Prev Page--Petrography || Next Page--Conclusions, References
Coal Petrography as Related to Coal Utilization
As early as 1923, Slopes and Wheeler had observed differences in the banded components of coal with respect to distillation and coking qualities. It is now recognized that the petrographic components of coal have differing chemical and physical properties and that these properties are of importance in effective coal utilization. Some of the physical and chemical properties of the petrographic components are summarized in the following discussion. Since a practical concern of coal petrography is to determine the significance of petrographic differences with regard to various utilization practices, some fields of application are suggested and the significance of mineral matter in coal is evaluated.
Physical Properties
Specific Gravity
Variations in specific gravity inherently due to type differences have been little explored according to McCabe (1945, p. 313). Anthraxylon is usually found to have the lowest apparent specific gravity whereas fusain has the highest. These differences largely reflect the ash content although pure fusain has a distinctly higher specific gravity than the other components. Some application of the property may be found in dust removal since a large part of the dust is composed of fusain. It is also possible that specific gravity differences could be utilized to prepare pure ingredients from Kansas coal should the need arise for a coal with particular properties.
Resistivity
McCabe (1937, p. 277) reports that moisture-free vitrain (anthraxylon) and clarain (translucent attritus) are nonconductors. Fusain, in contrast, exhibited a low restivity. Davis and Younkins (1929) were thus able to separate fusain electrostatically from associated bone and mineral matter in the high specific gravity fraction. No efforts have been made to determine the feasibility of cleaning Kansas coal refuse by this method.
Friability
One measure of the strength of coal is its ability to withstand disintegration during handling. This is called friability and depends on toughness, elasticity, and fracture characteristics as well as on strength. However, the friability test is the measure of strength most frequently used. The work of McCabe (1936) on Illinois coals has shown that the petrographic components differ markedly in friability: fusain is structurally the weakest; vitrain (anthraxylon) is brittle but stronger than fusain; clarain (translucent attriitus) is relatively strqng and nonfriable; and durain (opaque attritus) is the strongest of the group. The counterparts of Thiessen's (1920) classification possess the same relative friability.
This information is of immediate significance in the mining process. For example, a coal bed high in fusain and anthraxylon responds more readily to cutting than does one high in opaque and translucent attritus. One the other hand, mines in splint coals are not as dusty, the proportion of lump is higher, not as much is lost in cleaning, but a larger charge is required in shooting. These factors influence total cost, ultimate recovery, and mine safety.
Another concern is in the operation of washing plants. A coal high in anthraxylon and fusain will produce an excess of fines which have little market value and may cause a plant to operate below capacity.
The most important significance of variable friability lies in the field of coal preparation. McCabe (1936) has shown, from a petrographic study of screenings of the Illinois Herrin (No. 6) Seam coal, that the concentration of certain constituents can be brought about by mechanical processing, thus producing a type of coal concentrate having vastly different characteristics from the bed coal. He reported that, although the vitrain (anthraxylon) content was only 20 percent in the bed coal, in the 1.25-0.75 inch screen size it had increased to 42 percent and reached 79 percent in the minus 48 mesh size. Extended studies have also shown that, in the natural fine sizes of Illinois coals, the vitrain content increases as indicated above but that, in the minus 48 mesh size, the fusain content increases and finally exceeds the vitrain in the minus 100 mesh size.
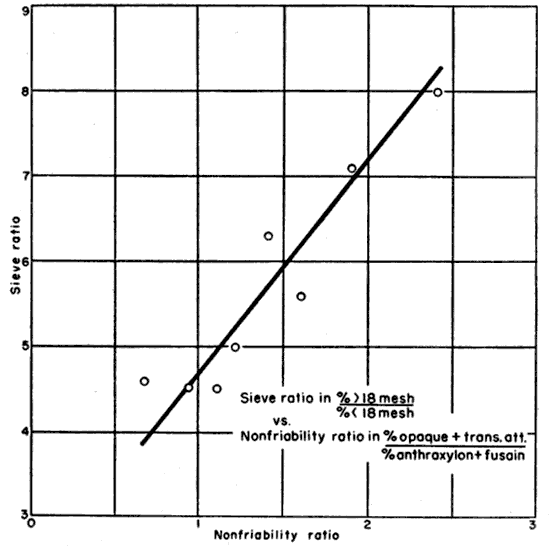
This distribution of vitrain and fusain in the fine sizes of Illinois coals suggested that it might be possible to show the reverse relationship in Kansas coals--e.g., the distribution of fine sizes should be some function of the distribution of anthraxylon and fusain in the bed sample. Therefore, the ratio of opaque attritus plus translucent attritus to anthraxylon plus fusain was calculated as the independent variable for each of the column samples and designated the "nonfriability ratio." These data are tabulated in Table 10. It would have been more desirable to use the inverse relation and designate it the "friability ratio." However, within the range of Kansas coals the distribution of values is too narrow for convenient manipulation.
Table 10--Friability data. Nonfriability ratio--ratio of opaque attritus plus translucent attritus to anthraxylon plus fusain. Sieve ratio--ratio of percent of sample plus 18 mesh to percent minus 18 mesh.
| Sample no. | Anthraxylon, percent |
Fusain, percent |
Nonfriability ratio |
Minus 18 mesh, percent |
Sieve ratio |
|---|---|---|---|---|---|
| Bn-2-B | 54.4 | 5.4 | 0.67 | 17.8 | 4.6 |
| Bn-3-B | 45.5 | 6.1 | 0.94 | 18.2 | 4.5 |
| Ck-4-M | 41.1 | 5.9 | 1.1 | 18.2 | 4.5 |
| Cr-13-B | 49.8 | 10.5 | 1.2 | 16.8 | 5.0 |
| Cr-9-B | 38.3 | 3.5 | 1.4 | 13.1 | 6.3 |
| Cr-6-B | 33.7 | 5.1 | 1.6 | 15.1 | 5.6 |
| Cr-1-C | 21.0 | 13.1 | 1.9 | 12.3 | 7.1 |
| Cr-4-M | 21.3 | 8.2 | 2.4 | 11.1 | 8.0 |
From the range of ratios, eight samples were selected which were representative of the possible values. The samples were crushed to one-half inch size in a Blake jaw crusher, cut to about 200 grams in Jones splitter, and sieved through an 18-mesh screen (1 mm). The minus 18 mesh size was weighed and the percent by weight determined. In order to narrow the spread of points in plotting the data, a ratio of plus 18-mesh size to minus 18-mesh size was calculated as the dependent variable and designated the "sieve ratio." These ratios are tabulated with the nonfriability ratios in Table 10. Figure 5 is the plot of nonfriability ratio versus sieve ratio and shows that there is a linear relation between the two. It is somewhat surprising that the relationship is shown so well since there are variables which have not been considered. For example, it might be expected that fusain would actually contribute more to the fine sizes than anthraxylon. Another difficult factor to evaluate is the mineral content of the coal. In the larger sizes it is probable that the mineral matter would act as a binder and reduce the percentage of fines whereas with finer crushing it might contribute appreciably to the fines. Since the ash content of the samples does not have a range of greater than 5 percent and since all the samples were subjected to standard treatment, it may be that the mineral matter contributed equally to all the samples.
Fig. 5--Distribution of fines as a function of petrographic composition.
Because the petrographic components differ in chemical behavior, their segregation according to relative friability imparts different properties to the various sizes of commercial coal. The ash content, ash-fusing temperature, volatile content, and coking qualities may differ. An understanding of such variations is important to the producer who is interested in maintaining a uniform product and to the consumer who is interested in a coal with particular characteristics. The petrographic composition is thus an important element of coal preparation since different coals can be blended and different sizes of the same coal can be mixed to obtain the desired characteristics.
As an example of practical importance, McCabe, Konzo, and Rees (1942) investigated the possibility of adapting banded ingredients for combustion in underfed domestic stokers since a high anthraxylon content results in excessive caking. Cady (1945, pp. 124-130) reports that other fields of application lie in coking or briquetting since fusain is noncoking and in excess of 15-20 percent acts to weaken the structure of the coke and briquettes.
Chemical Properties
There is much evidence that differences in chemical composition or behavior may be correlated with differing petrographic components. However, the results of such studies are sometimes regarded with reservation due to the inadequacy of chemical methods to determine completely the complex chemical structures. In addition, the petrographic purity of the material studied is not always beyond question.
The general chemical nature of the components of coal is known largely through the research of Thiessen (1947) and his coworkers who have been able to relate coal structures to the structures of modern plants. Pollen, spores, cuticle, and algae are of a waxy and fatty character and hence are resistant to alteration. The waste products or resins of plant materials also fall in this general category. Probably the principal plant materials are lignin or cellulose since they predominate in the cell walls of the higher plants. Cellulose is a relatively unstable compound but it is at least represented by residues. The lignins alter to humic acid or humins.
Proximate and Ultimate Analysis
Sprunk and others (1940, pp. 28-36) report that the average analyses of a number of associated bright and splint coals reveal significant differences if compared on an ash- and moisture-free basis. Splint coals are higher in fixed carbon, generally high in ash and ash-fusion temperature, and have a higher calorific value. Bright coals are lower in sulfur and higher in moisture, hydrogen, nitrogen, and oxygen and may thus have a higher total volatile content. However splints often contain abundant spores and resin so that the low volatile content due to the opaque constituents is increased and may be similar to that of anthraxylon. The higher sulfur and ash of splint coals may be attributed to the influx of clay, pyrite, and other mineral matter. The ash content of fusain is often unusually high because of mineral infiltration into open spaces and high adsorption capacity. Fusain is also lowest in volatiles and highest in fixed carbon. Since the Kansas coals are rather uniform in petrographic composition, it might be expected that their chemical composition would be fairly uniform as shown earlier.
Carbonization
According to Sprunk and others (1940, pp. 37-49), splint coals yield smaller amounts of water of decomposition than associated bright coals and usually a smaller amount of gas. The coal tar and oil products seem to vary in either direction; doubtlessly a reflection of the spore and resin content since these constituents are high in waxes and oil. The relative coking qualities of the different types of coal are not certain. However, the coke yield seems to increase regularly with an increase in fixed carbon in both bright and splint coals. It is known that fusain does not coke and that its presence in large quantities prevents coking of the other ingredients. Hoffmann (1930) has shown that, although there are varieties of vitrain (anthraxylon) which will not coke, generally speaking it forms a better coke than durain (opaque attritus). On the basis of petrographic analysis, it seems that the coke yield of the Mineral, Croweburg, and Bevier coals should be good. However, the high ash and sulfur contents are responsible for an unsatisfactory product.
Chemical Reactivity
Chemically, coals are natural polymers which may be investigated by studies of thermal decomposition in the presence or absence of solvents, oxidation, and reduction reactions. Lowrey (1942, p. 384), in a summary of such studies, shows that chemical reactivity usually decreases from brights to dulls to fusain and that there is no indication of the presence of essentially distinct types of chemical compounds peculiar to any of the ingredients of banded coal. They usually show a gradation of properties from one to the next.
Hydrogenation
Hydrogenation is a reduction reaction which would be discussed under "Chemical Reactivity" except that it merits special attention. In areas of dwindling oil reserves, the conversion of coal to liquid fuel by hydrogenation has long been a subject of intensive investigation. Realizing that such a process might, at some future date, become essential to the power requirements of the United States, the Bureau of Mines has investigated the amenability of various types of coal to hydrogenation. Storch and others (1941) have described the process, the experimental plant, and assays of typical coals.
The essential differences between bituminous coal and petroleum are the higher ratio of the number of carbon atoms to hydrogen atoms in coal; the lower oxygen, nitrogen, and sulfur content of petroleum; and the lower molecular weight of the petroleum molecules. In order to produce petroleum-like material from coal, it is necessary to double the number of hydrogen atoms; to remove most of the oxygen, nitrogen, and sulfur; and to crack the coal molecules until their weight equals that of the petroleum molecules. In 1913, Bergius (1926) discovered how to add hydrogen to coal at a temperature of 450°C. and at 200 atmospheres hydrogen pressure. Under such conditions, most of the oxygen was eliminated as water, the nitrogen as ammonia, and the sulfur as hydrogen sulfide. A petroleum-like liquid resulted. Suitable contact catalysts subsequently were developed to increase the speed of hydrogen addition to the cracked coal.
Fisher and others (1942) have shown, on the basis of small-scale bomb tests of 129 samples, that bright coals are more suitable than splint coals for conversion to liquid products. The petrographic components may be classed into two groups with respect to yield. The first group is easily liquefied and includes anthraxylon and all the organic constituents of translucent attritus, such as woody degradation matter, leaves, spores, pollens, cuticle, and algae. This applies to coal containing less than 89 percent carbon on the moisture-free and ash-free basis. The second group is more difficult to liquefy and includes opaque attritus and fusain. The average yield of opaque attritus from splints is about 60 percent at 430°C. for 3 hours in the presence of stannous chloride with an initial hydrogen pressure of 1,000 psi. Individual samples of opaque attritus, however, show a range in yield from 39 to 79 percent. This was attributed to variation in opacity since the less opaque matter would be expected to hydrogenate more readily. Seven samples of fusain gave a yield ranging from 15 to 27 percent, indicating that it is the most resistant of all the petrographic components.
Considerable success has been attained in selecting the best coals for large-scale tests. Further, with the aid of microscopic analysis, it has been possible to predict the yield of acetone insoluble residue with a fair degree of accuracy. Predictions of this kind are made on the following basis, provided proximate analysis indicates less than 89 percent fixed carbon on a moisture-free and ash-free basis:
| Ash and fusain | Should yield 100 percent residue |
| Opaque attritus | Should yield 38 percent residue |
| All other constituents | Should yield no residue |
The extent to which liquefaction yield from hydrogenation can be correlated with and predicted from petrographic analysis depends upon the accuracy and completeness of the analysis. Better correlations should be obtained when the opacity of the petrographic components is more completely defined by physical criteria. Although the method is not completely accurate, it is adequate for rejecting unsuitable coals. In addition, the concentration of high liquid-yield components by mechanical processing based on petrographic analysis seems to be an imminent possibility.
No experimental hydrogenation yield data are currently available for Kansas coals but the probable yield h^s been calculated according to the method described above. The calculated probable residue yield for each column sample and the average calculated probable yield for the bed are tabulated in Table 11.
Table 11--Predicted hydrogenation residues. Hydrogenation residue determined as follows: opaque attritus, 38 percent; fusain, 100 percent; all other constituents, none.
| Locality no. |
Sample no. |
Translucency ratio |
Predicted yield, percent |
|---|---|---|---|
| Bevier coal | |||
| 19 | Bn-3-B | 6.1 | 9.3 |
| 20 | Bn-2-B | 8.8 | 7.3 |
| 21 | Bn-1-B | 4.7 | 16.6 |
| 22 | Cr-9-B | 11.7 | 5.3 |
| 25 | Cr-5-B | 4.4 | 16.1 |
| 26 | Cr-6-B | 8.2 | 7.4 |
| 27 | Cr-7-B | 11.1 | 5.6 |
| 30 | Cr-12-B | 7.9 | 8.6 |
| 31 | Cr-14-B | 6.0 | 8.6 |
| 32 | Cr-11-B | 8.9 | 7.0 |
| 33 | Cr-13-B | 5.8 | 12.2 |
| 34 | Cr-10-B | 6.9 | 8.5 |
| 35 | Ck-1-B | 6.4 | 8.6 |
| 37 | Lt-1-B | 4.7 | 10.6 |
| Average | 7.2 | 9.4 | |
| Mineral coal | |||
| 1 | Cr-2-M | 13.3 | 5.4 |
| 2 | Cr-4-M | 9.4 | 8.8 |
| 3 | Cr-3-M | 7.7 | 8.9 |
| 9 | Cr-8-M | 4.4 | 13.9 |
| 15 | Ck-5-M | 9.7 | 7.7 |
| 16 | Ck-4-M | 12.7 | 6.5 |
| 18 | Ck-2-M | 13.3 | 6.1 |
| Average | 10.1 | 8.2 | |
| Croweburg coal | |||
| 8 | Cr-1-C | 2.8 | 16.2 |
Since the predicted liquefaction yield is largely a function of translucency, the ratio of anthraxylon plus translucent attritus to opaque attritus plus fusain has been calculated and designated the "translucency ratio." An opacity ratio might have been used except that such values would have a narrow distribution for Kansas coals. This devise provides a simple method for characterizing the translucency or opacity of a coal in terms of a single value and could be used as a basis for describing regional variation.
No attempt has been made to plot regional variation in the Mineral and Bevier coals because the values seem to be erratic on the basis of relatively few data. Certain general observations can be made nevertheless. (1) The Bevier coal has a lower average translucency ratio than the Mineral coal and could thus be expected to produce a lower liquefaction yield. (2) The Mineral coal data are suggestive of a trend which could be substantiated only with additional information. Just north of Frontenac, the coal has a low translucency ratio and hence probably a low liquefaction yield. Both north and south of this low, the ratios increase although there is a considerable interval between this point and the nearest point to the south. (3) The coals are probably amenable to hydrogenation and should produce good yields except for the high ash content which contributes to the total residue. Beneficiation would undoubtedly make them more desirable for this purpose.
Significance of Mineral Matter
The distribution of the mineral matter in the coal is important from the standpoint of beneficiation. It has been shown that the principal minerals are pyrite and calcite which are intimately associated with the coal. The larger quantity of pyrite is finely disseminated and much of the calcite occurs either in fusain or in the finely branching parts of the fractures and cleats. Such a distribution means that the coal must be crushed to a fine size if most of the mineral matter is to be released or that a substantial part of the coal must be rejected as refuse if it is cleaned in a low specific gravity zinc chloride solution. However, some of the pyrite and calcite can be removed in the nodular form. It thus seems that the coals can be improved to a certain extent but that preparation of a low-ash coal concentrate is not economically feasible. In an effort to test this contention, ash determinations were run on two of the minus 60 mesh samples which were the float fractions from the 1.70 specific gravity separation. Ash determinations had also been made on the original samples. In one sample the ash content decreased from 12.0 to 6.6 percent and in the other it decreased from 10.0 to 7.4 percent (decreases of 45 and 26 percent, respectively). In ordinary cleaning it would be impractical to grind the coal so finely or to use such a high-gravity solution.
No effort has been made to determine the effect of the mineral matter content on the chemical and physical properties of the coal. However, Gauger (1936) has been able to show the effect of mineral composition of the slagging characteristics of coal in carbonization studies.
Prev Page--Petrography || Next Page--Conclusions, References
Kansas Geological Survey, Geology
Placed on web November 2005; originally published May 1953.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/102_1/07_util.html