Prev Page--Previous Work || Next Page--General Geology
Chapter III—Petroleum and Natural Gas
Introduction
Petroleum and natural gas are naturally occurring materials of the greatest economic importance, consisting essentially of the elements carbon and hydrogen in very complex compounds known as hydrocarbons, or in mixtures of these compounds. Both may contain impurities of more or less widely different nature, such as various gases, sulphur, or oxidized and nitrogenous matter, the exact character of which is not clearly defined. Crude petroleum varies considerably in character, but as observed in most cases, is a dark, somewhat ill-smelling, more or less viscous liquid, slightly lighter than water. In chemical composition there is but slight variation between different petroleums, but there is a very wide range in the character of the hydrocarbons present. Natural gas is an inflammable mixture of the very lightest hydrocarbons, consisting chiefly of marsh gas or fire damp. It is colorless, almost odorless, and burns with a rather luminous flame. Both of these very valuable natural resources are widely, though not uniformly, distributed in the rocks of the earth. In some cases they are found under enormous pressures and in almost incredible quantities, but in general their occurrence is limited. At the present time so very great is man's use of petroleum and natural gas that they have become almost indispensable to him.
[Note: Petroleum (Latin, petra, rock, and oleum, oil) in its widest sense embraces all of the hvdrocarbons, gaseous, liquid and solid, cccurring in nature. The term is in practice limited to the liquid forms, and is synonymous with the simple word "oil" so widely used by geologists and the industry in general. Natural gas, as the term is applied, has come to have a technical meaning, referring only to those gases occurring in rocks which are sufficiently inflammable to be used as a fuel or illuminant. Volcanic gases, or the gases of the atmosphere, are also "natural," but are not included in the common use of the term.]
Properties of Petroleum
Chemical
Even prior to the discovery of commercial quantities of petroleum, a number of determinations of its chemical composition were made. These analyses, supplemented by recent investigation, show that petroleum consists of about 84 to 87 percent carbon and 11 to 13 percent hydrogen, with varying small proportions of sulphur, nitrogen and oxygen.
| Ultimate Analyses of Petroleum A chemical analysis of petroleum showing the irreducible elements of which it is composed is known as all ultimate analyses. |
|||||
|---|---|---|---|---|---|
| Locality | Carbon | Hydrogen | Oxygen, nitrogen |
Specific, gravity |
Degrees, Baume |
| Pennsylvania | 82.0 | 14.8 | 3.2 | .816 | 39 |
| West Virginia | 84.3 | 14.1 | 1.6 | .841 | 36 |
| West Virginia | 83.5 | 13.3 | 3.2 | .873 | 30 |
| Pennsylvania | 84.9 | 13.7 | 1.4 | .886 | 28 |
| Ohio | 84.2 | 13.1 | 2.7 | .887 | 28 |
| Texas a | 84.6 | 10.9 | 2.9 | .921 | 22 |
| California b | 81.5 | 10.0 | 6.9 | .965 | 15 |
| (a) Contains 1.63 percent sulpbur (b) Contains .55 percent sulphur. |
|||||
The various hydrocarbons of which petroleum is chiefly composed fall primarily into a regular series, in which a certain number (n) of hydrogen (H) atoms are combined with a given number (n) of carbon (C) atoms in the ratio indicated by the following series of generalized formulae. [The analysis of petroleum which consists in the separation of its various components, and in their identification as hydrocarbons of definite constitution, is known as a proximate analysis. The exact nature of these various hydrocarbon compounds of which there are a very large number, is in most cases not easily determinable.]
| CnH2n+2 | Paraffin series |
| CnH2n | Olefine and naphthene series |
| CnH2n-2 | Acetylene series |
| CnH2n-4 | |
| CnH2n-6 | Benzene series |
| CnH2n-8 | |
| CnH2n-10 | |
| CnH2n-12 |
The series represented in these formulae have all been recognized in petroleum. Each includes many members, the first, for example, beginning with marsh gas or methane, CH4, ranges at least as high as the compound C35H72. The lower members in this instance are gaseous, the middle members are liquids with regularly increasing boiling points, and the higher members are solids, like ordinary paraffin. Gaseous and solid hydrocarbons present in petroleum are in solution. The paraffins are found in all crude oils, in many cases composing the largest proportion of the oil. [Petroleum which contains chiefly the paraffin hydrocarbons. and which yields paraffin when the heavier distillates are subjected to a freezing temperature, is said to have a paraffin base. Crude oil containing asphaltic bodies yielding upon evaporation a residue consisting essentially of asphalt are said to have an asphaltic base. These terms, widely used formerly, are falling into disuse because some asphaltic oils may also yield paraffin wax.] The olefine series occurs in the majority of petroleums and is important in a few. The acetylene series is represented in some petroleum, notably that from the Baku district of Russia. Members of the benzene series, especially benzene and toluene, occur in all petroleums, but not in large amounts. Certain crude oils have been found to contain camphenes, napthalenes and other so-called aromatic hydrocarbons.
Sulphur may be present in crude petroleum either as free sulphur, as a constituent of hydrogen sulphide, or as an organic sulphur compound. Petroleum entirely free from sulphur is extremely rare, but the amount of this constituent is commonly very small. Most petroleum contains some nitrogen, but it rarely exceeds 2 percent. In most cases it exists in the form of complex organic compounds. Small amounts of oxidized materials, as complex acids and phenols, are found in some oils.
Physical
The physical properties of petroleum vary greatly. Most crude oils are opaque by transmitted light except in very thin layers. Many of the lighter grades, however, notably certain grades from Pennsylvania, Wyoming and Alberta, range in color from a pale straw yellow, through red and brown, to dark green. By reflected light crude oils have a greenish cast.
Of considerable scientific interest and technical importance is the effect exerted by petroleum on light rays which are passed through it. Thus the refractive index has significance regarding the origin of various petroleums, and may be used in their identification. It is observed, also, that certain crude oils and their distillates rotate slightly the plane of polarized light, the direction of rotation being in general to the right. Certain crude petroleums are distinguished by odor to such a degree that it is possible in some cases to determine by this character the region from which they have come.
| Baumé, degrees |
Specific gravity |
Pounds in gallon |
|---|---|---|
| 10 | 1.000 | 8.33 |
| 15 | .965 | 8.04 |
| 20 | .933 | 7.77 |
| 25 | .903 | 7.52 |
| 30 | .875 | 7.29 |
| 35 | .848 | 7.07 |
| 40 | .823 | 6.86 |
| 45 | .800 | 6.66 |
| 50 | 778 | 6.48 |
| 55 | 757 | 6.30 |
| 60 | .737 | 6.13 |
| 65 | .718 | 5.98 |
| 70 | .700 | 5.83 |
| 75 | .683 | 5.69 |
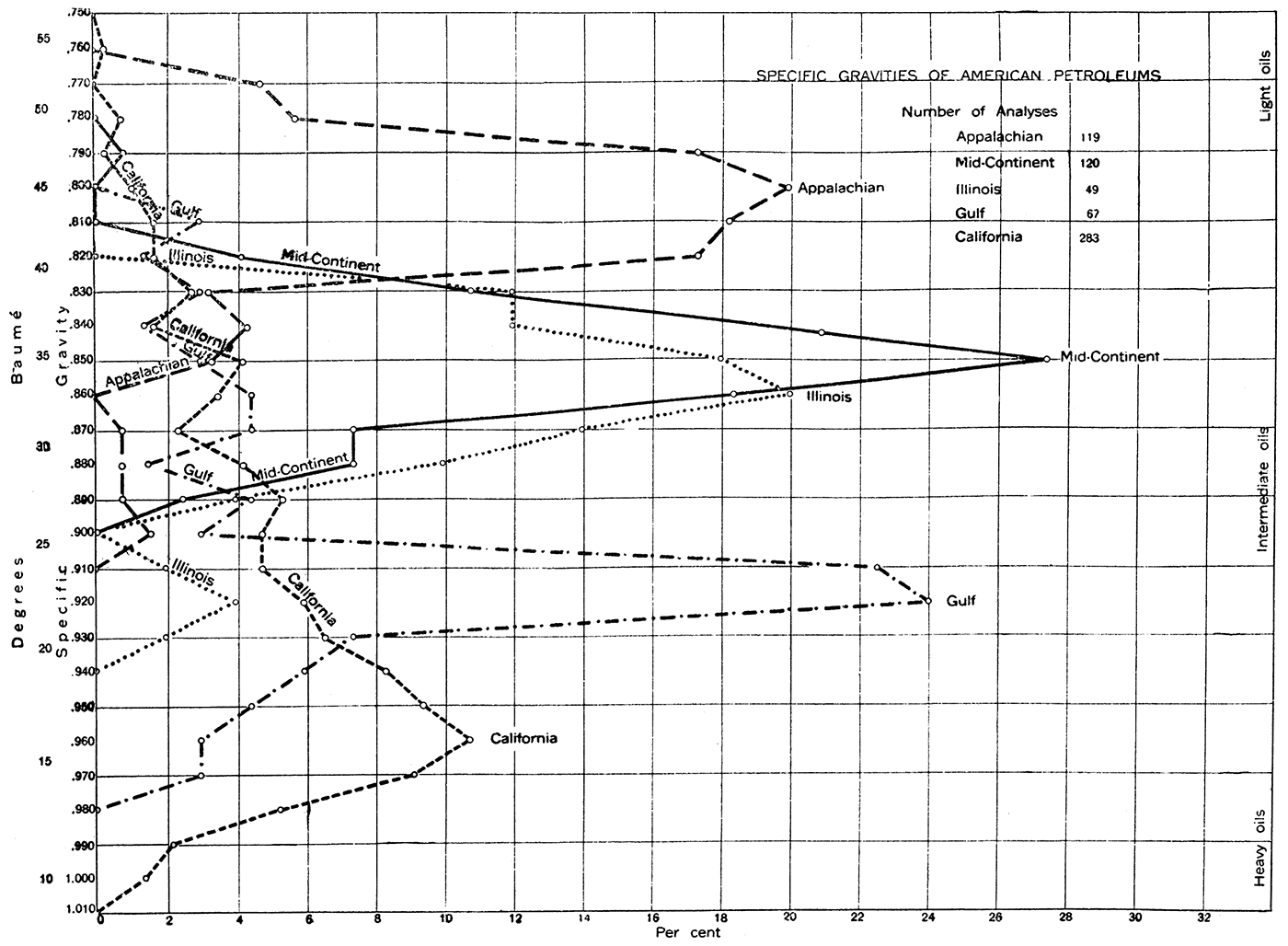
One of the important physical properties of petroleum, affording the basis for a rough classification which may be correlated with the proportion of more valuable light oils contained, is specific gravity. Crude petroleums show an extreme range in specific gravity from .771 to 1.06, the average, however, being between .8 and .98. Crude oil becomes denser on exposure to the air. The specific gravities of the crude oils from the various fields of the United States are shown in Plate II.
[Note: The specific gravity of petroleum is commonly given in terms of the Baumé scale, devised by Antoine Baumé, in 1768, for liquids lighter than water. A specific gravity of 1, compared with water, is arbitrarily chosen as 10° on the Baumé scale. Ordinary specific gravity determinations may he converted to the Baumé scale by dividing 140 by the specific gravity and subtracting 130; degrees Baumé may be converted to specific gravity by dividing 140 by Baumé degrees plus 130. Thus a heavy oil has it low specific gravity in degrees Baumé and a light oil a high one. Though the Baumé scale has no marked advantage over the rational scale, the refiner has adhered to its use through custom.]
Plate II—Specific Gravities of American Petroleums—Available determinations of the specific gravity of crude oils from each field have been separately computed to 100 percent for that field. Thus plotted they are directly comparable and show clearly the general average and the range of the crude oils of each field. About 27 percent of the oils reported from the Midcontinent field have a specific gravity of 0.850, or 35° Baume. Nearly 11 percent of California petroleums have a specific gravity of 0.960. or 16° Baumé, but the oils from this state range from 1.010 to 0.750. (Data from Mineral Resources, 1913, U. S. Geol. Surv., pp. 1126-1265,)
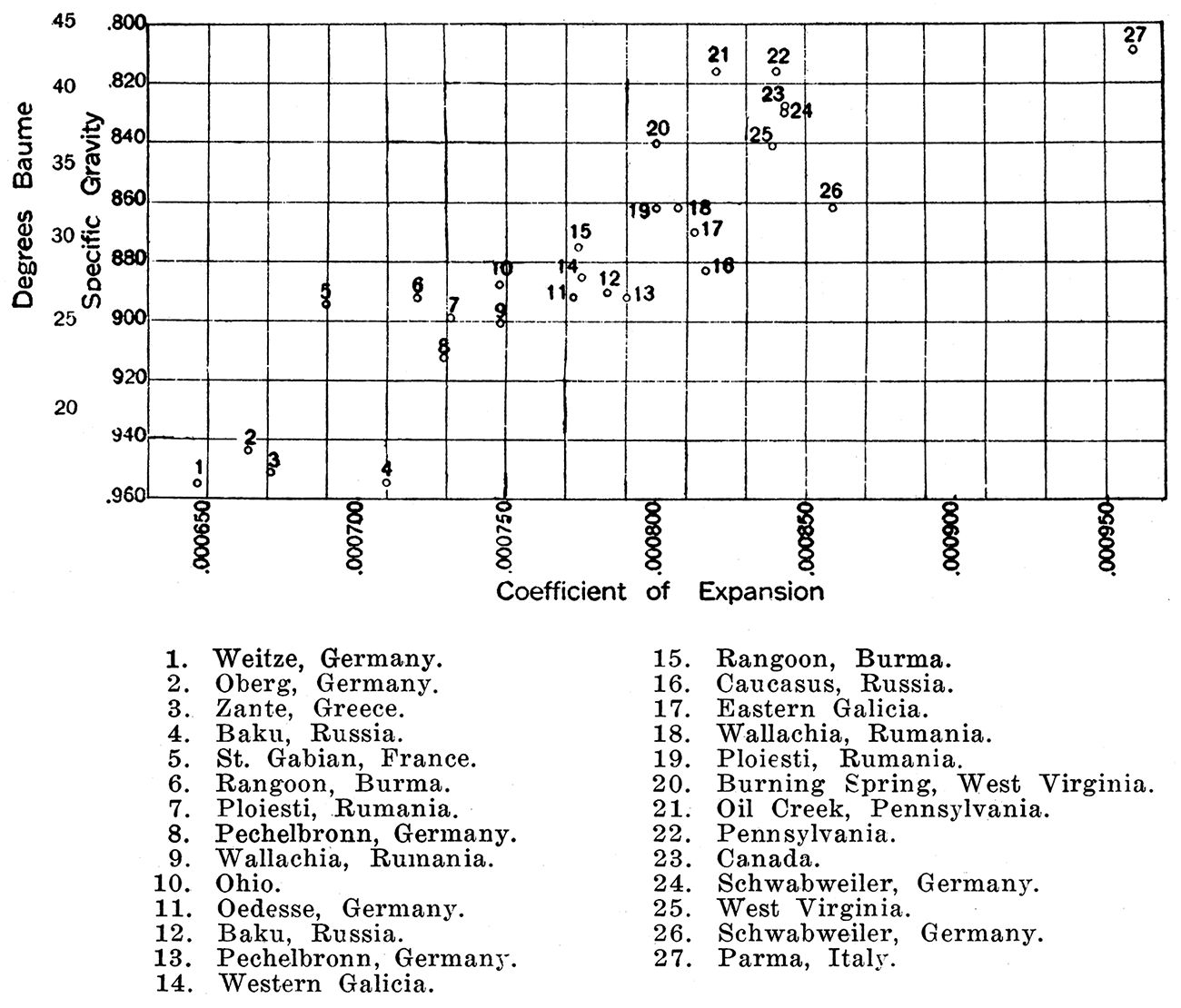
Changes of temperature produce a slight but variable change in the volume of petroleum. The coefficient of expansion of petroleums is important for determining the expansion space to be allowed in storage vessels and for transport. Observation shows that it decreases as the specific gravity rises. Thus a light oil has a high coefficient of expansion .and a heavy oil a low one. Figure 2 shows the relation of the coefficient of expansion of various crude oils to specific gravity. Certain exceptions which occur are attributable to chemical peculiarities of the oils.
Figure 2—Diagram showing relation of the coefficient of expansion of various crude oils to specific gravity. (Data from Bacon & Hamor, American Petroleum Industry.)
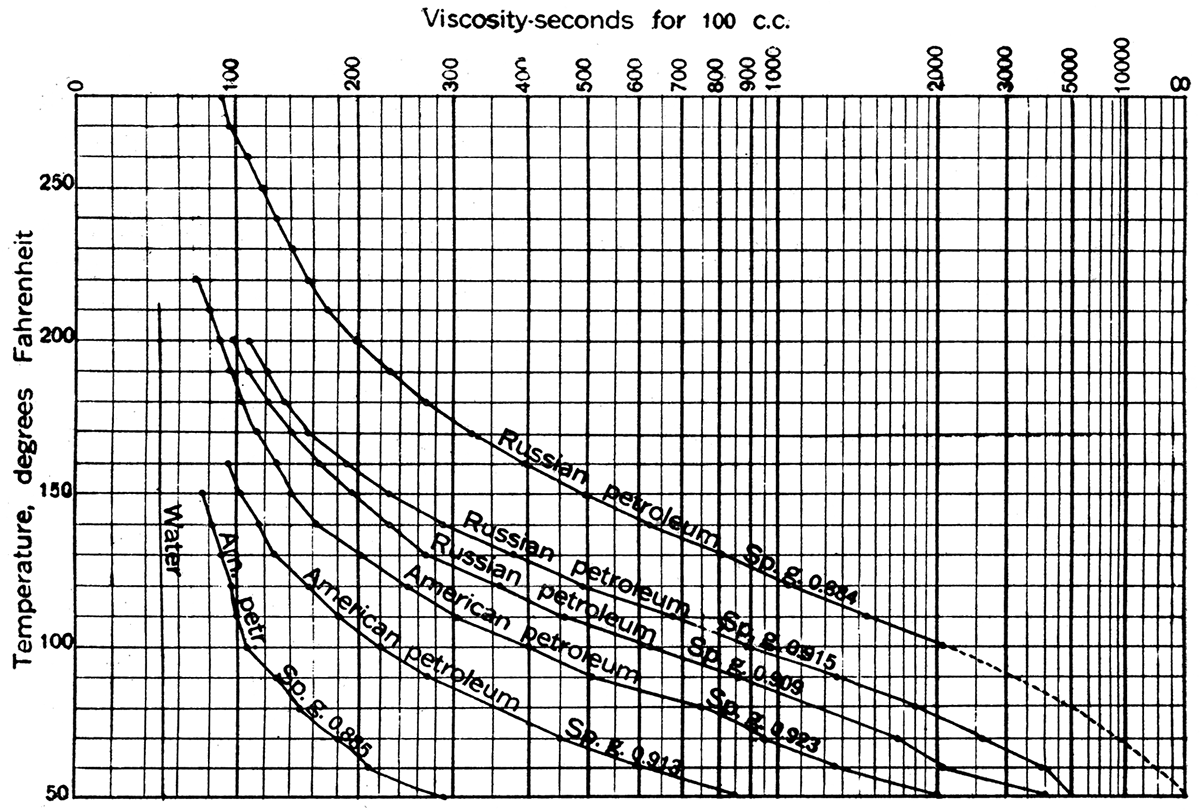
The viscosity of petroleums, like their specific gravity, shows a considerable range. Indeed, a somewhat direct relation appears to exist between these properties, certain of the very light oils being remarkably fluid and the heavier oils showing an increase of viscosity with specific gravity. Temperature, however, has an important effect on the viscosity of petroleum. The viscosity of petroleum is an important character because it determines the facility with which the oil can be pumped through pipe lines. Crude petroleum solidifies when cooled to a temperature varying from 82° F. in the case of some Burma oils to several degrees below zero in certain very light Italian oils. The relation of viscosity to temperature for various crude oils is shown in Figure 3.
Figure 3—Curves showing the relation of viscosity to temperature for various crude oils. The time in seconds required for 100 c. c. of oil to flow through an opening of given size, measured with Redwood's standard viscosimeter, is shown for various ternperatures. Note that Russian oil, sp. gr. 0.884, is semisolid at temperatures below 100° F. (Data from Redwood, A Treatise on Petroleum.)
The flashing point, or the lowest temperature at which inflammable vapors are given off, is a matter of concern in the case of certain oils. It has an extreme variation, from below zero in the Italian oils to more than 300° F. in certain oils from Africa.
The boiling points of petroleums and the distillates obtained at specified temperatures differ very considerably, also, in the crude oils from various sources. Distillation begins at lower temperatures in the lighter oils and at higher temperatures in the heavy oils.
The calorific or heating value of crude petroleum is an important character, especially in view of the rapid increase in the use of crude oil and residues for fuel in steam plants, and railway and marine engines. Some of the very heavy crude oils are used almost entirely for fuel and compete to advantage with coal. Many of the lighter, high-grade petroleums, after partial distillation, also, furnish a residue which is. sold as a fuel. The average heating value of crude petroleum from the Midcontinent field is about 10,900 calories, or 19,600 B. T. U. Good average coal has a heating value of about 7,500 calories, or 13,500 B. T. U. [Note: A calorie is the heat required to raise the temperature of 1 gram of water 1° Centigrade. The British thermal unit (B. T. U.) is the pound-degree, or the amount of heat required to raise a pound of water 1° Fahrenheit.]
Properties of Natural Gas
Chemical
Natural gas contains only the lightest and most volatile of the hydrocarbons. It consists chiefly of the lower paraffins, especially marsh gas, or fire damp, which has a chemical formula CH4. Ethane (C2H6) is present in considerable quantities in the natural gas from some regions. In association with these hydrocarbons there may be varying quantities of carbon dioxide (CO2), carbon monoxide (CO), hydrogen sulphide (H2S), hydrogen, nitrogen and oxygen. Some of the natural gas from Kansas is of more than usual interest because of its remarkably high nitrogen content, a sample from Dexter, Kan., containing 82.7 per cent of this element. Helium, argon and the rare element neon have been discovered in the natural gas of Kansas.
There are two broad, general types of natural gas: (1) "dry" gas, which consists principally of methane (CH4) with a small content of ethane (C2H6) and nitrogen, and (2) "wet" or casing-head gas, which contains besides methane varying amounts of the heavier hydrocarbons. The "dry" gas is the chief type produced in many localities. It does not contain any appreciable amount of readily condensible gasoline and is not usually closely associated with petroleum. "Wet" or casing-head gas contains readily condensible gasoline. It is characteristically associated with petroleum and is yielded with the oil in many wells. It also comes from gas wells in oil pools, producing from the oil-containing strata.
| Analysis of Natural Gas | ||||||||
|---|---|---|---|---|---|---|---|---|
| Locality | Methane (CH4) |
Other hydrocarbons |
Carbon dioxide (CO2) |
Carbon monoxide (CO) |
Oxygen | Nitrogen | Hydrogen sulphide |
Hydrogen sulphide (H2S) |
| Average Kansas | 93.65 | .25 | .30 | 1.00 | 4.80 | |||
| Average Pennsylvania and West Virginia |
80.85 | 14.00 | .05 | .40 | tr. | 4.60 | .10 | |
| Average Ohio and Indiana | 93.60 | .30 | .20 | .50 | .15 | 3.60 | 1.50 | .15 |
Physical
Although natural gas is somewhat closely related chemically to petroleum, its physical properties are very different. Of first importance, of course, is the gaseous character of its constituent hydrocarbons, which necessitates methods of handling commercially quite different from those used in oil production. The gas is colorless, practically odorless, and burns with a luminous flame. When mixed with air it is highly explosive.
In calorific value natural gas varies considerably, depending on the chemical character and proportion of its combustible hydrocarbons and the relative quantities which are present of such incombustible impurities. as nitrogen. The heating value of natural gas is high, as shown by numerous tests. "Dry" gas has a calorific value amounting to about 525 calories or 950 B. T. U. per cubic foot. Thus about 135 cubic feet of natural gas is equivalent in heating value to one gallon of average Midcontinent fuel oil. Casing-head gas may have a heating value as high as 2,500 B. T. U. and rarely falls lower than 1,000 B. T. U. Natural gas has a much higher heating power than manufactured gases. In many respects it may be regarded as an ideal fuel because of its cleanness, ease of handling, efficiency of combustion, and low content of poisonous constituents.
Origin of Oil and Gas
Note: For a very complete summary of all the hypotheses relative to the formation of petroleum, see Hofer, H. V., Das Erdol, 1906, vol. 1, pp. 160-229; and Engler, C., and Hofer, H. V., Die Geologic, Gewinnung und der Transport des Erdols, 1909, vol. 2, Leipzig, pp, 59-142. See, also, Clarke, F. W., Data of geochemistry: U. S. Geol. Surv., Bull. 616, 1916, pp. 726-737; Campbell, M. R, Historical review of theories advanced by American geologists to account for the origin and accumulation of oil: Econ. Geology. vol. 6, 1911, pp. 363-395; and White, David, Some relations in origin between coal and petroleum: Wash. Acad. Sci. Jour., vol. 5, 1915, pp. 189-212, map,
That the gaseous, liquid and solid hydrocarbons are closely related is evident from the fact that liquids identical with those distilled from petroleum may be condensed from natural gas, similarly that the gases given off by petroleum are like those predominating in natural gas, and finally that exposure of many petroleums to the air results in change to a viscous mass, which on drying becomes a solid asphalt or paraffin-like substance. Petroleum is rarely free from natural gas, although the gas may sometimes be formed alone, as in coal mines or from decaying vegetation. The question of the origin of these hydrocarbon compounds has great scientific interest as well as a fundamental importance in the economic development of these materials. It has engaged the attention of naturalists and others for more than a hundred years and has been the subject of considerable speculation and no little controversy.
The hypotheses which have been so far advanced for the origin of oil and gas may be divided readily into two main categories: (1) the inorganic, advanced chiefly by chemists on the basis of laboratory experimentation, and (2) the organic, held chiefly by geologists and those familiar with the geologic occurrence of oil and gas. The same evidence, interestingly enough, has been used in certain cases by persons holding opposite views. It is not possible or desirable in this report to consider in detail the almost innumerable hypotheses which have been advanced, but it will be valuable to review briefly the most important.
Inorganic hypotheses
That oil and gas may be derived entirely from inorganic sources was first definitely suggested by the French chemist, Berthelot in 1866 [Berthelot, E. M. P., Annales Chem. Phys., 4th ser. vol. 9, 1866, p. 481.]. On the assumption that the interior of the earth might contain free alkaline metal", he stated that mineral oil was produced by purely chemical action, similar to that employed in the manufacture of acetylene. Other hypotheses of like nature have been proposed by other chemists, one of the most noteworthy of which is that of the Russian chemist, Mendeléeff [Mendeléeff, D., Ber Deutch, Chem. Gesell., vol. 10, 1877. p. 229; Jour. Chem. Soc. vol. 32, p. 283; also, Mendeléeffs Principles of Chemistry, Eng. trans., 1891, pp. 364-366.]. This ascribes the formation of petroleum to the action of surface waters, which, percolating downward through the rocks to the heated interior of the earth, become converted into steam and attack iron carbides to form the hydrocarbons which make up oil and gas. From a chemical standpoint this more or less satisfactorily meets the requirements, but the circulation postulated is questionable and the actual existence of the carbides in nature remains to be proven. For example, if oil and gas were thus formed we should expect to find them most widely distributed and abundant in the oldest rocks of the earth's crust, an expectation which is distinctly contrary to fact. The production of hydrocarbons from cast iron (Hahn, Williams and Cloez, Compt. rend., vol. 75, p. 1003.), the formation of various metallic carbides in the electric furnace (Moissan, Compt. rend., vol. 122, 1896, p. 1362.), and various reported occurrences of gaseous and liquid hydrocarbons in association with volcanic emanations and igneous rocks (), seem to accord with inorganic hypotheses. [Note: Analyses of volcanic gases from Sicily, Santorin and West Indies (Clarke, F. W., Data of geochemistry: U. S. Geol. Surv., Bull. 616, 1916. pp, 263, 268) show the presence of methane (CH4), Basaltic lavas near Etna contain small amounts of oil and paraffin (Silvestri, O., Gazz. chim. ital., vol. 7, 1877, p. 1, vol. 12, 1882, p. 9, cited by Clarke, loc. cit., p. 727), and the petroleum of certain Javan and Mexican fields is closely associated with igneous rocks. (Clarke, F, W., loc. cit., p. 727).] The association of oil and gas with igneous rocks is not common, but even in the occurrences known there is no proof that the oil and gas originated in the igneous rock. Admitting that small quantities of different hydrocarbons may be formed by various inorganic agencies, the evidence seems to indicate clearly that this is not the origin of the large commercially important accumulations of the natural hydrocarbons.
Organic Hypotheses
An overwhelming and increasing majority of those who have studied the accumulating geologic evidence, and who are familiar with natural conditions under which petroleum occurs, are of the opinion that oil and gas are of organic origin. The organic hypotheses suggest that the natural hydrocarbons have been formed by the decomposition of organic matter buried in the rocks, though the precise character of the organisms and the exact nature of changes involved are not entirely certain. Petroleum has been prepared by the distillation of certain animal oils and is produced by the natural decomposition of some seaweeds. It is found chiefly in sedimentary rocks containing more or less abundant fossilized remains of various organisms. Gaseous hydrocarbons, especially methane, are certainly derived from decaying vegetation, either as "marsh gas" in swamps or "fire damp" in coal mines. Methane, carbon dioxide, hydrogen and nitrogen are produced in the decay of seaweeds (Phillips, F. C., Am. Chem. Jour., vol. 16, p. 427, 1894, cited by Clarke, Data of Geochemistry: U. S. Geol. Survey, Bull. 616, p. 729, 1916.). That hydrocarbons analogous to natural gas, petroleum and asphalt may be derived from either plant or animal matter or both has been demonstrated. The genesis of the larger accumulations of mineral oil, however, has not been proven. The fact that natural petroleum shows an optical activity similar to that of sugar, lactic acid and other organic compounds, which inorganically synthesized oil does not possess, is claimed to indicate undoubtedly the organic origin of petroleum.
While the evidence seems to indicate the organic origin of oil and gas, there is a difference of opinion as to whether they have been derived from accumulations of plants, the remains of animals, or both.
It was very early held that oil and gas were derived from the natural distillation of carbonaceous matter such as the remains of plants in coal. That plants have been very abundant in the past is shown by the numerous plant fossils which have been found in the rocks of almost every geologic age, including plants living on the land, and others found only in the sea. An apparent relation between oil and gas and certain land plants is indicated by the facts that hydrocarbons similar to those found in petroleum have been reported (Frazer, J. C. W., and Hoffman, E. J, The constituents of coal soluble in phenol: U. S. Bur. Mines, Tech. Paper 5, 1912.) in some bituminous coals, and that in at least one locality in West Virginia gas wells produce from the Pittsburg coal bed (Johnson, R. H., and Huntley, L. G., Principles of Oil and Gas Production, p. 19, 1916.). Plant spores, the so-called "algal remains" of boghead coal, are very abundant in some highly bituminous or petroliferous coals and shales, and may be in some form or other a source of petroleum (Jeffrey, E. C., On the composition and qualities of coal: Econ. Geology, vol. 9, p. 741, 1914.). Opposed to this it is observed that in general there is a striking lack of any association between petroleum and coal or lignite, that it requires a relatively high temperature, from a geologic standpoint, to convert wood into liquid bitumen without traces of its original structure, and that there is a great chemical difference between petroleum and the tar oils from coal or lignite. It does not seem that terrestrial vegetation would generally give rise to petroleum.
It is possible, in accordance with the arguments of many, that marine plants such as seaweeds are largely the source of petroleum and natural gas. Sea plants along the coasts of Sweden and Sardinia give rise to petroleum as a product of decomposition (Redwood, Sir Boyerton, Petroleum and its products, 3d edition, 1913. vol. 1, pp. 132, 148.). As observed in the California fields, oil is very closely associated in many instances with deposits of diatoms (Arnold, Ralph, and Anderson, Robert, Geology and oil resources of the Santa Maria oil district, Santa Barbara county, Cal: U. S. Geol. Survey, Bull. 322. p. 109, 1917. Anderson, Robert, and Pack, R. W., Geology and oil resources of the west border of the San Joaquin valley. north of Coalinga, Cal.: U. S. Geol. Survey, Bull. 603, p. 198, 1915.). The occurrence of petroleum in these diatomaceous deposits is so widespread that the diatom formations have been reliable guides in prospecting—a fact which furnishes the best evidence of the diatomaceous origin of the oil in these districts. Also the saline water associated with some oils carries iodine (Watts, Calif, State Min. Bur., BulL 19, p. 202, cited by Ries, H., Economic Geology, 4th edition, p. 82, 1916.), an element characteristically present in sea water. It seems likely that petroleum is at least in part derived from marine plants.
The theory that petroleum is formed by the decomposition or destructive distillation of animal matter entombed in the strata has many adherents. Under conditions easily reproducible in the chemical laboratory, animal matter of almost any sort can be decomposed into mixtures of oils closely resembling petroleum. The chemical processes involve the elimination of nitrogen and nitrogen compounds and a destructive distillation of the fats to form mixtures of hydrocarbons. Since there is indubitable evidence of the former existence of hosts of animal life of various kinds in the strata now containing petroleum, it seems very possible that at least in part the natural hydrocarbons are products of animal remains. In support of this belief it may be noted that certain petroliferous beds in Europe are very rich in fossil fish or the remains of mollusks (Clarke, F. W., loc. cit., p. 730.). Shells filled with petroleum have been observed by various writers (Phillips, F, C., On the occurrence of petroleum in the cavities of fossils: Am. Phil. Soc. Proc. vol. 36. pp. 121-126, 1897. Fraas, O, F., Bull. Soc. sci. nat. Neuchatel, vol. 8. 1868, p. 58. Clarke, F. W., loc. cit., 731-2.). The nitrogen bases of certain California petroleums furnish strong evidence that animal proteids contribute their share to its makeup and are indication of the animal origin of that particular oil.
Numerous objections, more or less valid, have been raised against an exclusively animal origin of the natural hydrocarbons. Since limestone is formed almost entirely by the accumulated shells and other hard parts of various sea animals, it might be supposed that oil and gas would be particularly closely associated with such formations. On the contrary, limestones are in most cases compact and massive, very ill-suited, except where rendered porous by strong jointing, solution or dolomitization, to act as reservoirs for oil or gas. In a few notable instances only, is petroleum found in limestone formations. It has been pointed out (Craig, E. H. Cunningham, Oil Finding, pp, 9-10, 1912) that were oil derived from animal remains to any large extent there should be a certain proportion of phosphates in the composition of petroleum, since these are characteristic constituents of almost all animal life. The accumulation and burying of the dead animals in such a manner as to account for the great quantity of petroleum deposits known is also to be considered. It seems necessary, however, to conclude that in certain cases petroleum and natural gas are largely derived from animal remains of various sorts.
Other hypotheses
The statements which have been made at once suggest an intermediate group of hypotheses which assume a mixed origin for petroleum—animal matter in some cases, vegetable matter in others, or both together. Suggestions of this sort have been made by a large number of writers (Haworth, Erasmus, Kan. Univ. Geol. Surv, vol. 1, 1896; Bownocker, J. A., Geol. Surv. Ohio, 4th ser., Bull. 1, 1903: Blatchley, W. S., Ind. Dept, Geol. and Nat. Hist., 28th Ann. Rept., 1904; McGee, W. J., U. S. Geol. Survey, 11th Ann. Rept., 1891. p. 589; Campbell, M. R., Econ. Geol., vol. 6. 1911, pp. 363-395.), probably the most detailed statement being that of Engler and Hofer (Engler, O., and Hofer, H., Das Erdol, vol. 2, pp, 59-102, 1909. See, also, Engler, O., Petroleum, vol. 7, p, 399, 1912; Bacon, R. F., and Hamor, W. A., The American Petroleum Industry, vol. 1, pp. 24-26, 1916.). This states that petroleum is derived from the natural decomposition in situ of the fatty remains of marine organisms, both animal and vegetable, and indicates the probable chemical stages of the change. Some contend that oils having an asphalt base are derived from animal matter, and that those having a paraffin base from vegetable matter, but little can be advanced in proof. It seems clear that under the proper conditions both plants and animals may supply the essential hydrocarbon constituents of oil and gas.
Differences in the quality of the oils are possibly in part due to differences of capillarity, heat, pressure and extent of the migration of the oil but none of the suggestions along this line have been definitely proved. [Note: Petroleum can be separated by simple filtration through fuller's earth into fractions which differ in density, viscosity and composition, as shown by D. T. Day (Cong. internat. petrole, Paris, 1900, p. 53.] It has been intimated recently by Richardson (Richardson, C., Jour. Ind. Eng. & Chem., vol. 8, p. 4, 1916, cited by Bacon and Hamor, loc. cit., p. 32.) that phenomena of surfaces and films, as demonstrated by recent developments of colloidal chemistry, open an entirely new viewpoint for the interpretation of the origin of petroleum. According to this view the origin of all forms of petroleum must be attributed to surface action between a natural gas and the "sands" (using this term in a general sense) with which it comes in contact.
An interesting and apparently a genetically important correlation between the quality of the oil and the degree of deformation of the containing strata has been made by David White (White, David, Some relations in origin between coal and petroleum: Wash. Acad. Sci., Jour., vol. 5, pp. 189-212, 1915). He has shown that in general the oils associated with the low volatile coals are lighter. This is attributed to the formation of a new very light oil of dynamo-chemical (The word dynamo-chemical is used to include the action of the heat generated by the pressure as well as the pressure itself.) origin which is contributed to the reservoir or else to the dynamic transformation of the old oil.
[Note: The following important conclusions are given by David White (Wash, Acad. Sci., Jour., vol, 5, p. 189, 1915), White has considered the ingredient materials of coals and oil rocks, the biochemical and dynamo-chemical processes of alteration of the organic detritus, its devolatilization, its regional alteration, and the corresponding regional differences in petroleums, and the occurrence of higher-rank oils in regions of greater alteration of the carbonaceous residues. His conclusions are as follows: (1) Petroleum is a product generated in the course of the geodynamic alteration of deposits of organic debris of certain types buried in the sedimentary strata. (2) The quantity and characters of the oils generated are determined by: (a) the composition of the organic deposit at the beginning of alteration; (b) the stage in the progress of this; (c) the elimination of the heavier and more viscous hydrocarbons through filtration incident to migration. It is probable that the composition of the mother organic deposit largely regulates the types of oils; it may account for the nitrogen and sulphur content, color, etc. (3) The rank of the oils is proportional to the degree of alteration of the carbonaceous deposits. (4) The change is marked by concentration of hydrogen in the distillates and of carbon in the residues. (5) Abnormally light oils are in most cases due to filtration. (6) In general, the oils found in successive underlying formations are progressively higher in rank. (7) In regions where the progressive devolatilization of the organic deposits in any formation has passed a certain point (usually 65 to 70 percent fixed carbon) commercial oil pools are not present in that or underlying formations, although gas may occur. (8) Wherever the regional alteration of the carbonaceous residues passes the point marked by 65 to 70 percent of fixed carbon in the pure coals, the light distillates appear in general to be gases at rock temperatures.]
General Conclusions
In conclusion it may be said that nearly all of the proposed theories to account for the origin of petroleum include certain elements of truth; in regard to some of the theories considerable experimental proof and geologic field evidence has been adduced. Hydrocarbons similar to those found in petroleum and natural gas may be formed by chemical processes from entirely inorganic sources. The volcanic hypothesis is supported by the fact that hydrocarbons occur among volcanic emanations, and perhaps by the limited occurrence of hydrocarbons in certain igneous rocks. As pointed out by Clarke (Clarke, F. W., loc. cit., p. 735), however, any attempt to discover the origin of petroleum must take into careful account the quantitative adequacy of the suggested sources. On this basis it seems clear that in none of the known petroleum fields are any of the inorganic hypotheses competent to explain the origin of the oil and gas. The organic origin of petroleum appears to be best maintained by the geologic relations of the hydrocarbons and by a consideration of all the factors involved. On the whole, the Engler-Höfer dual theory has the largest number of adherents. Campbell, who has considered the available evidence in a searching manner (Campbell, M, R., Petroleum and Natural Gas Resources of Canada; Canada Dept. of Mines, Mines Branch, vol. 1. p, 76, 1914), states that the testimony favors the animal origin of most petroleum, although a certain amount has probably been derived from the fatty portions of plants. The factors which must be considered are of extreme variety and complexity, and it is doubtful if any dogmatic statement concerning the origin of the natural hydrocarbons applicable to all occurrences can be made.
Prev Page--Previous Work || Next Page--General Geology
Kansas Geological Survey, Geology
Placed on web Aug. 10, 2018; originally published 1917.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/3/04_petro.html