in the soil and aquifers.
Sodium and chloride were present in water from most wells at higher concentrations than in the irrigation water before irrigation began, but the ratios increased rapidly during the first two or three years of irrigation when the salts leached from the soil reached the aquifer. In 1966, the concentration ratios exceeded 10 for sodium and 30 for chloride. As the supply of salt that accumulated before irrigation began was depleted, progressive dilution in some waters accompanied displacement of the mixture down the groundwater gradient. Greater fluctuations in the concentrations of chloride than of sodium in water from the deeper wells probably represent greater mobility of the anion in the aquifer.
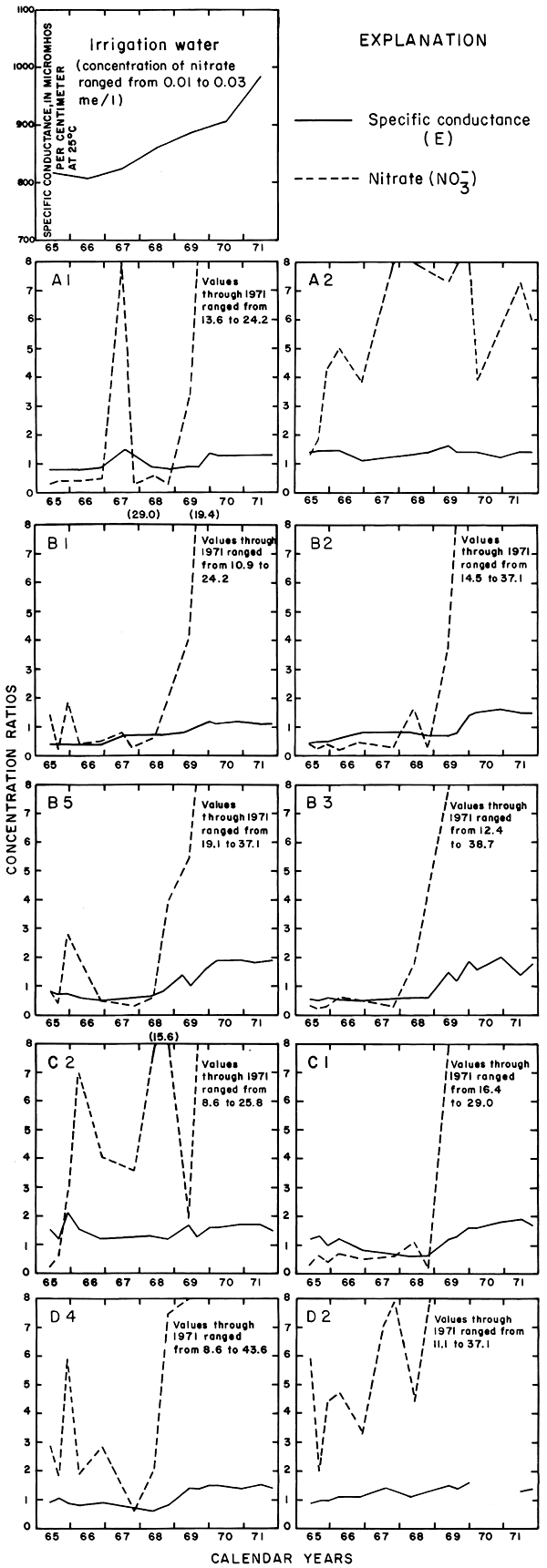
The curves for nitrate and potassium, both plant nutrients and components of fertilizer, differ markedly from the other curves. Small variations in the concentrations of nitrate in the well waters caused wide variations in the ratios because the concentration of nitrate in the irrigation water was less than 2 mg/l. The general increase in the concentration of nitrate in 1969 probably reflects leaching of fertilizer nitrogen carried below the root zone by irrigation water during the preceding dry year. The concentration of potassium in the well waters remained uniformly lower than the concentration of about 18 mg/l in the irrigation water.
According to calculations based on changes in the saturated thickness and in concentrations of chloride in the wells, the average concentration of chloride in the ground water underlying the field decreased and the outflow of chloride exceeded the inflow. The chloride was evidently displaced laterally toward plot 3 and the river.
PLOT 3
The subsurface geology of plot 3 is less complex than that of plot 1 (fig. 13) and the effects of irrigation alone are more readily discernible. The screened wells range in depth from about 14 to 60 feet (4 to 18 m) and the saturated zone lies entirely within relatively homogeneous sand and gravel. Although rainfall and crop use for the two plots were about the same, far more water was applied per acre to plot 3 than to plot 1 except during the dry 1968 season (table 4).
Figure 13--Configuration of bedrock surface and water table in plot 3.
Since 1965, the water table beneath plot 3 has risen about 5 feet (2 m) (fig. 14); the minimum depth to water was 29 feet (9 m) in well A1, which was measured in September 1971. Most of the water-table rise occurred during the first 3 years of irrigation. The configuration of the water table, which normally slopes gently toward the southeast, changed in delayed response to the application of irrigation water. According to continuous records for 1971 from well B3 in the center of the field, the water level declined to about 32 feet (10 m) below land surface by June 5, 1971. Irrigation began on June 9, but the water level remained constant until June 28 when it began to rise. Irrigation of the field was discontinued on August 25, but the water level continued to rise until September 14 to 30.4 feet (9.3 m) after which it again began to decline. Perhaps, coincidentally, the start and finish of irrigation for that year preceded the response at the water table by 19 days.
Figure 14--Fluctuations of water levels in observation wells in plot 3, 1965-71.
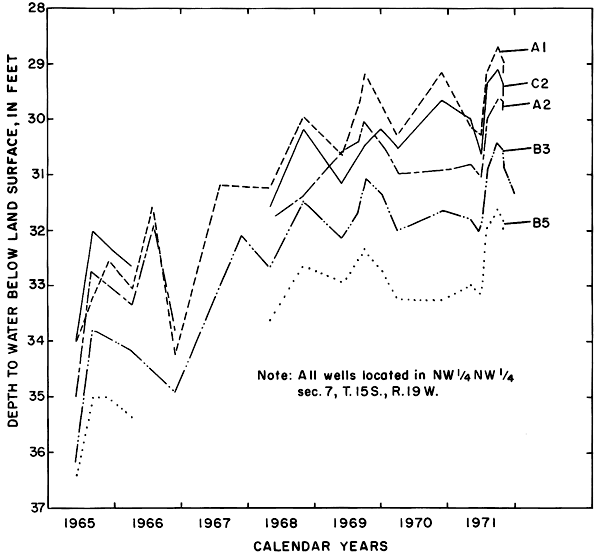
Figure 15--Specific conductance, sodium-adsorption ratio, and predominant ions in water from wells in plot 3.
The progressive changes in water type, increases in specific conductance, and reduction of the sodium-adsorption ratios in the well water (fig. 15) reflect more uniform vertical and lateral movement of larger volumes of water through more homogeneous and permeable aquifers than in plot 1. Analyses of samples from closely spaced wells with screens set at different levels imply no significant vertical stratification of the major ions in the saturated zone. The apparent southeastward migration of the water and the trend toward conformity in the composition of the ground water with the calcium-sulfate type irrigation water indicate percolation of excess water to the aquifer and displacement of the altered ground water down gradient.
In virtually all the wells, calcium, expressed as percent of total cations, increased at the expense of sodium, while magnesium remained relatively constant at percentages lower than the irrigation water. Sulfate and chloride, expressed as percent of total anions, varied inversely, with the percent sulfate always less than in the irrigation water and the percent chloride greater. By 1969, all of the waters were of the calcium-sulfate type.
Following the moisture deficiency in 1968, when the predicted annual concentration ratio for specific conductance was 1.8 (table 4), the ratio for specific conductance of most of the well waters remained relatively constant between 1.5 and 2.0. Unlike the ratios in plot 1 (fig. 7), however, the ratios for the ions in plot 3 do not appear to converge toward a common value (figs. 16, 17, and 18). Instead, each of the ratios for the cations remained relatively constant, while the ratios for some of the anions varied more widely. Evidently, the salts in the well waters were not derived solely from salts accumulated in the soil or carried into the plot by the applied irrigation water. The plot is located over the south bank of an extensive buried channel in the Cretaceous bedrock and is down gradient from other irrigated areas (pls. 1 and 2); therefore, changes caused by irrigation in the plot probably are superimposed on changes caused by lateral migration of ground water.
Figure 16--Concentration ratios for cations in water from wells in plot 3, 1965-71.
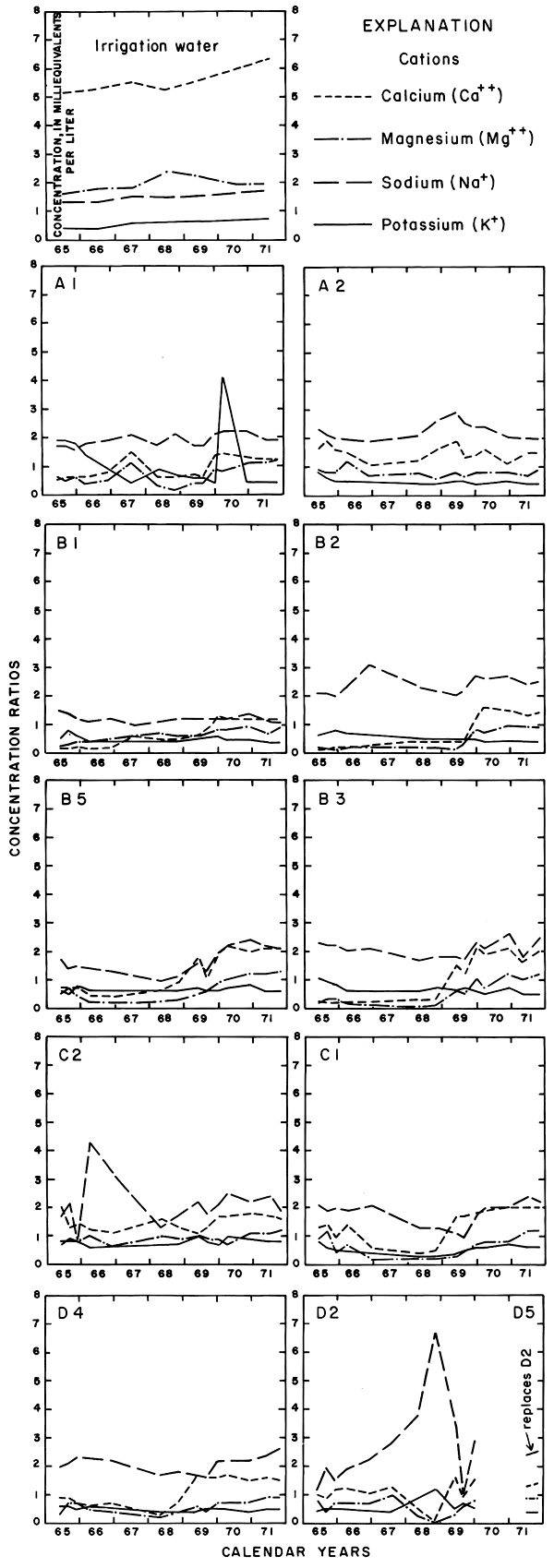
Figure 17--Concentration ratios for selected anions in water from wells in plot 3, 1965-71.
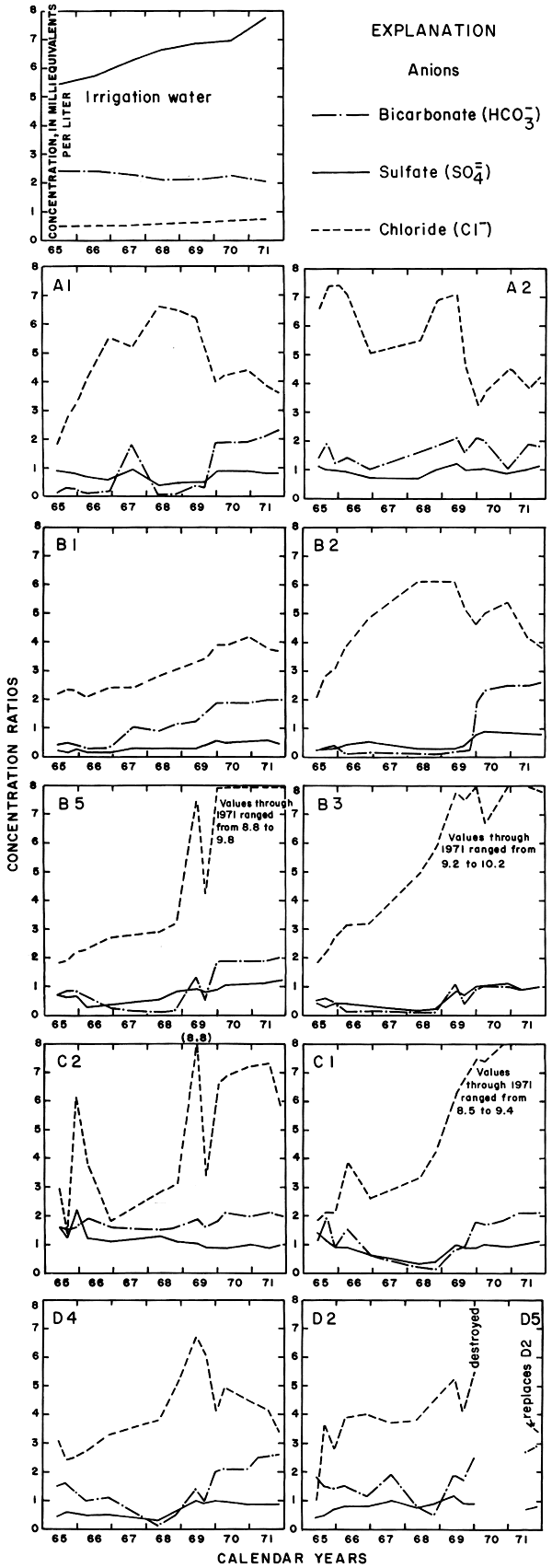
Figure 18--Concentration ratios for the specific conductance and nitrate in water from wells in plot 3, 1965-71.
The arrival of large quantities of irrigation water in the aquifers evidently caused the concentrations of calcium and sulfate in most of the well waters to increase rapidly during 1968 and 1969, but concentration ratios less than one for sulfate indicate loss of sulfate to the soil, lateral inflow of ground water containing less sulfate than the irrigation water, or both. During the entire 1965-71 period, the ratio for sodium remained near two. After 1966 the ratio for chloride exceeded two in all samples and reached peaks of more than 10 (more than 260 mg/l) in some of the wells in 1969. The high ratios indicate a source other than the irrigation waters. The decline in the ratios after 1969 may represent depletion by leaching of accumulated salts in the soil. The highest concentrations of chloride were recorded in waters from wells in the southwest part of the plot where the water table is discontinuous on an erosional remnant of bedrock.
Variations in the concentrations of the nutrients, potassium and nitrate, were somewhat similar to those in plot 1. The ratio for potassium generally was less than one, but the ratios for nitrate in nearly all the wells increased rapidly in 1968 to values exceeding eight. Anhydrous ammonia used as fertilizer probably is the main source of the nitrate.
PLOT 2
Plot 2 is on the flood plain of the Smoky Hill River and overlies a buried channel in the bedrock (pl. 2 and fig. 19). The lithologic characteristics of the alluvium differ vertically and laterally; therefore, the contact between clay, sandy clay, and sandy silt shown between wells D6 and D7 may be gradational rather than erosional.
Figure 19--Location of water table and configuration of the bedrock surface in plot 2.
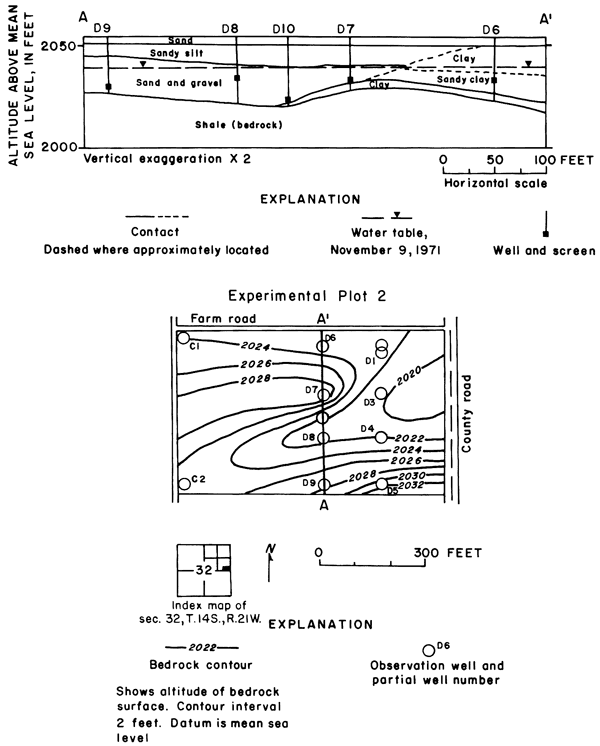
Far more water was applied to the alfalfa (1965-70) and grain sorghum (1971) in plot 2 than was applied to crops in plots 1 and 3 (table 4). Water readily infiltrated the sandy surface soil and was transpired by the deep-rooted alfalfa, but a large excess percolated to the underlying sand and gravel aquifer and moved laterally to the river, which is about 600 feet (180 m) from the southern boundary. Water levels fluctuated within a range of about 2 feet (0.6 m) at a depth of about 16 feet (4.9 m) below the irrigated field.
The main sources of ions in the well waters appear to be salts delivered in the irrigation water and the calcium and bicarbonate ions derived from fragments of limestone in the alluvium. Lesser amounts of salts have accumulated in the sandy soil at plot 2 than at the other sites owing to periodic flushing by river water during floods. Calcium and sulfate were the predominant ions in virtually all samples, although bicarbonate was the predominant anion in several samples containing calcium carbonate that bad precipitated in the well or sample bottle. The concentration ratios for specific conductance generally ranged between 1.2 and 1.6 (figs. 20, 21, and 22), which is lower than was predicted from the consumptive use (table 4). The limited quantity of calcium carbonate available may have affected the ratios in the well waters.
Figure 20--Concentration ratios for cations in water from wells in plot 2, 1965-71.
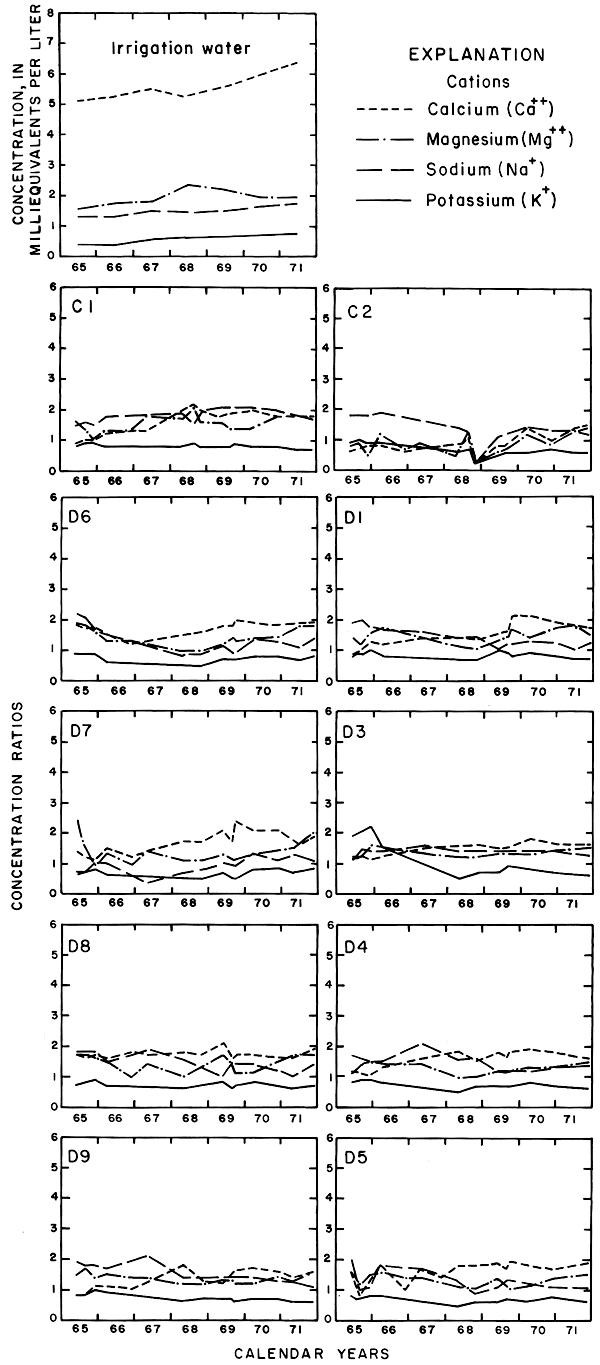
Figure 21--Concentration ratios for selected anions in water from wells in plot 2, 1965-71.
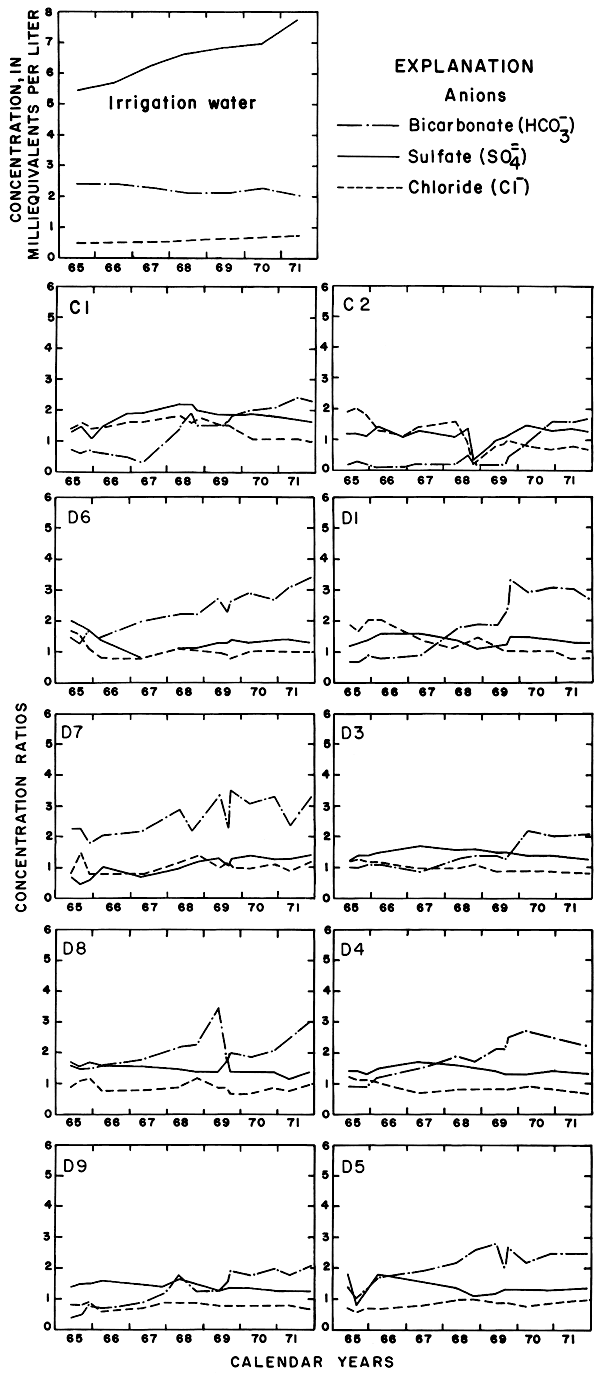
Figure 22--Concentration ratios for the specific conductance and nitrate in water from wells in plot 2, 1965-71.
Unlike well waters from the other plots, the concentration ratios for calcium and bicarbonate generally exceeded the ratios for the other major ions. The concentration of bicarbonate increased more rapidly in the well waters than in the irrigation water. The curves describing the ratios for sulfate are similar to the curves for specific conductance, and the ratios or sodium and chloride indicate that the irrigation water was an adequate source of those ions. After 1968, the ratio for chloride from well water was unity or less, indicating dilution of the irrigation water with respect to that ion or underestimation of rainfall.
As shown for the other plots, the concentration ratio for potassium in plot 2 generally was less than one. Potassium from the irrigation water may have been used by the crops. The ratio for nitrate fluctuated widely. Fixation in the soil of atmospheric nitrogen by alfalfa is a well-known phenomenon. The observed wide fluctuations in the concentration of nitrate in the ground water beneath the root zone are not readily evaluated.
To some extent, the difference in the effects of irrigation on the chemical quality of ground water beneath plots 1 and 3 on the high terrace and plot 2 on the flood plain are predictable. In the semiarid environment, soluble salts could accumulate in the sediments on the high terrace until irrigation provided excess water to transport them to the ground-water reservoir. Most of the accumulated salts would have been washed away during erosion and redeposition as the younger alluvium on which plot 2 is located. Periodic inundation and leaching by river water before construction of the dam forestalled significant reaccumulation of soluble salts other than those carried by the river water, which is generally similar in quality to the irrigation water. By analogy, the chemistry of the ground water beneath plot 2 may closely resemble the chemistry of ground water under the irrigated land on the high terrace after prolonged application of irrigation water has leached away previously accumulated salts.
Comparison of the chemistry of the irrigation water and well waters from plots 1 and 3 using graphical methods and a "mix-match" computer program (Lowell, et al., 1971) showed that the well waters were not simple mixtures of the original ground water and infiltrated irrigation water. Excessive concentrations of some of the ions evidently were derived from the soil. In particular, evidence that disproportionately high concentrations of sodium and chloride in well waters could be attributed to leaching of natural salts from the soil by excess irrigation water should allay suspicion that deleterious effects of oil-field and livestock operations were widespread or that sources of the sodium and chloride were perennial.
Relations of the Chemical Quality of Ground Water to Soil Chemistry
Soil samples from the experimental plots and from adjacent nonirrigated fields were analyzed to determine if the natural salt content of the soil profile in the irrigated areas had been depleted sufficiently by leaching to account for the observed changes in the chemistry of the well waters. The soils in the experimental plots are typical of a large part of the irrigated acreage; therefore, extrapolation of the results to describe a wider area probably is justified. Drs. O. Bidwell, R. Ellis, H. Jacobs, L. Murphy, and D. Whitney, Kansas State University (oral commun., 1972), contributed valuable information to this phase of the project.
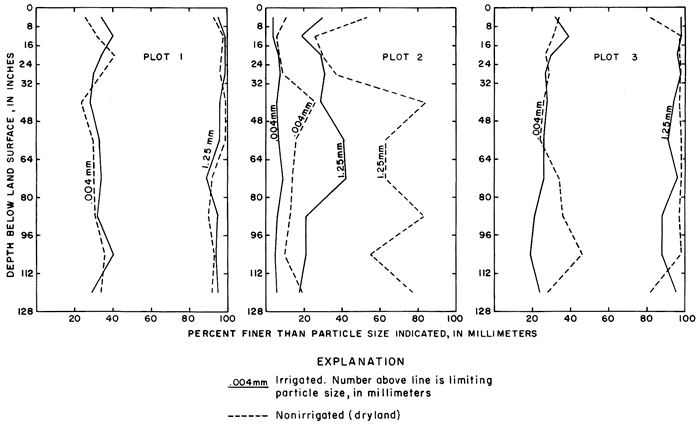
Corresponding soil profiles in each of the experimental plots and adjacent nonirrigated areas were sampled in March 1971. Samples were taken to a depth of 128 inches (325 cm) with a power probe loaned by the Agricultural Experiment Station at Hays. Mr. Carlyle Thompson, Soils Specialist, selected the sampling sites and supervised the sampling operation. At the selected sites, the chemistry of the profile should be unaffected by oil-field or livestock waste or by upward movement of salts from the deep-lying water table. Results of particle-size analyses for each of the profiles are shown on figure 23. The profiles in plots 1 and 3 contain material that is mainly silt and clay sized, and the profiles in irrigated and in adjacent nonirrigated sites are similar. Profiles in the coarse-grained alluvium for the irrigated and nonirrigated sites in plot 2 are dissimilar.
Figure 23--Particle-size distribution in soil profiles in and adjacent to experimental plots.
The soils in and adjacent to the experimental plots are classified as listed below according to an unpublished soil map of Ellis County provided by Mr. Bert Soderbloom, Soil Conservation Service, Hays.
| Plot | County | Owner | Soil type in irrigated field |
Soil type in nonirrigated field |
|---|---|---|---|---|
| 1 | Ellis | V. Moore | Harney silt loam (M3B) |
Harney silt loam (M3A) |
| 2 | Trego | P. Nicholson | Alluvium (unclassified) |
Alluvium (unclassified) |
| 3 | Ellis | V. Moore | Harney silt loam (M3A) |
Armo loam (M31D) |
The Harney and Armo are classified as medium-textured soils; the sandy soil in plot 2, which was highly disturbed by leveling, has not been classified.
Soil-saturation extracts of samples were analyzed by the Agronomy Department, Kansas State University. Saturation percentage, soluble (distilled water) and extractable (ammonium acetate) cations, and nitrogen were determined for samples representing 8-inch (20-cm) intervals to a depth of 32 inches (81 cm) and 16-inch (40-cm) intervals to a depth of 128 inches (325 cm). The methods used were those described by M. L. Jackson (1958). The pH, and the concentrations of phosphate, zinc, and copper also were determined for samples representing comparable depth intervals.
Duplicate soil-saturation extracts (distilled water) were analyzed for pH, specific conductance, the major anions, and for boron by the Kansas State Department of Health. The laboratory results are included in the basic data report (Leonard and Stoltenberg, 1972).
Analyses of samples of the 0 to 8-inch (20-cm) "A" soil horizon in plot 3 and an adjacent uncultivated field, as given in table 5, by E. A. Jenne, U.S. Geological Survey, indicate minor enrichment of the trace-element content of the soil by cultivation under irrigation. The methods of analyses were those of the U.S. Geological Survey, 1970. This single pair of samples, which may not be conclusive, were collected mainly to test results from field and laboratory methods.
Table 5--Non-silicate trace element content of samples of the "A" horizon in plot 3 and an adjacent uncultivated field, [Analyses by E. A. Jenne, U.S. Geological Survey, July 1972. Concentrations in micrograms per gram, oven dried at 110 °C.]
| Location | Beryllium (Be) |
Cadmium (Cd) |
Cobalt (Co) |
Copper (Cu) |
Iron (Fe) |
Lead (Pb) |
Manganese (Mn) |
Mercury (Hg) |
Molybdenum (Mo) |
Nickel (Ni) |
Silver (Ag) |
Zinc (Zn) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15-19W-7BBA (irrigated soil) |
0.26 | 0.32 | 4.0 | 2.6 | 9,900 | 9.3 | 360 | 0.07 | 1.2 | 7.2 | 0.04 | 17 |
| 15-19W-7BDA (nonirrigated soil) |
.16 | .22 | 3.3 | 2.9 | 9,300 | 8.0 | 270 | .11 | 1.1 | 6.4 | .13 | 17 |
If the accumulated soluble salts were leached from the soil by the irrigation water, the salt content of the profile and the concentrations of those salts in the saturation extract should be less in the irrigated area than in the nonirrigated area. However, as a result of evapotranspiration, the concentration of the ions in the soil solution in an irrigated area normally exceeds the concentration of the same ions in the irrigation water. To relate variations in the composition of the soil water to the irrigation water, the concentrations of the soluble ions in the saturation extracts (distilled water) are expressed as ratios to concentrations of the corresponding ions in the irrigation water in 1970. The concentration ratios are comparable to those shown in tables 2 and 4 and in figures 10 to 12, 16 to 18, and 20 to 22 describing variations in the concentration ratios of ions in the well waters and irrigation water.
Figure 24--Concentration ratios for specific conductance and soluble ions in saturation extracts of samples from irrigated and nonirrigated soil profiles in and adjacent to experimental plots, March 1971. [A larger version of this figure is available (Acrobat PDF).]
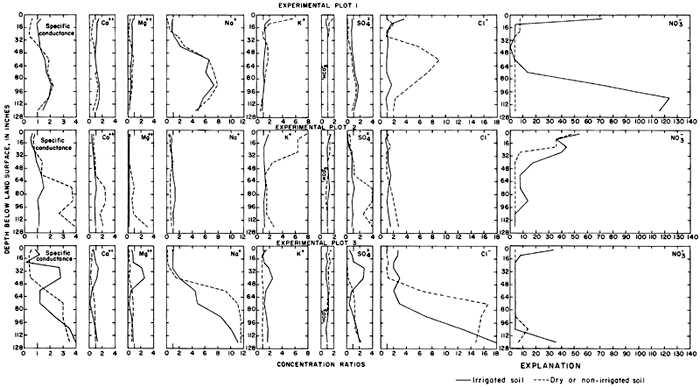
Table 6--Exchangeable cation content and sodium percentages of samples from irrigated and nonirrigated soil profiles in and adjacent to experimental plots. [Cations extracted with I N. ammonium acetate minus soluble cations extracted with distilled water. Cation content given in milliequivalents per 100 grams of soil.]
| Plot number |
Depth interval (inches)1 |
Calcium (Ca) (me/100 g) |
Magnesium (Mg) (me/100 g) |
Sodium (Na) (me/100 g) |
Potassium (K) (me/100 g) |
Exchangeable sodium percentage |
Soluble sodium percentage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | ||
| 1 | 0-8 | 14.18 | 16.05 | 3.09 | 4.17 | 0.06 | 0.23 | 2.32 | 1.20 | .33 | 1.04 | 3.6 | 17.0 |
| 8-16 | 15.99 | 20.67 | 3.88 | 6.10 | .09 | .31 | 1.79 | 1.14 | .42 | 1.11 | 11.3 | 19.7 | |
| 16-24 | 19.32 | 21.94 | 5.57 | 5.89 | .28 | .29 | 1.18 | .97 | 1.05 | 1.00 | 31.0 | 19.5 | |
| 24-32 | 19.20 | 21.96 | 5.98 | 5.98 | .41 | .33 | 1.22 | .98 | 1.52 | 1.12 | 53.1 | 22.0 | |
| 32-48 | 21.42 | 21.28 | 5.58 | 5.87 | .81 | .74 | 1.19 | 1.07 | 2.79 | 2.55 | 48.4 | 41.2 | |
| 48-64 | 21.11 | 22.35 | 5.48 | 4.77 | 1.43 | 1.18 | 1.22 | .95 | 4.88 | 4.02 | 64.3 | 68.9 | |
| 64-80 | 21.78 | 23.15 | 4.93 | 4.12 | 1.51 | 1.35 | 1.08 | .81 | 5.16 | 4.58 | 69.8 | 66.2 | |
| 80-96 | 23.59 | 23.80 | 4.55 | 3.70 | 1.50 | 1.28 | .92 | .85 | 4.92 | 4.34 | 66.7 | 64.6 | |
| 96-112 | 23.00 | 22.28 | 3.83 | 3.58 | 1.39 | 1.28 | .84 | .80 | 4.78 | 4.58 | 67.0 | 59.4 | |
| 112-128 | 24.37 | 25.08 | 4.05 | 3.38 | 1.50 | 1.01 | .84 | .68 | 4.86 | 3.35 | 73.1 | 61.8 | |
| 2 | 0-8 | 15.08 | 9.61 | .97 | .64 | .05 | .05 | .61 | .22 | .28 | .43 | 4.6 | 11.4 |
| 8-16 | 11.96 | 9.91 | .48 | .57 | .06 | 0.5 | .61 | .22 | .28 | .43 | 13.0 | 25.6 | |
| 16-24 | 12.80 | 12.69 | .53 | .77 | .06 | .07 | .37 | .24 | .41 | .52 | 9.8 | 19.0 | |
| 24-32 | 13.23 | 12.47 | .48 | .73 | .07 | .07 | .41 | .24 | .48 | .50 | 13.7 | 18.3 | |
| 32-48 | 21.44 | 10.91 | 1.46 | .48 | .18 | .06 | .89 | .20 | .77 | .55 | 15.8 | 19.6 | |
| 48-64 | 18.53 | 12.63 | 1.86 | .44 | .12 | .08 | .53 | .20 | .57 | .57 | 11.2 | 18.5 | |
| 64-80 | 15.57 | 13.85 | 1.19 | .44 | .07 | .06 | .31 | .22 | .40 | .44 | 4.7 | 20.8 | |
| 80-96 | 18.49 | 11.64 | .63 | .32 | .05 | .04 | .33 | .16 | .26 | .37 | 4.2 | 20.9 | |
| 96-112 | 14.51 | 11.78 | 1.07 | .36 | .05 | .06 | .28 | .16 | .33 | .48 | 6.3 | 21.2 | |
| 112-128 | 17.28 | 11.41 | 3.25 | .40 | .08 | .07 | .5o | .16 | .39 | .61 | 6.2 | 23.3 | |
| 3 | 0-8 | 16.10 | 15.55 | 1.71 | 3.70 | .07 | .21 | .90 | 1.11 | .35 | 1.03 | 3.9 | 23.4 |
| 8-16 | 17.82 | 21.39 | 2.12 | 5.16 | .06 | .28 | .72 | .02 | .31 | 1.00 | 10.0 | 20.9 | |
| 16-24 | 21.95 | 23.12 | 3.23 | 4.74 | .10 | .22 | .68 | .94 | .37 | .75 | 7.8 | 16.3 | |
| 24-32 | 18.56 | 21.51 | 4.09 | 5.07 | .13 | .20 | .54 | 1.00 | .57 | .71 | 19.1 | 8.4 | |
| 32-48 | 15.78 | 20.01 | 4.10 | 5.50 | .50 | .37 | .53 | 1.14 | 2.37 | 1.38 | 56.3 | 18.2 | |
| 48-64 | 16.51 | 18.14 | 4.20 | 4.62 | .86 | 1.00 | .57 | 1.00 | 3.90 | 4.04 | 79.1 | 67.7 | |
| 64-80 | 18.09 | 17.68 | 3.85 | 3.77 | 1.38 | 1.47 | .57 | 1.08 | 5.79 | 6.13 | 72.2 | 78.2 | |
| 80-96 | 20.61 | 16.20 | 3.90 | 2.52 | 9.30 | 1.06 | .72 | .70 | 26.9 | 5.20 | 72.8 | 71.7 | |
| 96-112 | 26.55 | 17.31 | 4.42 | 2.46 | 11.34 | 1.05 | .93 | .70 | 26.2 | 4.90 | 71.8 | 64.4 | |
| 112-128 | 20.72 | 18.21 | 2.87 | 2.35 | 7.83 | 1.12 | .76 | .71 | 24.3 | 5.00 | 67.4 | 61.5 | |
| Inches X 2.54 equal centimeters. | |||||||||||||
Variations in the ratios for the soluble ions with depth in each profile are shown in figure 24. Dilution by rainfall, variations in the solubility of the salts, and ion-exchange reactions within the soil complex alter the ionic composition of the soil water, but the major differences between irrigated and nonirrigated profiles probably are attributable to leaching. The concentration ratios for the specific conductance of the saturation extracts from the upper 2 feet (0.6 m) of the irrigated profiles are generally similar to the predicted annual concentration ratios (CRa) shown in table 4.
Variations in the concentrations of calcium and bicarbonate ions are probably governed by the solubility of calcium carbonate in each profile. The tops of calcareous zones of varying thickness were found in the soil profiles at depths from 18 to 35 inches (46 to 89 cm) in and adjacent to plots 1 and 3 and at the land surface in plot 2. Magnesium carbonate is more soluble than calcium carbonate; ratios less than one for magnesium throughout most of the profiles may represent ion exchange for sodium, which forms more soluble salts. Relatively low concentration ratios for specific conductance, sulfate, and other ions near the surface probably represent leaching by rainfall after the irrigation season. The concentration of sulfate in soil profiles in plots 1 and 3 was increased by application of irrigation water.
In plots 1 and 3 the concentration ratios for soluble sodium, chloride, and nitrate ranged more widely than for the other ions. In plot 1, the concentration of soluble sodium was only slightly lower in the irrigated than in the nonirrigated profile, but the concentration of chloride was much lower. In plot 3, high concentrations of sodium and chloride appear to have been displaced downward by the excess water. In plots 1 and 2, excess potassium seems to have been leached from the irrigated profile, but the concentration of potassium in plot 3 is higher in the irrigated than the nonirrigated profile, which could be a result of fertilization.
High ratios for nitrate near the surface of irrigated plots 1 and 3 reflect application of anhydrous ammonia fertilizer before soil sampling. The high ratios at the base of the sections probably represent excess fertilizer that was leached below the root zone.
In this report, the exchangeable cation content is defined as the difference between the extractable and the soluble cation content of each sample of soil. Extractable cations are the total cationic content of that part of a soil sample which was removed by ammonium acetate; soluble cations are those removed from the soil sample by distilled water (table 6). The soluble anions also were removed by distilled water. The exchangeable sodium percentages generally increased with depth and the percentages for the irrigated profiles exceeded the percentages for the dry profiles in the upper part of the section. The results substantiate the prediction by Jacobs and Whitney (1968, p. 5) that the exchangeable sodium percentage of the upper layers would not exceed 10 percent subsequent to irrigation with water of the type applied.
The extractable cation content was reported in terms of milliequivalents per 100 grams of soil (me/100 g). For purposes of comparison, the soluble ion content of the soil (M) in each depth increment was converted to the same units from the saturation percentage (SP), the concentration (C), and a units conversion factor (K) characteristic of each ion.
M (me/100 g) = [C(mg/l) X SP (%) X K(me/mg)] / 1000
The soluble cation content generally represented less than 10 percent of the extractable cation content for each profile.
Differences in content of the exchangeable cations, the soluble cations, and the soluble anions for corresponding depth intervals in the irrigated and nonirrigated profiles are shown in figure 25. Values representing ionic content of nonirrigated profiles were subtracted from corresponding values representing ionic content of irrigated profiles. Positive values probably indicate accumulation of salt and negative values probably indicate leaching of salts by irrigation water if the profiles are equivalent. At depths greater than 48 inches (120 cm), most of the differences are negative or near zero. Calcium, magnesium, and sulfate from the irrigation water evidently augmented the natural accumulation of the ions in the upper part of the section.
Figure 25--Differences in the exchangeable cation content and the soluble cation and anion content of irrigated and nonirrigated soil profiles in and adjacent to experimental plots. [A larger version of this figure is available (Acrobat PDF).]
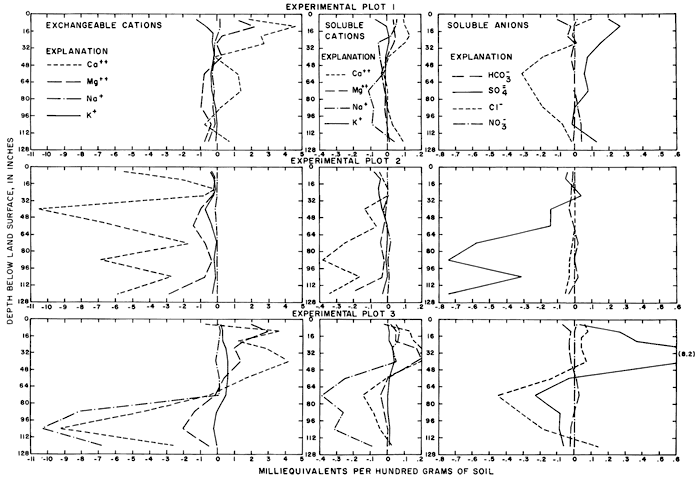
By cumulating the differences for each interval of corresponding profiles, weighted according to the size of the interval, the average net differences (in me/100 g) were calculated for each plot. Negative differences are recorded in table 7 for major ions except calcium in plot 1, magnesium and potassium in plot 3, sulfate in plots 1 and 3, and nitrate for all plots.
Table 7--Net differences in the content of major ions in adjacent irrigated and nonirrigated soil profiles to a depth of 128 inches.
| Plot | Ion type | Content, in milliequivalents per 100 grams dry soil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca++ | Mg++ | Na+ | K+ | HCO3- | SO4-- | Cl- | NO3- | ||
| 1 | Extractable | 1.12 | -0.14 | -0.18 | -0.26 | ||||
| Soluble | .04 | .01 | -.03 | -.01 | -0.01 | 0.07 | -0.13 | 0.02 | |
| Exchangeable | 1.08 | -.15 | -.15 | -.25 | |||||
| 2 | Extractable | -4.89 | -.90 | -.02 | -.30 | ||||
| Soluble | -.17 | -.04 | .00 | -.03 | -.01 | -.35 | -.02 | .00 | |
| Exchangeable | -4.72 | -.86 | -.02 | -.27 | |||||
| 3 | Extractable | -.86 | .24 | -3.25 | .27 | ||||
| Soluble | .05 | .04 | -.14 | .01 | -.01 | 1.24 | -.10 | .00 | |
| Exchangeable | -.91 | .20 | -3.11 | .26 | |||||
Under the tenuous assumption that the negative differences represent the quantity of salts leached from the upper 128 inches (325 cm) of soil in each plot, the amounts by which the concentrations of the ions in the leachate increased can be estimated. Undisturbed soil samples were not available, but a bulk density of 1.45 grams per cubic centimeter is a reasonable estimate for the silt loam sampled in plots 1 and 3. Approximately 13,150 cubic meters of dry soil, about 1.91 X 1010 grams, underlies each acre to a depth of 128 inches (325 cm). The amount of an ion leached from the soil (in grams per acre) is the product of the weight of the soil in grams, the concentration of the ion (in equivalents per gram of soil) and the gram equivalent weight for the ion.
According to table 7, the concentration of soluble chloride in the irrigated profile in plot 1 was 0.13 me/100 g less than in the nonirrigated profile. This is equivalent to a difference of about 900 kilograms per acre, computed as follows:
1.91 X 1010 (grams of soil/acre) X 1.33 X 106 (equivalents/ gram) X 35.46 (grams/ equivalent)
= 9.01 X 105 grams/acre = 900 kilograms/acre
According to table 4, excess water during irrigation seasons 1965-70 was about 3 acre-feet per acre or 3.7 X 106 liters per acre. If the 900 kilograms of chloride were dissolved in the excess water, the concentration of chloride would increase by about 240 mg/l. By similar calculations, based on the difference for soluble sodium, the increase for sodium would be about 40 mg/l. Comparable increases for plot 3 would be about 110 mg/l for both soluble chloride and sodium. If the calculations were based on differences for extractable sodium, the increase in concentration would be about 200 mg/l for plot 1, and more than 2,000 mg/l for plot 3 as a result of anomalously high concentrations of exchangeable sodium reported in the deepest cores from the nonirrigated profile for plot 3.
Figures 12 and 24 and table 7 show that the content of nitrate in the soil and the concentration of nitrate in well waters in plot 1 increased with the application of irrigation water. The apparent additions in the soil, mainly below the root zone, are equivalent to about 178 kilograms per acre of nitrate or 40 kilograms per acre of nitrogen. The increase may indicate excessive application of nitrogen fertilizer or, more likely, applications of water in excess of that required for plant growth.
Peaks in the concentrations of sodium and chloride in the well waters (figs. 12 and 16) after 1 to 3 years of irrigation indicate that the concentrations of those ions in the leachate probably increased rapidly during the first years of irrigation, then decreased as the supply of ions in the soil was depleted. More rigorous quantitative analysis of the relations of changes in the concentrations of the ions in the well waters to changes in the salt content of the soil could probably be made with a relatively small amount of additional data. However, the estimates presented above indicate that leaching of accumulated natural salts and applied fertilizer from the upper part of the soil profile by excess irrigation water, and lateral movement of the displaced ions are the major cause of observed changes in the concentrations of sodium and chloride in the ground water. The effects of the arrival of the chemically altered ground water on low flow of the Smoky Hill River was recorded during a series of seepage-salinity surveys.
Prev Page--Distribution and Use of Water || Next Page--Surface Water
Kansas Geological Survey, Geohydrology
Placed on web Nov. 2012; originally published 1975.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS1/06_gw.html