Prev Page--General Discussion--Beverages || Next Page--Waters of Kansas--Sulfate
Part II--The Mineral Waters of Kansas, Arranged and Classified, with Analyses
Waters from the springs and wells referred to in Part II of this report are classified as follows:
- The Chlorid Group.
- The Sulfate Group.
- The Chlor-sulfate Group.
- The Carbonate Group.
- The Chlor-carbonate Group.
- The Sulfid Group.
- The Chalybeate Group.
- The Special Group.
- The Soft-water Group.
See Methods of Classification.
As has been previously stated, it is not possible to make a sharp and perfect classification of the waters, as the ingredients vary in quality and quantity, so that, while in most cases there is no doubt where a water should be classified, there are some waters that belong with equal propriety to two or possibly three groups. In some cases, therefore, a water is mentioned in several groups, but the description of locality, analysis, etc., is given only once, in what appears to be its most proper location. When it occurs in other lists it is bracketed, thus (......).
Chapter X--The Chlorid Group
Waters of this class are generally called" brines," although many of them do not contain a sufficient quantity of sodium chlorid to be workable for salt, or they contain too many foreign ingredients to be of use.
The chlorid waters are obtained from the solution of the salts that have been left after the drying up of some ancient ocean. It is said that they show their relation to the ancient seas rather than the modern, because they contain calcium chlorid or sulfate, as well as chlorids of the alkalies and magnesium chlorid, salts which are present in modern sea water. Waters of this class are frequently called "saline," from the abundance of common salt which they contain, although some have objected to this term as a misnomer, since all mineral waters contain "salts" in the ordinary chemical acceptation of the term.
Since the ocean is the great storehouse of bromids and iodids, we find that these waters often contain both these ingredients. We look here also for waters rich in magnesium. Waters of the chlorid group have found their most important use for bathing purposes, although, diluted with soft water, they may be used internally. Some waters that are classified here as brines are already diluted in nature, and as such may be used internally.
This group is represented by the following waters:
- Abilene, Dickinson county, artesian well.
- Arkansas City, Cowley county.
- Atchison, A. B. C. laundry well.
- Atchison, Becker's well.
- Atchison, diamond drill prospect well.
- Eureka, Greenwood county.
- Fay, Russell county.
- Fredonia, Wilson county.
- Geuda Springs, Cowley county.
- Independence, Montgomery county, Brom-magnesian well.
- Lawrence, Douglas county, artesian well.
- Leavenworth, "Ocean Spray."
- Marion, Marion county, lower vein.
- Mound City, Linn county.
- Mound Valley, Labette county.
- Overbrook, Osage county, No. 2 well.
- Rosedale, Johnson county, Geyser well.
- Saint Paul, Neosho county.
Abilene Artesian Well, Dickinson County
In the fall of 1901 the Abilene Oil and Gas Company drilled a five-inch well, or prospect hole, 1260 feet in depth. A pipe twenty feet long extended above the surface of the ground, and the water rises in the well and overflows this pipe. It was proposed to drive the boring still deeper, but on account of the discovery of such an abundant sale-water supply, the work has been stopped for the present. The water is nearly clear, and has a salt and astringent taste. The reaction is strongly acid, and upon being heated the water becomes yellow from the presence of iron chlorid, and gives off free hydrochloric-acid gas. The analysis is as follows:
| Abilene Artesian Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 56.4721 | Sodium oxid (Na2O) | 76.1080 | |
| Potassium (K) | trace | Potassium oxid (K2O) | trace | |
| Calcium (Ca) | 8.6709 | Calcium oxid (CaO) | 12.1340 | |
| Magnesium (Mg) | 2.7264 | Magnesium oxid (MgO) | 4.5840 | |
| Iron (Fe) | .0801 | Iron oxid (FeO) | .1030 | |
| Manganese (Mn) | trace | Manganese oxid (MnO) | trace | |
| Chlorin (Cl) | 110.2785 | Chlorin (Cl) | 110.2785 | |
| Bromin (Br) | .5152 | Bromin (Br) | .5152 | |
| Iodin (I) | .0063 | Iodin (I) | .0063 | |
| Sulfuric acid ion (SO4) | .1249 | Sulfuric anhydrid (SO3) | .1040 | |
| Silicic acid ion (SiO3) | .0114 | Silicic anhydrid (SiO2) | .0090 | |
| Hydrogen ion | trace | Oxygen equivalent | 24.9853 | |
| Total | 178.8567 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 142.9970 | 8352.454 |
| Sodium bromid (NaBr) | .6633 | 38.743 |
| Sodium iodid (NaI) | .0074 | .432 |
| Potassium sulfate (K2SO4) | trace | trace |
| Calcium chlorid (CaCl2) | 23.9070 | 1396.408 |
| Calcium sulfate (CaSO4) | .1768 | 10.326 |
| Magnesium chlorid (MgCl2) | 10.8870 | 635.910 |
| Iron chlorid (FeCl2) | .2092 | 12.219 |
| Manganese chlorid (MnCl2) | trace | trace |
| Silica (SiO2) | .0090 | .526 |
| Totals | 178.8567 | 10447.018 |
| Specific gravity, 1.112. Analysis by E. H. S. Bailey and F. B. Porter. |
||
Arkansas City Well
This well is located on the farm of Rheinhold Hess, southeast of the city. It is bored to a depth of 250 feet, the last part through a loose gravel and rock. The water is used for bathing and drinking purposes. Arkansas City is on the line of the Frisco, Santa Fe and the Missouri Pacific railroads.
Improvements
The proprietor has built here a small bath-house, in which hot, cold, shower and vapor baths are given. Water escapes continually from the well. In order to get large quantities it is necessary to use a pump. Considerable gas is given off from the water when it is first drawn.
The composition of the water is as follows:
| Grams per liter | ||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 13.1735 | Sodium oxid (Na2O) | 17.1349 | |
| Calcium (Ca) | .3060 | Calcium oxid (CaO) | .4284 | |
| Magnesium (Mg) | .2581 | Magnesium oxid (MgO) | .4302 | |
| Aluminum (Al) | .0141 | Aluminum oxid (Al2O3) | .0266 | |
| Sulfuric acid ion (SO4) | 3.6226 | Chlorin (Cl) | 18.774.0 | |
| Silicic acid ion (SiO3) | .2671 | Sulfuric anhydrid (SO3) | 3.1230 | |
| Silicic anhydrid (SiO2) | .2118 | Less oxygen equivalent | 4.2427 | |
| Total | 36.4862 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Calcium sulfate (CaSO4) | 1.0414 | 60.8282 |
| Magnesium sulfate (MgSO4) | 1.2916 | 75.4423 |
| Sodium sulfate (Na2SO4) | 2.9298 | 171.1290 |
| Sodium chlorid (NaCl) | 30.9850 | 1809.8339 |
| Alumina (Al2O3) | .0266 | 1.5534 |
| Silica (SiO2) | .2118 | 12.3712 |
| Totals | 36.4862 | 2131.1589 |
| Analysis by E. H. S. Bailey. | ||
Atchison County Mineral Waters
In a paper read before the Kansas Academy of Science (Trans. Kans. Acad. Sci., vol. IV, p. 88), Prof. E. B. Knerr says: "The drift in Atchison county is quite uniform in structure, being a heavy compact clay for the most part, with but little sand and gravel intermixed. Water will pass through it very slowly; hence the wells dug into it are deep, as a rule, usually from forty to sixty feet in depth, and the water generally stands quite low, though about three feet of water may generally be counted upon in the dryest months. Such wells at these seasons may easily be pumped dry, but in the course of several hours the water will collect to the depth of a foot or two again. Analysis of this drift water presents nothing of unusual interest.
"There are numerous springs in Atchison county. Where these issue from the limestone they are of interest only as furnishing good, cool drinking water. Several such springs occur within the city limits of Atchison and have always supplied the neighborhoods in the vicinity with water."
The A. B. C. Laundry Well, Atchison
This is an example of a comparatively shallow well yielding salt water, for the depth is only sixty-three feet. Wells 200 feet in depth in this locality generally yield salt water. As an example of water from the deepest well, attention is called to the diamond-drill prospect boring.
This water has the following composition:
| A. B. C. Laundry Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | .4827 | Sodium oxid (Na2O) | .6506 | |
| Potassium (K) | trace | Potassium sulfate (K2SO4) | trace | |
| Calcium (Ca) | .0340 | Calcium oxid (CaO) | .0475 | |
| Magnesium (Mg) | .0067 | Magnesium oxid (MgO) | .0110 | |
| Iron (Fe) | .0231 | Iron oxid (FeO) | .0296 | |
| Chlorin (Cl) | .7450 | Chlorin (Cl) | .7450 | |
| Sulfuric acid ion (SO4) | .0219 | Sulfuric anhydrid (SO3) | .0182 | |
| Silicic acid ion (SiO3) | .0558 | Silicic anhydrid (SiO2) | .0447 | |
| Water of combination (H2O) | .0235 | |||
| Carbonic anhydrid (CO2) | .1152 | |||
| Oxygen equivalent | .1676 | |||
| Total | 1.5177 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 1.2278 | 71.7158 |
| Potassium sulfate (K2SO4) | trace | trace |
| Calcium sulfate (CaSO4) | .0310 | 1.8107 |
| Calcium bicarbonate (CaH2(CO3)2) | .1005 | 5.8702 |
| Magnesium bicarbonate (MgH2(CO3)2) | .0404 | 2.3598 |
| Iron bicarbonate (FeH2(CO3)2) | .0733 | 4.2814 |
| Silica (SiO2) | .0447 | 2.6109 |
| Totals | 1.5177 | 88.6488 |
| Analysis by E. B. Knerr. | ||
Atchison Diamond Drill Prospect Boring
In the summer of 1900 a well 1353 feet in depth, penetrating into the Mississippian limestone for the last thirty-eight feet, was bored at Atchison, for the purpose of investigating the coal seams beneath the city. The total cost of this well was about $4700, which amount was mostly raised by public subscription. The drill, which brought up a 2-inch core, penetrated in all fourteen feet and five inches of coal, a 36,inch seam being found at a depth of 1123 feet, and a 28-inch seam being found at a depth of 1188 feet. Samples of water were taken at different depths, and all proved to be brines. The analysis of the water taken at the greatest depth, which, of course, would be more or less a mixture of all the different streams which found their way into the well, is given herewith. A complete report made by A. E. Langworthy of the boring and log of the well has been preserved, and the core is deposited with the Geological Department of the University (Trans. Kans. Acad. Sci., vol. XVII, pp. 45-52).
The analysis is as follows:
| Atchison Diamond Drill Prospect Boring Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 9.8016 | Sodium oxid (Na2O) | 13.1937 | |
| Potassium (K) | trace | Potassium oxid (K2O) | trace | |
| Calcium (Ca) | .5699 | Calcium oxid (CaO) | .7846 | |
| Magnesium (Mg) | .1231 | Magnesium oxid (MgO) | .2058 | |
| Iron (Fe) | .0618 | Iron oxid (FeO) | .0795 | |
| Chlorin (Cl) | 15.0660 | Chlorin (Cl) | 15.0660 | |
| Iodin (I) | trace | Iodin (I) | trace | |
| Sulfuric acid ion (SO4) | .0188 | Sulfuric anhydrid (SO3) | .0157 | |
| Silicic acid ion (SiO3) | .3560 | Silicic anhydrid (SiO2) | .2812 | |
| Nitric acid ion (NO3) | trace | Nitric anhydrid (N2O5) | trace | |
| Carbonic anhydrid (CO2) | 1.7658 | |||
| Water of combination (H2O) | .3623 | |||
| Oxygen equivalent | 3.4052 | |||
| Total | 28.3494 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 24.8550 | 1451.780 |
| Sodium iodid (NaI) | trace | trace |
| Potassium nitrate (KNO3) | trace | trace |
| Calcium sulfate (CaSO4) | .0266 | 1.554 |
| Calcium bicarbonate (CaH2(CO3)2) | 2.2388 | 130.768 |
| Magnesium bicarbonate (MgH2(CO3)2) | .7518 | 43.912 |
| Iron bicarbonate (FeH2(CO3)2) | .1960 | 11.448 |
| Silica (SiO2) | .2812 | 16.424 |
| Totals | 28.3494 | 1655.886 |
| Analysis by E. H. S. Bailey and F. B. Porter. | ||
Beckel's Mineral Well, Atchison
In the western part of the city, in the valley of the White Clay, on West Main street, a well 125 feet deep has been drilled on the property of Peter Becker. When first drawn the water is surcharged with carbon-dioxid gas and is perfectly clear, but after standing a while the gas escapes and the water becomes turbid on account of the deposition of iron hydrate.
Improvements
The improvements on this property are convenient bathrooms, supplied with the usual facilities for taking hot and cold saline baths.
| Becker's Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 10.1000 | Sodium oxid (Na2O) | 13.6148 | |
| Potassium (K) | .0360 | Potassium oxid (K2O) | .0435 | |
| Ammonium (NH4) | .0200 | Ammonium hydroxid (NH4OH) | .0288 | |
| Calcium (Ca) | .4200 | Calcium oxid (CaO) | .5880 | |
| Magnesium (Mg) | .3100 | Magnesium oxid (MgO) | .5164 | |
| Iron (Fe) | .0420 | Iron oxid (FeO) | .0540 | |
| Chlorin (Cl) | 15.5500 | Chlorin (Cl) | 15.5500 | |
| Sulfuric acid ion (SO4) | 1.1088 | Sulfuric anhydrid (SO3) | .9240 | |
| Phosphate ion (PO4) | .0241 | Phosphoric anhydrid (P2O5) | .0180 | |
| Silicic acid ion (SiO3) | .0228 | Silica (SiO2) | .0180 | |
| Carbonic anhydrid (CO2) | 1.2254 | |||
| Water of combination (H2O) | .2528 | |||
| Oxygen equivalent | 3.5035 | |||
| Total | 29.3300 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Potassium sulfate (K2SO4) | .0804 | 4.6835 |
| Sodium sulfate (Na2SO4) | .0425 | 2.4813 |
| Sodium phosphate (Na2HPO4) | .0060 | 2.1000 |
| Sodium chlorid (NaCl) | 25.6264 | 1495.0000 |
| Ammonium sulfate (NH4)2SO4) | .0730 | 4.2580 |
| Calcium sulfate (CaSO4) | 1.3920 | 81.1950 |
| Calcium bicarbonate (CaH2(CO3)2) | .0431 | 2.5142 |
| Magnesium bicarbonate (MgH2(CO3)2) | 1.8851 | 109.9600 |
| Iron bicarbonate (FeH2(CO3)2) | .1335 | 7.7870 |
| Silica (SiO2) | .0180 | 1.0498 |
| Totals | 29.3300 | 1711.0288 |
| Specific gravity, 1.02007; temperature, 14° C. (57° F.) Analysis by E. B. Knerr. (Trans. Kans. Acad. Sci., vol. XV, p. 89) |
||
Plate 12--Fourth Avenue Hotel, Eureka

Plate 12--Bath-house Hotel, Geuda Springs.

Eureka Mineral Well
In 1887 a well 140 feet deep was bored in Eureka, Greenwood county. At a depth of thirty-six feet rock was struck, and the boring was continued through this for 100 feet, till an abundance of water was found. The water was for several years quite extensively used for bathing and drinking.
Improvements
The well is situated on the grounds of the Fourth Avenue hotel, opposite and directly north of the court-house. Bathrooms are provided, with modern improvements. The property was originally developed by A. P. Cogswell, was later owned by Dr. S. J. Carpenter, and has now passed into other hands. The hotel has accommodations for perhaps twenty guests, but the mineral water is not used extensively at the present time.
Eureka is the county-seat of Greenwood county, and is on the A. T. & S. F. and the Mo. Pac. railroads.
| Eureka Mineral Well (Trans. Kans. Acad. Sci., vol. XII, pp. 28, 29) Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Potassium (K) | .0691 | Potassium (K2O) | .0833 | |
| Sodium (Na) | 2.6983 | Sodium oxid (Na2O) | 3.6369 | |
| Calcium (Ca) | .2591 | Calcium oxid (CaO) | .3626 | |
| Magnesium (Mg) | .1101 | Magnesium oxid (MgO) | .1835 | |
| Iron (Fe) | .0008 | Iron oxid (FeO) | .0011 | |
| Aluminum (Al) | .0009 | Aluminum oxid (Al2O3) | .0017 | |
| Chlorin (Cl) | 4.3919 | Chlorin (Cl) | 4.3919 | |
| Bromin (Br) | .0004 | Bromin (Br) | .0004 | |
| Iodine (I) | .0001 | Iodine (I) | .0001 | |
| Sulfuric acid ion (SO4) | 4.5801 | Sulfuric anhydrid (SO3) | .4834 | |
| Phosphoric acid ion (PO4) | .0006 | Phosphoric anhydrid (P2O5) | .0003 | |
| Boric acid ion (BO4) | trace | Boric anhydrid (B2O3) | trace | |
| Nitric acid ion (NO3) | trace | Nitric anhydrid (N2O5) | trace | |
| Silicic acid ion (SiO3) | .0162 | Silica (SiO2) | .0137 | |
| Organic matter | trace | Carbonic anhydrid (CO2) | .2158 | |
| Water of combination (H2O) | .0409 | |||
| Oxygen equivalent | .9913 | |||
| Total | 8.4243 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Potassium sulfate (K2SO4) | .1272 | 7.4297 |
| Sodium chlorid (NaCl) | 6.8631 | 400.8736 |
| Sodium bicarbonate (NaH2(CO3)2) | trace | trace |
| Sodium nitrate (NaNO3) | trace | trace |
| Sodium bromid (NaBr) | .0005 | .0292 |
| Sodium iodid (NaI) | .0001 | .0058 |
| Sodium phosphate (NaHPO4) | .0006 | .0351 |
| Calcium sulfate (CaSO4) | .7225 | 42.2012 |
| Calcium bicarbonate (CaH2(CO3)2) | .1883 | 10.9986 |
| Magnesium chlorid (MgCl2) | .3107 | 18.1480 |
| Magnesium bicarbonate (MgH2(CO3)2) | .1924 | 11.2382 |
| Iron bicarbonate (FeH2(CO3)2) | .0035 | .2044 |
| Alumina (Al2O3) | .0017 | .0993 |
| Silica (SiO2) | .0137 | .8002 |
| Totals | 8.4243 | 492.0633 |
| Analysis by E. H. S. Bailey. | ||
Fay, Russell County
An artesian well on farm of C. H. Kellogg, called "Artesian Ranch," at Fay, was drilled in 1894 to a depth of 121 feet. It is located on southeast quarter of section 14, township 12, range 15 west, Paradise township. The flow is said to be 1000 barrels in twenty-four hours, and the water has a pressure of fifteen pounds per square inch. The water has not been advertised, but has a local reputation as a valuable medicinal agent.
The following is the composition:
| Fay Artesian Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 4.921 | Sodium oxid (Na2O) | 6.627 | |
| Potassium (K) | .039 | Potassium (K2O) | .048 | |
| Calcium (Ca) | .171 | Calcium oxid (CaO) | .240 | |
| Magnesium (Mg) | .282 | Magnesium oxid (MgO) | .470 | |
| Chlorin (Cl) | 6.742 | Chlorin (Cl) | 6.742 | |
| Sulfuric acid ion (SO4) | 2.068 | Sulfuric anhydrid (SO3) | 1.722 | |
| Carbonic anhydrid (CO2) | .297 | |||
| Total | 16.146 | |||
| Temperature, 13.3° C. (56° F.) ; specific gravity, 1.0109. Analysis by G. H. Failyer. |
||||
Hudson Well, Fredonia
This gas-well is four miles south and one mile west of Fredonia (Trans. Kans. Acad. Sci., vol. XV, pp. 86, 87). The depth is 1175 feet, and the salt water comes in at a depth of 400 feet. The flow is estimated at five barrels per hour. Gas was first struck at a depth of 325 feet, and a second and stronger stratum at a depth of 1150 feet. Oil was also struck at a depth of 1100 feet, in sufficient quantity to pump.
| Hudson Well, Fredonia Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 2.7907 | Sodium oxid (Na2O) | 37.4788 | |
| Calcium (Ca) | 1.4246 | Calcium oxid (CaO) | 2.0147 | |
| Magnesium (Mg) | 2.8443 | Magnesium oxid (MgO) | 4.7398 | |
| Iron (Fe) | .0561 | Iron oxid (FeO) | .0722 | |
| Chlorin (Cl) | 49.2850 | Chlorin (Cl) | 53.6533 | |
| Bromin (Br) | .0790 | Bromin (Br) | .0790 | |
| Iodin (I) | .0084 | Iodin (I) | .0084 | |
| Sulfuric acid ion (SO4) | .0397 | Sulfuric anhydrid (SO3) | .0314 | |
| Silicic acid ion (SiO3) | .0550 | Silicic anhydrid (SiO2) | .0434 | |
| Carbonic anhydrid (CO2) | .1097 | |||
| Water (H2O) | .0224 | |||
| Oxygen equivalent | 12.1307 | |||
| Total | 86.1224 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 70.5133 | 4118.6819 |
| Sodium bromid (NaBr) | .1016 | 5.9344 |
| Sodium iodid (NaI) | .0106 | .6191 |
| Sodium bicarbonate (NaHCO3) | .0414 | 2.4181 |
| Calcium chlorid (CaCl2) | 3.9461 | 230.4918 |
| Calcium sulfate (CaSO4) | .0533 | 3.1132 |
| Magnesium chlorid (MgCl2) | 11.2346 | 656.2130 |
| Iron bicarbonate (FeH2(CO3)2) | .1781 | 10.4029 |
| Silica (SiO2) | .0434 | 2.5349 |
| Totals | 86.1224 | 5030.4093 |
| Analysis by E. H. S. Bailey and H. E. Davies. | ||
Plate 13--The Seven Springs, Geuda Springs.
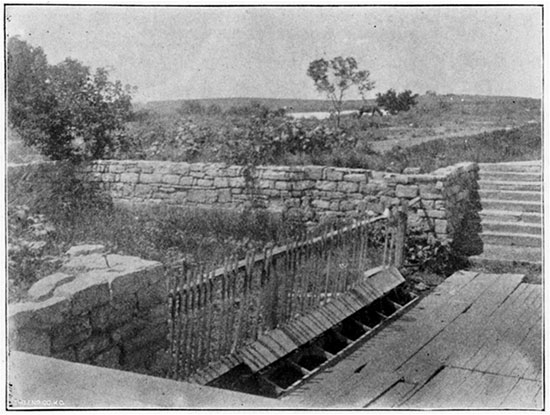
Plate 13--Lake above Geuda Springs.

Geuda Springs
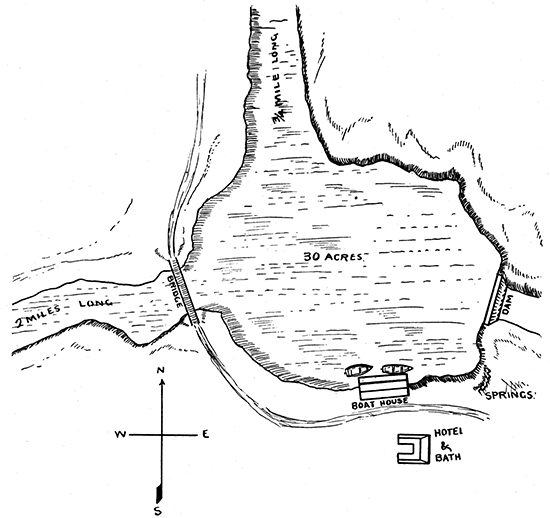
In the south-central part of the state, on the line between Cowley and Sumner counties, is situated a remarkable group of springs that has been known since the earliest settlement of the state. These springs may be easily reached from Arkansas City, seven miles, by a branch of the St. Louis & San Francisco railroad. The town is only eight miles from the undulating plains of Indian Territory. It is about a mile from the Arkansas river, near its confluence with Slate creek. In the vicinity, especially to the north, there are numerous salt springs, so that many of the streams are quite saline in character. A small lake something more than a half-mile long and from five to ten rods wide, in the bed of the creek, was for some time used for boating and fishing. This lake was some years ago artificially improved and enlarged by placing a dam across the creek, making a body of water covering probably fifty acres, the largest salt lake in the state or in the vicinity. On the shores of the lake the salt is crystallized out, and glistens in the sunlight like newly fallen snow. The name "Geuda" is said to come from the Indian word "Ge-u-da," meaning healing springs, and it is believed that this locality was a favorite camping ground with the aborigines.
Improvements
In the year 1886 and for a year or two following, many improvements were made about the town and springs by the Geuda Springs Town and Water Company. A commodious bath-house and hotel combined was built, capable of accommodating forty guests. The dam for the lake was raised, approaches to the springs were improved, drives were laid out, and trees planted. More recently, other improvements have been made, and it is proposed to connect the town with Arkansas City by an electric road.
The owners are building an addition to the bottling works and are putting in a gasoline-engine for pumping, etc., so that now the capacity of the plant is fifty cases of fifty bottles each per day. They also make lemon sour, ginger ale, and other carbonated beverages, which are shipped to points in Kansas and adjacent states.
Salt Lake, Geuda Springs
On the high land west of the springs a beautiful and far-extending view of the vicinity may be obtained, with the village, hotels and stream in the foreground, and in the background the valley of the Arkansas, fading away toward the rich grazing lands of the Territory. The mineral springs at the north end of the principal street are unique in their situation and their properties. They are located in a space not more than twenty-five by thirty-two feet in area, and afford, at all times of the year, an abundant supply of clear water. The flow of each spring is from 100 to 450 gallons per hour. The waters are all brought above the surface by means of large earthen pipes cemented to the rock below, and the overflow is brought out in a series of parallel pipes, as shown in the cut, into a common waste-pipe which carries away quite a stream from the combined waters. The composition of the water, with the temperature and specific gravity, is given below.
Something over two years ago a dam with flume outlet was built across the "salt" depression directly north of the springs, and this caused the whole of the salt marsh to be covered with water, besides backing the water up the creek about two miles, and up the north arm of Salt marsh about three-quarters of a mile. This gives excellent boating for about three-quarters of a mile north and over one and one-quarter miles west from the boat-house. The lake, which, with its arms, covers about fifty acres, in dry seasons becomes quite salty, but the west arm, being a creek, in time of high water flows into the main body of the lake and over the dam, thus leaving mostly fresh water in the lake. This lake presents quite an attraction for boating and bathing. It has also been well stocked with fish.
Plate 14--Hotel Geuda.

Plate 14--Central Hotel and Bath-house, Geuda Springs.

| Geuda Springs No. 1 Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 6.9452 | Sodium oxid (Na2O) | 9.3899 | |
| Potassium (K) | .0163 | Potassium oxid (K2O) | .0196 | |
| Lithium (Li) | trace | Lithium oxid (Li2O) | trace | |
| Calcium (Ca) | 1.0230 | Calcium oxid (CaO) | 1.4322 | |
| Magnesium (Mg) | .1599 | Magnesium oxid (MgO) | .2655 | |
| Iron (Fe) | .0004 | Iron oxid (FeO) | .0006 | |
| Aluminum (Al) | .0006 | Aluminum oxid (Al2O3) | .0011 | |
| Chlorin (Cl) | 10.9284 | Chlorin (Cl) | 10.9284 | |
| Bromin (Br) | .0003 | Bromin (Br) | .0003 | |
| Iodin (I) | trace | Iodin (I) | trace | |
| Sulfuric acid ion (SO4) | 2.8121 | Sulfuric anhydrid (SO3) | 2.3225 | |
| Phosphoric acid ion (PO4) | .0003 | Phosphoric anhydrid (P2O5) | .0002 | |
| Nitric acid ion (NO3) | .0051 | Nitric anhydrid (N2O5) | .0045 | |
| Boric acid ion (B4O7) | .0021 | Boric anhydrid (B4O6) | .0020 | |
| Silicic acid ion (SiO3) | .0131 | Silicic anhydrid (SiO2) | .0104 | |
| Carbonic anhydrid (CO2) | .0399 | |||
| Water (H2O) | .0080 | |||
| Oxygen equivalent | 2.4687 | |||
| Total | 21.9564 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 17.6793 | 1032.6479 |
| Sodium phosphate (Na3PO4) | .0004 | .0237 |
| Sodium bromid (NaBr) | .0004 | .0237 |
| Sodium iodid (NaI) | trace | trace |
| Sodium nitrate (NaNO3) | .0066 | .3860 |
| Sodium bicarbonate (NaHCO3) | .0071 | .4155 |
| Sodium biborate (Na2B4O7) | .0029 | .1693 |
| Potassium sulfate (K2SO4) | .0364 | 2.1261 |
| Lithium chlorid (LiCl) | trace | trace |
| Calcium sulfate (CaSO4) | 3.4233 | 199.9549 |
| Calcium bicarbonate (CaH2(CO3)2) | .0653 | 3.8141 |
| Magnesium sulfate (MgSO4) | .4383 | 25.6011 |
| Magnesium chlorid (MgCl2) | .2836 | 16.5650 |
| Iron bicarbonate (FeH2(CO3)2) | .0013 | .0759 |
| Alumina (Al2O3) | .0011 | .0642 |
| Silica (SiO2) | .0104 | .6074 |
| Organic matter | trace | trace |
| Totals | 21.9564 | 1282.4733 |
| Free carbon dioxid | 34.956 cu. in. |
| Free hydrogen sulfid | 1.018 cu. in. |
| Specific gravity | 1.018 |
| Temperature | 17.3° C. (63.25° F.) |
| Analysis by E. H. S. Bailey and E. C. Franklin. | |
| Geuda Springs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | |||||||
| Grams per liter |
Grains per gallon |
Grams per liter |
Grains per gallon |
Grams per liter |
Grains per gallon |
Grams per liter |
Grains per gallon |
Grams per liter |
Grains per gallon |
Grams per liter |
Grains per gallon |
|
| Sodium chlorid (NaCl) | 18.1122 | 1057.9336 | 13.9865 | 816.9514 | 11.5186 | 672.8014 | 6.1397 | 358,6198 | 7.4112 | 432.8882 | 8.6460 | 505.0128 |
| Sodium phosphate (Na3PO4) | .0004 | .0233 | .0004 | .0233 | .0005 | .0292 | .0007 | .0108 | .0004 | .0233 | .0014 | .0818 |
| Sodium bromid (NaBr) | .0004 | .0233 | .0004 | .0233 | trace | trace | ||||||
| Sodium iodid (NaI) | trace | trace | trace | trace | trace | trace | ||||||
| Sodium nitrate (NaNO3) | .0070 | .4088 | .0031 | .1810 | .0005 | .0292 | .0006 | .0354 | .0006 | .0350 | ||
| Sodium bicarbonate (NaHCO3) | .0055 | .3215 | .0077 | .4500 | .0119 | .6950 | .0084 | .4906 | .0053 | .3095 | .0103 | .6016 |
| Sodium biborate (Na2B4O7) | .0029 | .1691 | .0029 | .1692 | .0015 | .0876 | .0022 | .1285 | .0022 | .1285 | .0043 | .2510 |
| Potassium sulfate (K2SO4) | .0289 | 1.6880 | .0327 | 1.9100 | .0327 | 1.9100 | .0142 | .8294 | .0093 | .5432 | .0082 | .4789 |
| Lithium chlorid (LiCl) | trace | trace | trace | trace | trace | trace | trace | trace | trace | trace | trace | trace |
| Calcium sulfate (CaSO4) | 3.5107 | 205.0599 | 2.8734 | 167.8075 | 2.7145 | 158.5539 | 2.5010 | 146.0834 | 2.5247 | 147.4677 | 2.7466 | 100.4289 |
| Calcium bicarbonate (CaH2(CO3)2) | .1013 | 5.9169 | .1028 | 6.0045 | .1176 | 6.8694 | .1832 | 10.7007 | .2144 | 12.5231 | .0946 | 5.5255 |
| Magnesium sulfate (MgSO4) | .4157 | 4.2810 | .4893 | 28.5800 | .4569 | 26.6875 | .4284 | 25.0228 | .4189 | 24.4679 | .3921 | 22.9025 |
| Magnesium chlorid (MgCl2) | .3424 | 19.9995 | .1717 | 10.0289 | .1354 | 7.9087 | .1449 | 8.4636 | .0850 | 4.9648 | .1658 | 9.6843 |
| Iron bicarbonate (FeH2(CO3)2) | .0030 | .1752 | .0013 | .0759 | .0010 | .0584 | .0007 | .0408 | .0010 | .0584 | .0015 | .0874 |
| Alumina (Al2O3) | .0003 | .0175 | .0158 | .9228 | .0002 | .0116 | .0002 | .0116 | ||||
| Silica (SiO2) | .0148 | .8650 | .0140 | .8172 | .0136 | .7943 | .0136 | .7943 | .0126 | .7359 | .0137 | .8010 |
| Organic matter | trace | trace | trace | trace | trace | trace | trace | trace | trace | trace | trace | trace |
| Sodium sulfid (NaHS) | .0110 | .6425 | ||||||||||
| Total | 22.5455 | 1316.8826 | 17.6862 | 1033.0510 | 15.0200 | 877.3182 | 9.4377 | 551.2555 | 10.6966 | 624.7884 | 12.0853 | 705.9023 |
| Free carbon dioxid (cu. in.) | 27.692 | 18.917 | 17.642 | 23.983 | 22.158 | 29.040 | ||||||
| Specific gravity | 1.016 | 1.012 | 1.012 | 1.009 | 1.009 | 1.009 | ||||||
| Analyses by E. H. S. Bailey and E. C. Franklin | ||||||||||||
Plate 15--View East from Bridge, Geuda Springs.
Plate 15--Sanitarium at Independence.

Bromo-magnesium Well, Independence
In 1884 a well 1100 feet deep was bored in the northern part of Independence, Montgomery county. The tube extends 400 feet from the surface, and a pipe used for drawing the water extends several hundred feet further into the well. The well is artesian ill character, as a small stream flows from it most of the time. Independence is on the lines of the A. T. & S. F. and the Missouri Pacific railroads.
Improvements
A sanitarium and bath hotel have been erected here, with seven bath-rooms, and facilities for using brine either directly or mixed with fresh water. The water is raised to the surface by windmill power. As will be noticed by the analysis which is given below, the well is interesting as containing a comparatively large quantity of bromids. It was the first water of this kind discovered in this region. Others have been found recently to contain bromids and iodids. A comparison of this water with some other well-known waters will be of interest:
| Fabian, N. Y. |
Hawthorne, Saratoga |
Congress, Saratoga |
Dead Sea |
Bromo- magnesium well |
|
|---|---|---|---|---|---|
| Sodium bromid (NaBr) | 4.655 | 1.534 | 8.559 | 156.53 | 13.711 |
| Sodium iodid (KI) | .235 | .198 | .138 | trace | .092 |
Comparing this well with the water of the Atlantic ocean, it is seen to be somewhat similar in composition, though the Independence water contains a larger quantity of calcium salts, twice as much magnesium, more sodium iodid, and nearly one-half as much sodium bromid. The mineral strength of this water is about twice as great as ocean water, as may be seen from the following analysis:
| Bromo-Magnesium Well, Independence Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 23.4678 | Sodium oxid (Na2O) | 31.6278 | |
| Potassium (K) | .1060 | Potassium oxid (K2O) | .1279 | |
| Calcium (Ca) | 2.7620 | Calcium oxid (CaO) | 3.8710 | |
| Magnesium (Mg) | 1.5095 | Magnesium oxid (MgO) | 2.5159 | |
| Iron (Fe) | .0090 | Iron oxid (FeO) | .0117 | |
| Aluminum (Al) | trace | Aluminum oxid (Al2O3) | trace | |
| Chlorin (Cl) | 45.0811 | Chlorin (Cl) | 45.0811 | |
| Bromin (Br) | .1826 | Bromin (Br) | .1826 | |
| Iodin (I) | .0013 | Iodin (I) | .0013 | |
| Sulfuric acid ion (SO4) | .2395 | Sulfuric anhydrid (SO3) | .1995 | |
| Silicic acid ion (SiO3) | .0251 | Silicic anhydrid (SiO2) | .0198 | |
| Organic matter | trace | |||
| Carbonic anhydrid (CO2) | .2998 | |||
| Water (H2O) | .0610 | |||
| Oxygen equivalent | 10.1993 | |||
| Total | 73.8001 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 59.4476 | 3472.433 |
| Sodium bromid (NaBr) | .2351 | 13.733 |
| Sodium iodid (NaI) | .0016 | .093 |
| Potassium sulfate (K2SO4) | .2361 | 13.793 |
| Calcium chlorid (CaCl2) | 7.1872 | 419.806 |
| Calcium sulfate (CaSO4) | .1551 | 9.061 |
| Calcium bicarbonate (CaH2(CO3)2) | .5236 | 30.586 |
| Magnesium chlorid (MgCl2) | 5.9651 | 348.323 |
| Iron bicarbonate (FeH2(CO3)2) | .0289 | 1.684 |
| Alumina (Al2O3) | trace | trace |
| Silica (SiO2) | .0198 | 1.157 |
| Organic matter | trace | trace |
| Totals | 73.8001 | 4310.669 |
| Specific gravity 1.052; Temperature 16.6° C. (62° F.) Analysis by E. H. S. Bailey. |
||
Lawrence, Douglas County, Artesian Well
Several years ago a well was bored on the bank of the Kaw river, northeast of the Santa Fe railroad depot, for the purpose of testing the underlying strata. After it reached the depth of about 1400 feet, the boring was abandoned. A small stream of salt water continually runs from this well. This water is used locally with good results for rheumatism, etc. This water has the following composition:
| Lawrence Artesian Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 4.2160 | Sodium (Na2O) | 5.6898 | |
| Calcium (Ca) | 2.7924 | Calcium oxid (CaO) | 3.9144 | |
| Magnesium (Mg) | 6.3096 | Magnesium oxid (MgO) | 10.5160 | |
| Iron (Fe) | .0481 | Iron oxid (FeO) | .0619 | |
| Chlorin (Cl) | 30.0656 | Chlorin (Cl) | 30.0656 | |
| Bromin (Br) | trace | Bromin (Br) | trace | |
| Silicic acid ion (SiO3) | .1076 | Silicic anhydrid (SiO2) | .0852 | |
| Carbonic anhydrid (CO2) | .0757 | |||
| Water (H2O) | .0154 | |||
| Oxygen equivalent | 6.7945 | |||
| Total | 43.6295 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 10.7147 | 625.845 |
| Sodium bromid (NaBr) | trace | trace |
| Calcium chlorid (CaCl2) | 7.5712 | 452.748 |
| Magnesium chlorid (MgCl2) | 24.9254 | 1455.893 |
| Iron bicarbonate (FeH2(CO3)2) | .1530 | 8.937 |
| Silica (SiO2) | .0852 | 4.976 |
| Totals | 43.6295 | 2548.400 |
| Temperature 18.5° C. (65.5° F.); Specific gravity 1.0355 Analysis by E. Bartow and H. M. Thompson. |
||
Leavenworth Natatorium, Leavenworth County
A natatorium has been established on Third street and Metropolitan avenue, near the United States military reservation, by the Home-Riverside Coal-mining Company, to utilize the waters from their mines. These mines are about 750 feet deep, and the salt water or "ocean spray" is pumped from this depth. The pump for this water has a two-inch discharge and is run from four to six hours per day, to pump the water into cisterns, and from thence to the natatorium.
The building is 41 x 100 feet, with dressing-rooms 15 x 40 feet and 6 x 100 feet. There is a swimming pool 30 x 75 feet, with a depth of from 2 to 7 feet. This place is operated more as a resort than for medical or cleansing purposes. There is such an abundance of water as to admit of a continuous flow through the pool. This natatorium is in operation during the summer months, and last season served about 6000 people.
| "Ocean Spray" (Mine Water), Home-Riverside Coal-Mining Company, Leavenworth |
||||
|---|---|---|---|---|
| Ions | Grams per liter | |||
| Sodium (Na) | 9.2962 | |||
| Calcium (Ca) | .5412 | |||
| Magnesium (Mg) | .2315 | |||
| Iron (Fe) | .0084 | |||
| Chlorin (Cl) | 15.7171 | |||
| Sulfuric acid ion (SO4) | .0362 | |||
| Silicic acid ion (SiO3) | .0548 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 23.6065 | 1378.86 |
| Calcium chlorid (CaCl2) | 1.1753 | 68.66 |
| Calcium bicarbonate (CaH2(CO3)2) | .3386 | 19.78 |
| Magnesium chlorid (MgCl2) | .8793 | 51.36 |
| Magnesium sulfate (MgSO4) | .0453 | 2.65 |
| Iron bicarbonate (FeH2(CO3)2) | .0267 | 1.56 |
| Silica (SiO2) | .0426 | 2.49 |
| Totals | 26.1143 | 1525.36 |
| Analysis by O. F. Stafford. | ||
Marion Mineral Well
The city of Marion and vicinity is rich in springs, some of which are strongly impregnated with mineral matter. The city is on the line of the A. T. & S. F. rail way, and also on the C. R. I. & P. In the northern part of the city, three blocks from the Elgin hotel, a commodious three-story stone building was erected, and used as a sanitarium and bath-house. This was formerly managed in connection with a deep well. This well is about fifty feet from Luta creek, one of the streams that unite to form the Cottonwood river, just below the city. The well was drilled as a prospect hole, and is 175 feet deep, and has connected with it two pumps, one taking the water from a depth of fifty feet, at a point justabove the rock, and the other taking the water from a point, twenty-five feet above the bottom. Both waters contain hydrogen sulfid (H2S) when first drawn. The upper water is utilized for drinking and the other for bathing purposes. The analysis given below shows an important difference between these two waters. The upper water is a saline water while the lower is a concentrated brine stronger even than sea water. For analysis of upper vein, see chapter XII.
| Marion Well (Lower Vein) (Trans. Kan. Acad. Sci., vol. XII, p. 26) Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 22.4131 | Sodium oxid (Na2O) | 31.6871 | |
| Calcium (Ca) | .9898 | Calcium oxid (CaO) | 1.3858 | |
| Magneslum (Mg) | .4663 | Magnesium oxid (MgO) | .7772 | |
| Iron (Fe) | .0031 | Iron oxid (FeO) | .0038 | |
| Chlorin (Cl) | 33.1232 | Chlorin (Cl) | 33.1232 | |
| Sulfuric acid ion (SO4) | 8.3932 | Sulfuric anhydrid (SO3) | 6.9946 | |
| Silica (SiO2) | .0128 | Silica (SiO2) | .0128 | |
| Organic matter | trace | Carbonic anhydrid (CO2) | .0046 | |
| Water (H2O) | .0009 | |||
| Oxygen equivalent | 7.4854 | |||
| Total | 66.5046 | |||
| Analysis by E. H. S. Bailey. | ||||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium sulfate (Na2SO4) | 6.1427 | 358.7951 |
| Sodium chlorid (NaCl) | 54.6433 | 3191.6851 |
| Calcium sulfate (CaSO4) | 3.3648 | 196.5379 |
| Magnesium sulfate (MgSO4) | 2.3316 | 136.1887 |
| Iron bicarbonate (FeH2(CO3)2) | .0094 | .5491 |
| Silica (SiO2) | .0128 | .7476 |
| Totals | 66.5046 | 3884.5035 |
Mound City Well, Linn Connty
No. 1
There is a salt well on the property of Doctor Trego, at Mound City. This well, which is 340 feet deep, was bored with an eightinch drill. The brine comes into the well at a depth of 210 feet, and is forced out at the top of the well by the gas which accompanies it. Since 1886, when the well was bored as a prospect hole, the water has been flowing at the rate of about forty gallons per hour. The brine is used locally for medicinal purposes. This water yields 1020 grains of mineral matter to the gallon, upon evaporation. Of this, 1000 grains is common salt. The reaction of the water is slightly alkaline. Besides the salt, it contains small quantities of sodium carbonate, calcium carbonate, and magnesium carbonate, as well as traces of sulfates, bromids, and iodids.
No. 2
Another salt well, about 400 feet from No. 1, on the property of Robert Fleming, has been recently bored, to the depth of 144 feet, and it has about the same flow as the former. This, as well as the former well, showed thirty-five pounds per square inch of gas pressure. The water is a brine, not as salt as No. 1, however. It contains 719 grains of mineral matter per gallon, and of this, 686 grains is common salt. The other ingredients are carbonates of iron, calcium, magnesium, and sodium, rather more in proportion than No. 1.
Mound Valley, Labette County, Salt Well
At Mound Valley is a salt well 600 feet deep, the water from which was forme:rly used in a health home neal' by, which was built to utilize the water. As it has not been extensively advertised, its use does not extend beyond the immediate vicinity. The water gushes out of the top of the well with sufficient force so that it can be piped to the hotel. The home is pleasantly situated a short distance east of the village, and the latter is easily accessible by two lines of railroad. It has been proposed to utilize the gas that comes up with the water for heating purposes. There are other gas wells in the vicinity which yield gas for local consumption, but the pressure is not very great.Overbrook, Osage County, Well
Atchison Well
There is a well two and one-half miles northwest of the town on the farm of John Atchison. The water has been recommended by some of the local physicians on account of its therapeutic properties. The well is 180 feet deep.
| Overbrook, Atchison Well Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 4.0502 | Sodium oxid (Na2O) | 5.4572 | |
| Calcium (Ca) | .1248 | Calcium oxid (CaO) | .1773 | |
| Magnesium (Mg) | .0817 | Magnesium oxid (MgO) | .1361 | |
| Iron (Fe) | .0515 | Iron oxid (FeO) | .0663 | |
| Chlorin (Cl) | 6.2398 | Chlorin (Cl) | 6.2398 | |
| Sulfuric acid ion (SO4) | .3298 | Sulfuric anhydrid (SO3) | .2745 | |
| Silicic acid ion (SiO3) | .3072 | Silica (SiO2) | .2429 | |
| Carbonic anhydrid (CO2) | .3586 | |||
| Water (H2O) | .0726 | |||
| Oxygen equivalent | 1.4103 | |||
| Total | 11.6150 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 10.2941 | 601.2413 |
| Calcium bicarbonate (CaH2(CO3)2) | .5020 | 29.3218 |
| Calcium sulfate (CaSO4) | .0041 | .2394 |
| Magnesium sulfate (MgSO4) | .4082 | 23.8488 |
| Iron bicarbonate (FeH2(CO3)2) | .1637 | 9.5640 |
| Silica (SiO2) | .2429 | 14.1877 |
| Totals | 11.6150 | 678.4030 |
| Analysis by E. H. S. Bailey and Watson Sellards. | ||
Geyser Mineral Bath-house, Rosedale
Plate 16--Blazing's Artesian Wells, Riley County.

Plate 16--Geyser Mineral Well, Rosedale.

A well about 300 feet deep was bored here several years ago for the purpose of getting gas. Well is situated on the south side of the rather narrow valley of Turkey creek, just north of the stream, at a point where it runs close to the high bluffs on the south. The water is pumped from the well and stored in large wooden tanks in the second story of the building.
Improvements
A few years ago a bathing establishment was erected here, and it receives good patronage. The bath-house is divided into two sections, so as to accomodate both men and women. There are about a dozen bath-rooms, with cots for resting or sleeping in adjacent rooms.
There is a sufficient quantity of gas arising from the well, so that it is stored in tanks and used for running a gas-engine for pumping the water. The gas is also burned under the boiler for generating steam for heating purposes. There is sufficient gas in the water so that it effervesces like soda-water, and if the stopper be taken out of a bottle that has been nearly filled with water, the gas may be lighted at the mouth. The gas has practically no odor, and the water gives no reaction for hydrogen sulfid. This mineral spring company also manufactures "pop" and effervescent drinks from ordinary water.
| Geyser Mineral Well, Rosedale, Johnson County Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium(Na) | 9.1991 | Sodium oxid (Na2O) | 12.3977 | |
| Calcium (Ca) | .2975 | Calcium oxid (CaO) | .416,1 | |
| Magnesium (Mg) | .1890 | Magnesium oxid (MgO) | .3155 | |
| Barium (Ba) | .0193 | Barium oxid (BaO) | .0216 | |
| Strontium (Sr) | .0020 | Strontium oxid (SrO) | .0024 | |
| Aluminum (Al) | .0095 | Aluminum oxid (Al2O3) | .0178 | |
| Iron (Fe) | .0032 | Iron oxid (FeO) | .0042 | |
| Chlorin (Cl) | 14.1400 | Chlorin (Cl) | 14.1400 | |
| Bromin (Br) | .0260 | Bromin (Br) | .0260 | |
| Iodin (I) | .0010 | Iodin (I) | .0010 | |
| Silicic acid ion (SiO3) | .0139 | Silicic anhydrid (SiO2) | .0110 | |
| Carbonic anhydrid (CO2) | 1.3698 | |||
| Water (H2O) | .2822 | |||
| Oxygen equivalent | 3.1981 | |||
| Total | 25.8070 | |||
Hypothetically combined as follows:
| Grams per liter |
Grains per gallon |
|
|---|---|---|
| Sodium chlorid (NaCl) | 23.3362 | 1363.067 |
| Sodium bromid (NaBr) | .0334 | 1.951 |
| Sodium iodid (NaI) | .0012 | .070 |
| Calcium bicarbonate (CaH2(CO3)2) | 1.2046 | 70.361 |
| Magnesium bicarbonate (MgH2(CO3)2) | 1.1516 | 67.265 |
| Barium bicarbonate (BaH2(CO3)2) | .0365 | 2.132 |
| Strontium bicarbonate (SrH2(CO3)2) | .0048 | .280 |
| Iron bicarbonate (FeH2(CO3)2) | .0104 | .607 |
| Alumina (Al2O3) | .0178 | 1.040 |
| Silica (SiO2) | .0110 | .643 |
| Totals | 25.8075 | 1507.416 |
| Specific gravity 1.018; Temperature 15.5°C. (60°F.) Analysis by E. H. S. Bailey. |
||
Saint Paul, Neosho County
A well 700 feet deep was bored at this place. The well is located on the second bottom of the Neosho river and produces considerable gas, which bubbles up with the water.
| Saint Paul Deep Well (Trans. Kan. Acad. Sci., vol. XV, p. 87) Grams per liter |
||||
|---|---|---|---|---|
| Ions | Radicals | |||
| Sodium (Na) | 10.8198 | Sodium oxid (Na2O) | 14.5554 | |
| Calcium (Ca) | .4652 | Calcium oxid (CaO) | .6514 | |
| Magnesium (Mg) | .3410 | Magnesium oxid (MgO) | .5684 | |
| Iron and aluminum (Fe and Al) | .0498 | Iron and aluminum oxids (Al2O3 and Fe2O3) |
.0640 | |
| Chlorin (Cl) | 17.1240 | Chlorin (Cl) | 17.1240 | |
| Sulfuric acid ion (SO4) | .0224 | Sulfuric anhydrid (SO3) | .0187 | |
| Silicic acid ion (SiO3) | .0215 | Silicic anhydrid (SiO2) | .0170 | |
| Temperature 12.7° C. (55° F.) Analysis by E. H. S. Bailey and H. E. Davies. |
||||
Comparison of Similar Waters
Waters of the chlorine group or brines are common in very many parts of the world. For comparison, it is of interest to notice the analysis of some typical waters in the United States and abroad; the results in all cases are expressed in grains per gallon.
Sulpho-magnesium Well, Excelsior Springs, Mo.
| Analysis by E. H. S. Bailey | |
|---|---|
| Sodium chlorid | 644.554 |
| Sodium bromid | 1.050 |
| Sodium iodid | 0.840 |
| Sodium bicarbonate | 1.994 |
| Sodium hydrosulfid | 0.192 |
| Sodium sulfate | 5.248 |
| Potassium sulfate | 1.376 |
| Calcium bicarbonate | 49.768 |
| Magnesium bicarbonate | 5.686 |
| Magnesium sulfate | 23.566 |
| Iron bicarbonate | 0.869 |
| Silica | 0.647 |
| Total | 735.790 |
| Carbon-dioxid gas, abundant. Hydrogen sulfid gas, a trace. |
|
Moorman's Mineral Well, Ypsilanti, Mich.
| Analysis by F. M. Shepard | |
|---|---|
| Sodium chlorid | 1,573.62 |
| Sodium sulfid | 8.42 |
| Potassium sulfate | 35.33 |
| Calcium sulfate | 175.65 |
| Calcium carbonate | 57.26 |
| Calcium chlorid | 143.35 |
| Magnesium chlorid | 128.09 |
| Magnesium bromid | 10.97 |
| Magnesium sulfate | 103.76 |
| Silica | 19.81 |
| Total | 2,256.26 |
| Hydrogen-sulfid gas, 26.84 cubic inches. | |
Upper Blue Lick Springs, Nicholas County, Kentucky
| Analysis by T. F. Fudge and A. Fennel | |
|---|---|
| Sodium chlorid | 516.53 |
| Potassium chlorid | 1.80 |
| Potassium sulfate | 12.97 |
| Calcium carbonate | 25.06 |
| Calcium sulfate | 44.13 |
| Magnesium bromid | 3.80 |
| Magnesium iodid | 0.15 |
| Magnesium chlorid | 37.72 |
| Magnesium carbonate | 0.14 |
| Alumina, lime phosphate, iron peroxid | 1.96 |
| Silica | 1.00 |
| Loss on ignition | 14.88 |
| Totals | 660.14 |
| Carbon-dioxid gas, 48.16 cubic inches. Hydrogen sulfid gas, 8.16 cubic inches. |
|
Harrowgate, England. "Montpelier," Strong.
| Analyzed by A. W. Hoffman | |
|---|---|
| Sodium chlorid | 642.472 |
| Sodium sulfid | 11.528 |
| Potassium chlorid | 4.600 |
| Calcium carbonate | 19.344 |
| Calcium sulfate | 0.472 |
| Calcium chlorid | 49.528 |
| Magnesium chlorid | 43.736 |
| Silica | 1.472 |
| Total | 773.152 |
| Carbon-dioxid gas, 11.208 cubic inches Oxygen gas, 0.384 cubic inches Nitrogen gas, 3.856 cubic inches Marsh gas, 0.424 cubic inches |
|
Kreuznach, Oranienquelle, Rhenish Prussia
| Analyzed by Liebig | |
|---|---|
| Sodium chlorid | 869.640 |
| Potassium chlorid | 3.680 |
| Calcium carbonate | 2.040 |
| Calcium chlorid | 181.992 |
| Magnesium carbonate | 1.040 |
| Magnesium bromid | 14.240 |
| Magnesium iodid | 0.096 |
| Ferrous carbonate | 2.848 |
| Aluminum phosphate | 0.760 |
| Silica | 7.992 |
| Total | 1,084.328 |
Comparison of Abilene Artesian Well with some other Waters
| Atlantic ocean |
Dead Sea | Hutchinson brine |
Abilene artesian well |
Bromo- magnesium well |
|
|---|---|---|---|---|---|
| Sodium chlorid | 1671.34 | 6702.73 | 15978.640 | 8352.456 | 3472.433 |
| Potassium chlorid | 682.63 | ||||
| Ammonium chlorid | 3.35 | ||||
| Calcium chlorid | 1376.75 | 13.200 | 1396.408 | 419.806 | |
| Magnesium chlorid | 199.66 | 4457.23 | 143.105 | 685.909 | 848.328 |
| Aluminum chlorid | 31.37 | ||||
| Iron chlorid | 1.50 | 12.219 | |||
| Iron bicarbonate | 1.684 | ||||
| Manganese chlorid | 3.35 | ||||
| Sodium bromid | 31.16 | 156.53 | 38.743 | 13.733 | |
| Potassium sulfate | 108.46 | 13.793 | |||
| Magnesium sulfate | 34.99 | ||||
| Calcium sulfate | 93.30 | 38.07 | 341.990 | 10.327 | 9.061 |
| Sodium iodid | 0.432 | 0.093 | |||
| Calcium bicarbonate | 30.586 | ||||
| Organic matter | 34.59 | trace | |||
| Insoluble residue | 1.460 | 0.525 | 1.157 | ||
| Totals | 2138.91 | 13488.10 | 16478.395 | 10447.019 | 4310.669 |
| Specific gravity | 1.0275 | 1.172 | 1.112 | 1.052 |
Comparison of the Most Important Constituents of the Waters of the Chlorid Group
| Grains per gallon | |||||||
|---|---|---|---|---|---|---|---|
| Name | Total solids |
Sodium chlorid |
Calcium chlorid |
Calcium bicarbonate |
Calcium sulfate |
Magnesium chlorid |
Magnesium sulfate |
| Abilene artesian | 10447 | 8352 | 1396 | 10 | 365 | ||
| Arkansas City | 3131 | 1809 | 60 | 75 | |||
| Laundry, Atchison | 88 | 71 | 5 | 1 | |||
| Prospect, Atchison | 1655 | 1451 | 130 | 1 | |||
| Becker's, Atchison | 1711 | 1495 | 2 | 81 | |||
| Eureka | 492 | 400 | 11 | 42 | 18 | ||
| Fredonia | 5030 | 4118 | 230 | 3 | 656 | ||
| Geuda, No. 1 | 1282 | 1032 | 3 | 199 | 16 | 25 | |
| Bromo-magnesium | 4310 | 3472 | 419 | 30 | 9 | 348 | |
| Ocean Spray | 1525 | 1378 | 68 | 19 | 51 | 2 | |
| Marion | 3884 | 3191 | 196 | 136 | |||
| Overbrook | 678 | 601 | 29 | 23 | |||
| Geyser | 1507 | 1363 | 70 | ||||
| Excelsior Springs, Mo. | 735 | 644 | 49 | 23 | |||
| Ypsilanti, Mich. | 2256 | 1573 | 143 | *57 | 175 | 128 | 103 |
| Blue Lick, Ky. | 660 | 516 | *25 | 44 | 37 | ||
| Harrowgate, England | 773 | 642 | 49 | 19 | 43 | ||
| Kreuznach, Germany | 1084 | 869 | 181 | 2 | |||
| *Calcium chlorid | |||||||
These waters are evidently of different degrees of dilution, although common salt is a characteristic ingredient of all of them. Calcium salts are also present in most of them, although represented in various combinations. Calcium sulfate, as would be expected when we consider the origin of the waters from evaporated ocean water, is usually present. When the magnesium salt is not reported as chlorid or sulfate, magnesium bicarbonate is usually considered as being present. It is of interest to notice that iron bicarbonate, sometimes as much as eleven grains in a gallon, is found in these waters. The same thing is noticed in the saturated brines that are pumped up for the manufacture of salt. When these waters are used internally, it is evident that the iron salts present must have an important influence on the system.
The Arkansas City water is reported as containing 171 grains of sodium sulfate per gallon, so it would have the added properties of this cathartic salt. The Marion well is still richer in this substance, as it contains 358 grains per gallon. The presence of bromids and iodids in many of the chlorid waters has already been referred to, and, indeed, calls for a classification sometimes in the special group.
A glance at the table will show that the Kansas waters compare favorably in quantity of constituents and in variety with waters of the same class found elsewhere.
Prev Page--General Discussion--Beverages || Next Page--Waters of Kansas--Sulfate
Kansas Geological Survey, Geology
Placed on web April 7, 2017; originally published 1902.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/Vol7/12_chlorid.html