Prev Page--Mining Systems || Next Page--Value, Commerce
Chemical Properties of Kansas Coals
The following details relating to the chemical composition of coal, representing the various mining districts referred to in the present report, will be presented in the same order as the geological descriptions have been presented, namely: beginning with the Cherokee and closing with the Osage City and Burlingame shales. As will be seen on examining the lists of locations, from which samples for analysis have been obtained, only the more prominent ones are given. Most of these are located in the eastern part of the state, and especially that part lying within the Coal Measures proper. A large number of localities scattered over the eastern and central portions of the state, where coal is mined on a very limited scale principally for home consumption, are not represented here.
In the following series of determinations the greatest care has been exercised both in sampling and analyzing.
Sampling
The samples analyzed were obtained directly from the mines by the writer in person. In many instances samples were selected in the pit, which were put with samples taken from the cars at the mine, a peck of average samples being selected, representing most of the varieties, such as nut, lump, and egg. These samples were boxed and put away in a dry building where they were left for several months, in some cases from six to eight months. The coal was then removed from the boxes and broken up into pieces about the size of walnuts, then thoroughly mixed and spread out on a level surface. Pieces were then selected—not according to their appearance but according to position, that is picked uniformly from all over the lot. The selected portion was then put into a hand mortar and ground tolerably fine. After thoroughly mixing this finely ground coal, about one-half pint was taken as a fair representative of the coal from the mine in question. The resulting one-half pint was again put into the mortar and ground until it would pass through a sieve, 1000 meshes to the square inch, giving a very fine powder.
This method of sampling, while it is not an exact average of the mine run coal, yet is a fair mean of the coal produced. Not as large a percent of sulphur and mineral matter will be found in coals thus chosen as in the marketable product, since there seems to be no systematic method of removing the gross impurities by the mine operators in general; only a few of the better equipped mines remove these impurities with anything like system.
The analyses herein given will probably differ somewhat from analyses made elsewhere. But it must be borne in mind that the method of sampling IS one of the chief causes of this discrepancy, and also that the coal produced, by one and the same mine, will constantly vary, which fact is especially true of certain localities.
Kinds of Coal
There are three varieties of coal found in the state, namely: (1) Bituminous, (2) Semi-Anthracite, and (3) the Lignite of the Cretaceous and Tertiary deposits of central and western Kansas.
The bituminous coal deposits of the Lower Coal Measures yield the great bulk of coal placed upon the market in Kansas. The semi-anthracite and the peacock coals are not of very much value commercially. The semi-anthracite is a variety harder than bituminous and is found 10 localities generally near the Missouri state line, showing traces of orographic movements of coal and associated strata. The bituminous coals of the Upper Coal Measures are not, at present, very extensively mined; while the lignite of the Cretaceous and Tertiary areas is mostly consumed by local trade.
Chemical Analysis
As the percent of fixed carbon and volatile products is a basis of comparison for coal, it is not necessary to find the actual percent of constituents of the coal, such as carbon, oxygen, nitrogen, sulphur, hydrogen, iron, etc. The ordinary method is to find the actual percent of substances eliminated under similar conditions, thus affording a convenient and valuable means of comparison.
The percent composition of the ash may be determined, very readily, by the usual analytical method employed with ores, minerals, etc. The volatile sulphur must be determined by special methods, and separately, as it is driven off with the volatile matter. The determinations made are:
| Substance | How Determined |
|---|---|
| 1. Moisture | By weight |
| 2. Volatile and Combustible matter | By weight |
| 3. Fixed Carbon | By weight |
| 4. Ash | By weight |
| 5. Sulphur (volatile) | By analysis |
| 6. Sulphur (fixed) | By analysis |
| 7. Iron as Fe2O3 | By analysis |
| 8. Iron as Fe | By analysis |
| 9. Phosphorus | By analysis |
Methods of Analysis
Determination of Moisture
One gram of coal is heated, in an air-bath, to 110° C., in an uncovered crucible—platinum is best—for fifteen minutes, is then cooled in a desiccator and weighed as quickly as possible, is again heated for ten minutes, then cooled and weighed. This operation is repeated to constant weight, or until the weight begins to increase.
Determination of Volatile and Combusttble Matter
Weigh out one gram of undried coal (the air-dried sample should be used in all cases), place in a platinum crucible and cover closely, heat for about three and five-tenths minutes over a Bunsen burner, then transfer to blow-pipe flame without cooling, for the same length of time. The crucible is then cooled in a desiccator and weighed. The weight thus obtained subtracted from the original weight gives the volatile and combustible matter and moisture. The percent of moisture being known, taken from the combined weight of volatile and combustible matter and moisture, the remainder will be the volatile and combustible matter. Several such tests may be conducted at one and the same time, as but one weighing is required.
Determination of Fixed Carbon
To determine the fixed carbon, the crucible as last weighed, after determining the volatile and combustible matter, may be used. After all the volatile matter has been driven off the residue will consist entirely of fixed carbon and ash. The method of procedure must, therefore, be one which will produce a complete combustion of all the carbon remaining in the crucible. After such a combustion another weighing should be made, and the difference in weight of the crucible, after the volatile matter has been driven off and after the carbon has been completely oxidized, represents the weight of the fixed carbon. Care should be taken, however, not to heat the ash to too high a temperature as some of it may be volatilized.
To produce such an oxidation one of two general methods may be employed, either of which will give correct results if properly applied. These methods are: 1st, burning in free oxygen; 2d, burning in the air. Choice of the two methods should depend upon facilities at the command of the analyst; in the analyses of the Kansas coal free oxygen was employed in the combustion. The process may be described briefly as follows:
The oxygen was retained in an ordinary gas receiver, from which it was drawn through a washing apparatus to produce pure oxygen, and finally delivered through a flexible rubber tube, into the free end of which was inserted a glass tube with the outer end drawn out so as to form a small opening. The valve was then opened until a proper flow of gas was obtained. While the crucible was still red hot from a Bunsen burner the current of oxygen was carefully directed against the residue in the crucible. In this way complete oxidation of all the carbon in the crucible may be obtained in a short time. A common method of applying the oxygen in such analyses is by the use of a crucible lid through which a small tube projects. The rubber supply tube is connected with this tube on the outside, the lid placed on the crucible, when the operation can be conducted about as mentioned above. Under such circumstances it is necessary to avoid a too rapid oxidation, or little explosions may take place with sufficient violence to throw Some of the material out of the crucible, producing thereby a loss which would destroy the value of the analysis.
If the analyst should find it difficult to obtain oxygen for such a combustion equally good results may be obtained by simply heating the crucible over a Bunsen burner until the fixed carbon is entirely oxidized by the oxygen in the air. It is difficult to obtain a proper circulation of the air within the crucible and therefore to obtain a complete combustion by such a process. Where time is of any considerable value and the number of analyses to be made is great the analyst would be justified in going to a considerable expense to obtain the oxygen in order that the combustion may be carried on more rapidly than can possibly be done by using the oxygen of the air.
Determination of Ash
If from the last weight the weight of the crucible be taken the resulting weight will be the ash.
From the ash the percent of iron may readily be determined. Most of the ash can easily be removed from the crucible, and, if any adheres to the sides and bottom it can be removed by filling the crucible one-half full of nitric acid, HNO3, or sulphuric acid, H2SO4 and gently heating it. When dissolved it may be rinsed into a beaker, into which the remainder of the ash is placed. From the resulting solution the percent of iron as Fe and Fe2O3. may be found.
Determination of Total Sulphur
Treat finely, powdered coal with concentrated nitric acid, evaporate to dryness, dilute with water, digest well and, filter, wash residue thoroughly, then to filtrate add, barium chloride, BaCl2, which precipitates barium sulphate, BaSO4. Filter dry, and weigh the precipitate as barium sulphate. from which the percent of sulphur may be obtained.
Determination of Fixed Sulphur
Heat one gram of powdered coal in a platinum crucible: first over a Bunsen burner, then over the blow-pipe flame until all the volatile and combustible matter is driven off. The residue remaining is ash and fixed carbon, which contains the fixed sulphur. Treat the residue with concentrated nitric acid, HNO3, as in the determination of total sulphur, the resulting barium sulphate, BaSO4 will give the percent of fixed sulphur.
Determination of Volatile Sulphur
The difference between the percent of total sulphur and fixed sulphur will give the percent of volatile sulphur.
Determination of Iron, as Fe and Fe2O3
Treat one gram of powdered coal with hydrochloric acid to dissolve the iron, to this add sulphuric acid, and evaporate in an air-bath, until fumes of SO2 and SO3 (the di- and tri-oxides of sulphur) are driven off, then cool and add, with the greatest care, warm water. Rinse the resulting solution into a 200 c.c. flask, and treat with amalgamated zinc and platinum—to reduce the Fe2(SO4)3 to FeSO4, or from the -ic to the -ous form.
Test a portion of a drop with potassium sulphocyanide, KCNS, to see if all the iron exists in the -ous form. If all the iron exists in the ferrous form, titrate with potassium permanganate, K2Mn2O3, until the liquid turns red (light).
For illustration, say that the standard solution of potassium permanganate would oxidize .0050111 parts of iron, that is 1 c.c. of K2Mn2O3 oxidizes .0050111 parts of iron. Multiplying .0050111 by the number of cubic centimeters of K2Mn2O3 employed in a titration we get (by multiplying by 2 or dividing by 1/2) the percent of iron, Fe. In order to find Fe2O3, divide by .7 after multiplying by the cubic centimeters of K2Mn2O3, and then multiplying by 2, which gives Fe2O3. From the above it will be seen that in the same operation both the percent of iron as Fe and Fe2O3 may be determined. The following is a problem illustrating this:
0.005011 Strength of standard solution of K2Mn2O3.
3.500000 c.c, of K2Mn2O3 required to oxidize the iron solution.
0.005011 times 3.50 = .017538, which divided by .7 and multiplied by 100, and the product divided by 1/2, gives as a quotient 5.011, which is the percent of Fe2O3.
.005011 X 3.5 = .017538. .017538 ÷ .5 = .03506: .03506 X 100 = 3.506, which is the percent of Fe.
Determination of Phosphorus
Two methods were employed: (a) One method starts with powdered coal, before it has passed through any other process (b) The other method has to do simply with the ash. As most of the phosphorus exists as phosphates, being combined with inorganic parts of the coal, and as little or none of it passes off in the combustion of the volatile and combustible matter and fixed carbon, the latter is a method which seems most applicable.
Methods in Detail—(a) The powdered coal is digested with concentrated HNO3, is then filtered. and precipitated with NH4OH, which throws down the iron, Fe, aluminum, Al, etc. The precipitate is then dissolved with HNO3 precipitated with ammonium molybdate (NH4)2MoO4, dissolved again with ammonia, NH4OH, and finally precipitated with "magnesium mixture," the resulting compound being magnesium pyro-phosphate, MgS2O4, from which the percent of phosphorus may readily be determined,
(b) Treat the ash with concentrated hydrochloric acid, HCl, dissolve the residue and add sulphuric acid, HSO4, evaporate until sulphur di-oxide, SO2 fumes are given off, then precipitate with ammonium chloride, NH4Cl, and ammonium hydrate, NH4OH, and filter, dissolve in HNO3. Precipitate again with NH4Cl and NHPH and dissolve in HNO3, then precipitate with (NH4)2MoO4, dissolve the precipitate in NH4OH and precipitate with magnesium mixture. Magnesium-pyro-phosphate is thrown down, which is very soluble in water, therefore the solution must be kept concentrated.
There is little or no danger of the silicon, SiO2, being thrown down in this method as the precipitate for phospho-molybdate is so small. The solution must be cool before adding the magnesia mixture. From the magnesium-pyro-phosphate the percent of phosphorus may be obtained.
Specific Gravity
The specific gravity of coal may be determined by the ordinary method employed with insolubles, except that the coal must . be soaked in water for at least 12 hours previous to taking specific weight, in order to drive out all air from the crevices and cracks. Pieces weighing from .2 and .5 grams were taken.
Remarks
Many of the same coals, the analyses of which are given above, were analyzed by Prof. E. H. S. Bailey, of the University of Kansas, some years since. His results were published in the Transactions of the Kansas Academy of Science, Vol. XI, page 49, 1887-'88, and will be reproduced here for comparison.
He says: "If the water and ash are eliminated in the calculations from the above results, as is suggested in a recent report of the Pennsylvania Geological Survey, the coals of Kansas will be divided into five groups. In the first group are included the Cherokee coals; in the second, Fort Scott, Leavenworth and Linn counties; in the third, Osage county; in the fourth, Franklin county; and in the fifth, Cloud county." This arrangement agrees perfectly with our results, see Plates LII and LIII and accompanying text, and bears us out in saying that the value of coal, taking the percent of fixed carbon as the basis of comparison, decreases in passing from the southeast to the north and west.
Table IV—Showing chemical analyses of Kansas Coals
| Number | Name and location of mining company | Moisture in coal |
Volatile and combustible matter | Fixed carbon |
Total sulphur |
Fixed sulphur |
Volatile sulphur |
Iron as Fe2O3 | Iron as Fe | Specific gravity |
Color of ash | Form of ash | Ash | Phosphorus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Missouri, Kansas & Texas Mineral City |
2.15 | 32.42 | 58.38 | 1.007 | 0.245 | 0.752 | 5.011 | 3.5077 | 1.338 | Grayish pink | Light and flaky | 7.05 | Trace. |
| 2 | James O'Neil Cherokee |
1.35 | 36.85 | 52.40 | 2,678 | 0.403 | 2.275 | 5.001 | 3.5087 | 1.399 | Light reddish gray | Quite light and flaky | 9.40 | Trace. |
| 3 | Fidelity Land and Improvement Co. Cherokee |
1.96 | 40.62 | 53.30 | 1.460 | 0.235 | 1.225 | 1.234 | Dark gray | Light and loose | 5.12 | Trace. | ||
| 4 | Columbus Coal Co. Stepville |
2.13 | 36.71 | 57.55 | 0.681 | 0.210 | 0.474 | 5.001 | 3.5087 | 1.278 | Light bluish gray | Coarse ash and clinker shells | 3.61 | Trace. |
| 5 | Central Coal and Coke Co. Scammon |
3.03 | 33.,77 | 57.48 | 1.894 | 0.549 | 1.345 | 1.322 | Very dark colored | Flaky | 5.72 | Trace. | ||
| 6 | Durkee Coal Co., No. 4 Scammon |
3.33 | 35.91 | 54.70 | 1.616 | 0.530 | 1.086 | 1.336 | Light gray with a little reddish gray | Light and flaky | 6.06 | Trace. | ||
| 7 | Davis Coal Co., No. 3 Cherokee |
3.77 | 35.94 | 50.48 | 1.288 | 0.279 | 1.009 | 1.343 | Very light gray | Small clinker shells | 9.81 | Trace. | ||
| 8 | Kansas & Texas, No. 18 Weir City |
3.57 | 36.96 | 51.84 | 2.551 | 0.254 | 2.297 | 3.722 | 2.6058 | 1.296 | Pinkish gray | Quite light | 7.63 | Trace. |
| 9 | Kansas & Texas, No. 47 Weir City |
3.16 | 39.21 | 53.87 | 0.765 | 0.428 | 0.337 | 1.281 | Light bluish gray | Very light and flaky | 3.76 | Trace. | ||
| 10 | Durkee Coal Co., No. 4 Weir City |
2.34 | 36.88 | 55.69 | 1.056 | 0.403 | 0.653 | 1.299 | Light gray with bluish tint | Light and flaky | 5.09 | Trace. | ||
| 11 | Western Coal Co., No. 2 Fleming |
2.75 | 34.22 | 57.22 | 0.994 | 0.423 | 0.471 | 1.286 | Light bluish gray | Light and flaky | 5.79 | Trace. | ||
| 12 | Hamilton & Braidwood Coal Co., No. 2 Weir City |
2.63 | 38.80 | 53.74 | 1.244 | 0.520 | 0.724 | 2.862 | 2.0041 | 1.344 | Reddish gray with little bluish spots | Coarse ash but rather light | 4.83 | Trace. |
| 13 | The Central Coal and Coke Co., No. 5 Weir City |
3.14 | 34.87 | 55.39 | 2,557 | 0.385 | 2.172 | 1.313 | Grayish pink | Has tendency to clinker | 6.60 | Trace. | ||
| 14 | Durkee Coal Co., No. 1 Weir City |
2.57 | 36.34 | 54.99 | 2.787 | 0.823 | 1.961 | 1.319 | Dark reddish gray | Coarse but light ash | 6.10 | Trace. | ||
| 15 | The Excelsior Coal Co. Weir City |
2.58 | 36.73 | 55.02 | 1.715 | 0.280 | 1.435 | 1.285 | Bluish gray | Has a tendency to clinker | 5.62 | Trace. | ||
| 16 | Santa Fe Mine Chicopee |
3.17 | 34.83 | 55.10 | 1.381 | 0.209 | 1.172 | 4.234 | 2.964 | 1.382 | Quite dark gray | Clinker shell and a little fine ash | 6.90 | Trace. |
| 17 | Santa Fe Mine, No. 1. Frontenac |
3.06 | 35.92 | 54.89 | 2.329 | 0.358 | 1.971 | 1.337 | Bluish gray | Light and flaky | 6.13 | Trace. | ||
| 18 | Kansas & Texas, No. 20 Pittsburg |
3.32 | 31.57 | 57.08 | 1.867 | 0.318 | 1.549 | 1.310 | Reddish gray | Light and flaky | 5.03 | Trace. | ||
| 19 | Company unknown Pittsburg |
2.51 | 33.70 | 56.06 | 1.513 | 0,248 | 1.265 | 1.017 | Part light gray; part brick color | Quite light | 7.73 | Trace. | ||
| 20 | Pittsburg & Midway Coal Co., No. 4 Midway |
2.44 | 33.64 | 56.83 | 3.061 | 0.543 | 2.518 | 4.380 | 3.066 | 1.313 | Light red | Light and flaky | 7.09 | Trace. |
| 21 | Senott Bros., No. 3 Cornell |
3.10 | 34.76 | 55.86 | 1.560 | 0.431 | 1.129 | 1.321 | Dark brown and bluish gray | Light | 6.28 | Trace. | ||
| 22 | Morganville Coal Co. Morganville, Mo. |
2.93 | 31.99 | 59.43 | 0.942 | 0.209 | 0.733 | 1.261 | Light pink | Light and flaky | 5.65 | Trace. | ||
| 23 | Company unknown Arcadia (first south of town) |
2.55 | 33.42 | 56.22 | 2.376 | 0.778 | 1.598 | 1.276 | Reddish to pink | Amorphous flakes; light | 7.81 | Trace. | ||
| 24 | Company unknown Pleasanton |
4.99 | 36.65 | 54.23 | 0.956 | 0.261 | 0.695 | 2.044 | 1.431 | 1.352 | Dark chrome yellow | Light and flaky; a few clinkers | 4.13 | Trace. |
| 25 | State Mine Lansing |
6.58 | 33.52 | 33.91 | 1.197 | 0.368 | 0.829 | 1.319 | Pinkish gray | Light and flaky | 15.99 | Trace. | ||
| 26 | Blacksmith Mine Blacksmith |
13.42 | 39.83 | 39.29 | 5.550 | 1.136 | 4.414 | Reddish brown, nearly drab | Light | 7.46 | Trace. | |||
| 27 | Crane's Mine Dover (three miles west) |
9.50 | 31.72 | 21.51 | 1.108 | 0.244 | 8.864 | 3.436 | 2.405 | Light brown | Light | 37.27 | Trace. | |
| 28 | Pittsburg Commercial Pittsburg |
5.13 | 38.41 | 52.03 | 1.022 | 0.258 | 0.764 | 2.022 | 0.986 | 1.302 | Light red | Clinky | 4.03 | Trace. |
| 29 | Anthracite taken from seam next to "Bell" Leavenworth |
0.82 | 9.14 | 89.03 | 1.400 | White | Light | 1.01 | Trace. |
Table V—Showing Bailey's chemical analyses of Kansas Coals.
| Location | Number | Water | Volatile matter |
Fixed carbon |
Ash | Color of ash |
|---|---|---|---|---|---|---|
| Cherokee | 1 | 1.54 | 38.06 | 53.44 | 6.96 | Gray |
| Cherokee | 2 | 1.26 | 35.60 | 52.20 | 10.94 | Drab |
| Cherokee | 3 | 1.37 | 37.19 | 50.23 | 11.21 | Reddish gray |
| Cherokee | 4 | 2.59 | 39.12 | 51.54 | 6.75 | Brownish |
| Cherokee | 5 | 1.35 | 36.11 | 50.91 | 11.60 | Gray |
| Cherokee | 6 | 2.49 | 34.59 | 54.11 | 8.81 | Light gray |
| Cherokee | 7 | 2.76 | 36.21 | 51.91 | 6.12 | Light gray |
| Cherokee | 8 | 2.75 | 36.76 | 53.08 | 7.41 | Gray |
| Cherokee | 9 | 1.33 | 37.33 | 51.59 | 9.75 | Brownish gray |
| Cherokee (upper vein) | 1 | 2.25 | 34.17 | 49.51 | 14.07 | Grayish brown |
| Cherokee (upper vein) | 2 | 2.07 | 34.37 | 50.21 | 13.35 | Grayish brown |
| Cherokee (upper vein) | 3 | 1.91 | 37.44 | 46.19 | 14.46 | Gray |
| Fort Scott | 1 | 2.35 | 42.79 | 45.00 | 9.86 | Reddish |
| Fort Scott | 2 | 2.21 | 43.89 | 45.15 | 8 75 | Reddish brown |
| Fort Scott | 3 | 4.27 | 38.61 | 52.49 | 4.63 | Reddish brown |
| Leavenworth County | 1 | 3.22 | 41.55 | 49.32 | 5.91 | Dark red |
| Leavenworth County | 2 | 2.25 | 36.49 | 47.27 | 13.99 | Light red |
| Leavenworth County | 3 | 2.61 | 39.58 | 43.65 | 12.16 | Brick color |
| Linn County | 1 | 1.61 | 38.25 | 48.76 | 11.3. | Dark brown |
| Linn County | 2 | 2.36 | 40.14 | 48.88 | 8.62 | Reddish brown |
| Linn County | 3 | 2.39 | 42.19 | 42.05 | 13.37 | Yellowish brown |
| Linn County | 4 | 1.92 | 37.11 | 47.87 | 13.10 | Red |
| Osage County | 1 | 7.19 | 40.03 | 41.13 | 11.65 | Brown |
| Osage County | 2 | 7.71 | 41.56 | 39.92 | 10.81 | Light brown |
| Osage County | 3 | 9.29 | 42.05 | 40.89 | 7.77 | Red |
| Osage County | 4 | 4.70 | 44.86 | 42.11 | 8.33 | Dark brown |
| Osage County | 5 | 6.75 | 42.79 | 40.97 | 9.49 | Dark brown |
| Osage County | 6 | 7.27 | 41.45 | 41.35 | 9.93 | Dark red |
| Osage County | 7 | 5.56 | 42.79 | 39.32 | 12.33 | Dark red |
| Osage County | 8 | 5.83 | 43.26 | 41.75 | 9.16 | Reddish brown |
| Osage County | 9 | 7.36 | 38.33 | 38.54. | 15.77 | Dark brown |
| Osage County | 10 | 4.91 | 39.58 | 43.17 | 12.31 | Yellowish brown |
| Osage County | 11 | 7.77 | 40.85 | 40.29 | 11.09 | Light brown |
| Franklin County | 1 | 7.55 | 44.40 | 37.68 | 10.37 | Gray |
| Cloud County | 1 | 13.70 | 46.14 | 28.52 | 11.64 | Dark gray |
Physical Properties of Kansas Coals
The value of a coal depends largely upon what it is to be used for. The commercial value of coal differs widely from its actual value, or its value as a heat producer. In the first instance the method of mining and the ease with which it may be placed upon the market affects the value greatly, thus producing a specific value which is far from being constant. On the other hand the heat producing value of coal is to all practical purposes a fixed quantity, varying as the proportion of fixed carbon and volatile matter varies. As will be noticed by Tables IV and V, the proportion of constituents remains very nearly constant for the same horizon. We should expect therefore that the value as a heat producer would also in like manner remain constant, which is verified later in these pages. The amount of heat obtainable from coals taken even from one locality differs greatly, owing to the kind of furnace employed, the age of the furnace and boiler, and probably, most of all, upon the methods of firing, such as care of grate, ventilation, etc., which points need not be discussed here. But to the expert fireman the best indicator of correct firing is the smoke issuing from the stack. In other words incomplete combustion is responsible, in many cases, for the small amount of heat obtained, rather than the coal which generally receives most of the blame.
The duty test of a certain coal in a particular furnace will therefore give the amount of heat obtainable for the coal in that furnace, under the various conditions above mentioned, but will give data of little value for other furnaces with their attendant conditions. The results will simply be relative for the coal in the particular furnace. It will therefore appear to be necessary for the comparison of coals, first, to produce perfect combustion; second, to have the conditions governing the combustion constant.
Method Employed
There are several methods employed whereby fuels may be compared according to their evaporative power; that is, to determine how many pounds or kilograms of water may be converted into steam at 212 degrees F. or 100 degrees C., under atmospheric, or 760 mm. pressure, by means of one pound or kilogram of the fuel.
Thompson's calorimetric method was employed in obtaining the following results. The method consists in burning a fixed amount, generally two grams, of coal in the midst of 1934 grams of water, or 1 gram of coal in 967 grams of water, from which it will be seen that the rise of temperature of the water will give the number of grams of water which a gram of coal will be able to convert into steam at the boiling point, 212&def; F., thus giving directly the evaporative power of the coal. The apparatus, which is of English make, was first constructed to burn 30 grains in 29,010 grains (30 times 967) of water, only Fahrenheit thermometers being used. Later the grain proportions were changed to gramme, so that both the Fahrenheit and Centigrade thermometers might be used, and thus conform to a standard.
Principles upon which the Method is Based—Theory
The principles upon which the method is based are: first, that the latent heat—the amount of heat which disappears when water is converted into steam at 212 degrees F.—equals 967 degrees F., or 537.22 gram degrees or calories; and secondly, that when coal is burned in pure oxygen the same amount of heat is evolved as when perfectly burned in the air.
These two principles may be found fully explained in any first-class text hook on physics. The latent heat of water being 967&def; F., then 967 parts of water heated 1 degree F. indicates that there must have been sufficient heat employed to convert into steam 1 part of water at the boiling point, or 212&def; F. If this is true for 1&def; F. then it must hold good for any number of degrees F., which we will indicate by n. Therefore if 967 parts of water be heated n degrees F. enough heat has been, used to: evaporate n parts of water: Thus, by noting the number of degrees rise of temperature, it is evident that we have the number of parts of water capable of being converted into steam by the heat generated. As however the possibility of error increases with the smaller amounts of fuel taken, it is better to use as large amount as is practicable without increasing the errors in other directions, as, in having too much water, etc. The proportions which seem best adapted to the foregoing method are, two grams (30.865 grains) of fuel, and of course a corresponding quantity of water—1934 grams (967 x 2). The apparatus in question was constructed for these quantities.
To obtain the complete combustion of coal the neceesity of having it reduced to a fine state of subdivision is evident. It must, at least, be required to pass through a sieve 1000 meshes to the square inch. Coal will not ignite and burn freely in air, not considering the possibility of its so doing in a closed space, with only a limited supply of oxygen. To obviate this difficulty the finely powdered coal is intimately mixed with several (generally 11) times its weight of potassium chlorate, KClO3 and potassium nitrate, KNO3, in the proportion of 3 to 1. The potassium chlorate and nitrate furnish oxygen for the complete combustion of the coal. The burning taking place, of course, in the midst of the water. Thus all heat derived therefrom, either in the heating of the apparatus or given off as gaseous products must .needs pass first through the surrounding medium, namely, the water, before escaping into the air.
Apparatus
The burning of the coal takes place in a, copper cartridge, Figure 54, d, 1 and 2, about three inches long by three and onefourth inches in diameter, this fitting into a cup-shaped receiver, b, 1 and 2, so arranged as to allow another copper cylinder, c, 1 and 2, closed at one end, to fit diving-bell-like over the first. In the closed end of the second cylinder is fastened a small tube with a valve arrangement at the top, so as to allow the water to enter or be kept out of the cylinder at will. Around the base, that is the open end, a fraction of an inch above the edge, is a row of holes through which the gaseous product escapes. The vessel in which the combustion takes place and into which the above described apparatus is placed is a cylindrical, thick glass tube, formed into a foot below, lipped above, and graduated to hold 1934 or 2000 grams (2 litres) of water, a, 1, Figure 54. The temperature is determined by means of a thermometer reading to 1/20 of a degree F. A complete description of this method is given in the Encyclopaedia Britannica, under the head of "Coal."
Figure 54—Vertical sections and horizontal plans of containing jar, foot, valved cap, and cartridges of Thompson's calorimeter.
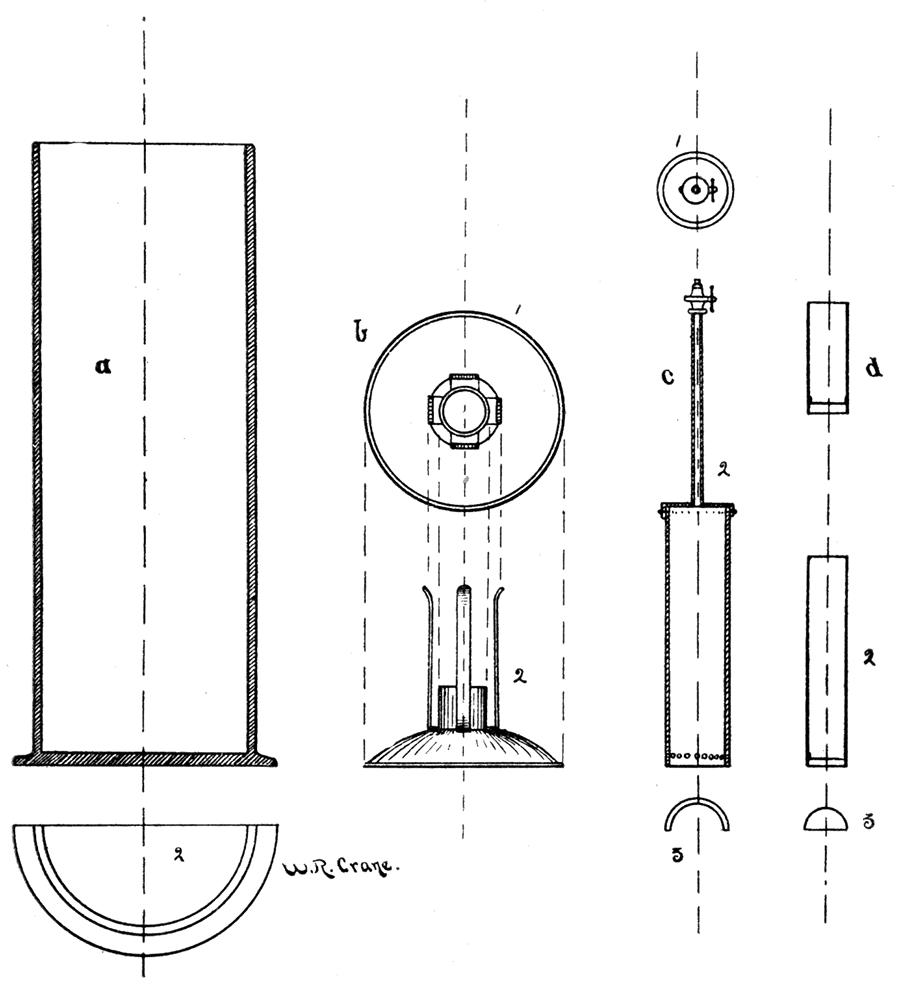
Details of Testing
The same coals were employed in the physical test that were used in the chemical test. See [previous] for methods of sampling. After having been reduced to a fine powder and sifted, the sample is weighed on a delicate balance. Two portions of two grams each are taken, a duplicate test being made in most cases. The potassium chlorate and nitrate, having been dried and thoroughly triturated, are mixed in the proportion of 3 to 1—16.5 grams KClO3 and 5.5 grams KNO3. To this mixture add the two grams of coal and mix intimately by means of a spatula. A smooth even grained glass plate is found to be the most satisfactory mixing board. When the three substances have become thoroughly intermixed, which fact can be determined from the grayish color of the powder, load into a cartridge, pressing well together with rammer but not tamping hard. The whole mass should be relatively compact but not packed together at intervals, which would produce very uneven and unsatisfactory burning. After the cartridge is loaded, take a knife blade or any flattened point and loosen up the powder in the center a quarter of an inch in depth; into this loosened powder press the fuse, heaping up the mixture around the base of it, but avoid pressing, in which case the fire is liable to be smothered out.
The fuse should be of sufficient length to insure the operator time to light and place the apparatus in the vessel of water. If the charge should begin to burn before immersed in the water the test is worthless. Each operator must judge for himself from experience what the proper length of fuse should be. The writer has found that the most satisfactory fuse—one which is more certain than the fuse generally employed—is made by taking a mixture of potassium chlorate, potassium nitrate, and coal, in the proportion of 2 1/2, 1 1/2, and 1 parts. Mix thoroughly and triturate so that the mixture will pass through a sieve 1000 meshes to the square inch. Take a quarter inch strip of tough tissue paper, moisten, and spread out upon a smooth surface, then sift some of the above mentioned mixture upon the strip and roll into a long tube. When nearly dry twist into a long tight string. This form of fuse has proved eminently satisfactory, seldom failing to ignite the charge, while it burns with a surprising regularity and freedom. The charge should ignite by the time the furnace reaches the bottom of the jar. Otherwise the fuse will not have been rightly timed. One must learn from practice rather than from precept what is the proper length for fuse, form of fuse, etc.
If the charge is properly ignited dense white fumes of carbon monoxide, CO, carbon dioxide, CO2, and sulphur dioxide, SO2, etc., the result of the chemical union of the carbon of the coal with the chlorate and nitrate, will be given off. Owing to the construction of the apparatus these fumes are forced to pass through the whole length of the column of water, from the bottom to the top, thus affording ample opportunity for the water to extract heat therefrom and attain a temperature common to both. Violent burning is not conducive to good results, neither is too slow burning. In the former case water is apt to be thrown out of the jar by the too rapid evolution of gases; in the latter case, especially when the time of burning extends up to four or five minutes, there is chance for loss of heat by radiation.
There are two ways to regulate the rate of combustion: first, by care in charging; second, by varying the proportion of the oxygen mixture. A well tamped charge burns less violently than one that has received little or no tamping, which fact is readily shown by tamping at intervals. Such a charge when ignited will burn very irregularly, but always less violently at the compressed portion. It has been suggested by some that 22 parts of oxygen mixture be used. This rule will not always hold good, for in some coals tested by the writer 22 parts would not produce combustion. In some such instances more of the oxygen mixture was required, while in others considerably less would produce the required result; in the former case often as high as 27 parts of the oxygen mixture were required, while in the latter not more than 18 parts. A thermometer is placed in the containing vessel as soon as the furnace is immersed, and is allowed to remain there until the temperature reaches the highest point possible, or in other words until the heat generated by the combustion has diffused throughout the apparatus and surrounding water and all parts have attained the same temperature. While the furnace is being immersed the stop cock in the above mentioned tube fitting to the diving-bell cap must be kept closed, so that no water can come in contact with the charge. As soon as the burning ceases open the stop cock and allow the water to enter and come in contact with the heated parts, cartridge, etc. Then churn the furnace up and down so as to distribute the heat evenly throughout the vessel.
The duration of burning should be noted. To get the time accurately there should be two persons: one to give the signal as the first indications of combustion are noticed, and a corresponding signal when the bubbles cease to escape. The difference of these two observations will give the duration of burning. The temperature of the room should be noted, and the temperature of escaping gas. The color of fumes, and the color and relative quantity of ash as seen floating in the water should also be noted, although these data do not play any part, directly, in reaching the results, yet they are always attendant upon the result and give a very approximate indication of the value of the coal as a heat producer. The temperature of the water in the testing vessel must, of course, be noted just before the furnace is immersed for combustion. The temperature of the water should always be lower than that of the room—six: degrees has been given as about the right difference, yet from observation it has been shown to make little or no difference if the temperature is not allowed to fall below 1 1/2° to 2° F. as a difference.
Corrections
The corrections upon the instrument have been determined as follows:
1. "Heat absorbed by the gases of combustion—This amount: can only be approximated. The volume of the escaping gas is. about 3300 c.c. The rate of combustion is slow enough to allow these gases to issue into the air at about the temperature of the water, or about 5.4° F. above the room temperature. Assuming their specific heat to be on the average that of carbon, dioxide, for about 12° rise in temperature 0.9 percent must be lost." [Blake, 1889]
2. The heat of the decomposition of the potassium chlorate and nitrate—Although this quantity cannot be determined exactly yet it can be quite closely approximated. The method employed is as follows: The combustion of two grams of the purest carbon obtainable—lamp black—gave 7930.4 thermal units or calories. It has been shown that the calorific powers of different forms of pure carbon, as determined by Favre and Silbermann (Groves and Thorp: Chemical Technology, vol. i, Fuels, p. 707, Philadelphia, 1889) are as follows:
| Forms of Carbon | Calorific Power | Analyst |
|---|---|---|
| Wood charcoal | 8,080.00 | Favre and Silbermann |
| Gas retort carbon | 8,047.30 | Favre and Silbermann |
| Artificial graphite | 7,762.30 | Favre and Silbermann |
| Native graphite | 7,796.60 | Favre and Silbermann |
| Diamond | 7,770.10 | Favre and Silbermann |
| Carbon vapor | 11,214.00 | Favre and Silbermann |
| Lamp black | 7,930.40 | W. R. Crane |
Taking lamp black, as being one of the purest forms of carbon, and carbon vapor, and averaging them, we have as nearly as can be determined the calorific power of pure carbon. This mean is 9572.20 calories. (A calorie is a gram of water raised 1 degree C.) The total loss therefore must be very nearly 15 percent.
As will be shown later in these pages, the loss due to absorption by the apparatus is about 1.18 percent, and as was mentioned in (1), a loss of 0.9 percent, thus making a loss of 2.08 percent due to causes other than the decomposition of the oxygen mixture. The loss due to the decomposition of the oxygen mixture must therefore be 12.92 percent (15—2.08).3. The error due to weighing the coal is practically negligible as the balances used read to .05 milligram and an error of 1 milligram would cause a loss of .005 part of 1 percent.
4. The error arising from taking too large or too small a quantity of water would not amount to over It to 2 tenths of 1 percent, a negligible quantity also.
5. Loss of heat from radiation and conduction was determined by experiment. 1934 grams of water were placed in the testing vessel, the temperature raised to about the average temperature obtained in testing, and the rate of cooling determined. The results are as follows:
| Time | Thermometer in Calorimeter | Temp. of Room |
|---|---|---|
| 8:30 A.M. | 87.90 F. | 74.8 F. |
| 8:35 A. M. | 87.85 F. | 74.8 F. |
| 8:40 A. M. | 87.85 F. | 74.8 F. |
| 8:50 A. M. | 87.0&def; F. | 74.9 F. |
| 9:30 A. M. | 86.50 F. | 75.0 F. |
| 10:00 A. M. | 85.00 F | 75.3 F. |
| 1 hr., 30 min | 2.9F |
The loss would therefore be 0.03° F. for one minute. An average burning requires about two minutes. A few extend from three and a half to four minutes. Taking the time a little above the average, we will say three minutes, then the loss would be .09° or 0.1° F. (3 times .03° F.)
6. The heat absorbed by apparatus—Several tests were made upon each of the separate pieces of the furnace to determine the amount of heat absorbed by them. The following tables give the results:
| Tests on Cap | ||
|---|---|---|
| No. 1 | Temperature of cap | 208. deg. F. |
| Temperature of water before heating | 70. deg. F. | |
| Temperature of water after heating | 71.04 deg. F. | |
| Difference in temperature of water | 1.04 deg. F. | |
| Difference 1.04 ÷ 208 = .005; or, .5 percent | ||
| No. 2 | Temperature of cap | 206.6 deg. F. |
| Temperature of water before heating | 70.4 deg. F. | |
| Temperature of water after heating | 71.4 deg. F. | |
| Difference in temperature of water | 1.04 deg. F. | |
| Difference 1.04 ÷ 206.6 = .0047; or, .47 percent | ||
| No. 3 | Temperature of cap | 201.2 deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.7 deg. F. | |
| Difference in temperature of water | 0.9 deg. F. | |
| Difference 0.9 ÷ 201.2 = .0044; or, .44 percent | ||
| Average percent heat absorbed by cap, .47 | ||
| Tests on Receiver or Base | ||
|---|---|---|
| No. 1 | Temperature of base | 208. deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.7 deg. F. | |
| Difference in temperature of water | 0.9 deg. F. | |
| Difference 0.9 ÷ 208 = .0043; or, .43 percent | ||
| No. 2 | Temperature of base | 199.4 deg. F. |
| Temperature of water before heating | 69.7 deg. F. | |
| Temperature of water after heating | 70.7 deg. F. | |
| Difference in temperature of water | 1.0 deg. F. | |
| Difference 1 ÷ 199.4 = .005; or, .5 percent | ||
| No. 3 | Temperature of base | 205.7 deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.8 deg. F. | |
| Difference in temperature of water | 1.0 deg. F. | |
| Difference 1 ÷ 205.7 = .0048; or, .48 percent | ||
| No. 4 | Temperature of base | 200.3 deg, F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.7 deg. F. | |
| Difference in temperature of water | 0.9 deg. F. | |
| Difference .9 ÷ 200.3 = .0044; or, .44 percent | ||
| Average percent heat absorbed by base, .46 | ||
| Tests of Small Cartridge | ||
|---|---|---|
| No. 1 | Temperature of cartridge | 209.3 deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.4 deg. F. | |
| Difference in temperature of water | 0.6 deg. F. | |
| Difference 0.6 ÷ 209.3 = .0028; or, .28 percent | ||
| No. 2 | Temperatnre of cartridge | 203.9 deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.3 deg. F. | |
| Difference in temperature of water | 0.5 deg. F. | |
| Difference 0,5 ÷ 203.9 = .0024; or, .24 percent | ||
| No. 3 | Temperature of cartridge | 206.6 deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.3 deg. F. | |
| Difference in temperature of water | 0.5 deg. F. | |
| Difference 0.5 ÷ 206.6 = .0024; or, .24 percent | ||
| Average percent heat absorbed by cartridge, .25 | ||
| Tests on Large Cartridge | ||
|---|---|---|
| No. 1 | Temperature of cartridge | 212. deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.3 deg. F. | |
| Difference in temperature of water | 0.5 deg. F. | |
| Difference 0.5 ÷ 212 = .0023; or, .23 percent | ||
| No. 2 | Temperature of cartridge | 210.2 deg. F. |
| Temperature of water before heating | 69.8 deg. F. | |
| Temperature of water after heating | 70.4 deg. F. | |
| Difference in temperature of water | 0.6 deg. F. | |
| Difference 0.6 ÷ 210.2 = .0028; or, .28 percent | ||
| No. 3 | Temperature of cartridge | 186.8 deg. F. |
| Temperature of water before heating | 69.9 deg. F. | |
| Temperature of water after heating | 70.4 deg. F. | |
| Difference in temperature of water | 0.5 deg. F. | |
| Difference 0.5 ÷ 186.8 = .0027; or, .27 percent | ||
| Average percent heat absorbed by cartridge, .26 | ||
| Summary | |
|---|---|
| Heat absorbed by receiver | .46 percent |
| Heat absorbed by cap | .47 percent |
| Heat absorbed by cartridges (average) | .25 percent |
| Total amount of heat absorbed | 1.18 percent |
In finding the amount of heat absorbed by the apparatus the following method was employed. The tests were made on the separate pieces of the furnace instead of the whole furnace together for the reason that they could be handled much more readily and rapidly, which are both very important points to be considered in the determinations. Each place was heated to a constant temperature in a copper oven, AB, Figure 55. The oven is about eight inches high, twelve inches long, and six inches wide. The door, BC, which takes up one whole side of the oven, is hinged on the shorter side, KL, thus opening lengthwise of oven. Two strips of copper, OP, are fastened lengthwise of the door, forming a groove an inch and a half in width. A corresponding groove, ZR, is arranged in both ends of the oven and on the stationary side of it. The pieces of the apparatus are so placed in these grooves that when the oven is turned upon its side—the door, forming the bottom (see dotted lines)—the piece falls into the groove, then when the door is opened it glides down the inclined plane of the door guided by the groove and is thus introduced into the receiving vessel. Figure 55.
Figure 55—Front view of Copper Oven, with door open, showing grooves on door and back of oven; also top view, with a portion of top cut away, showing grooves.
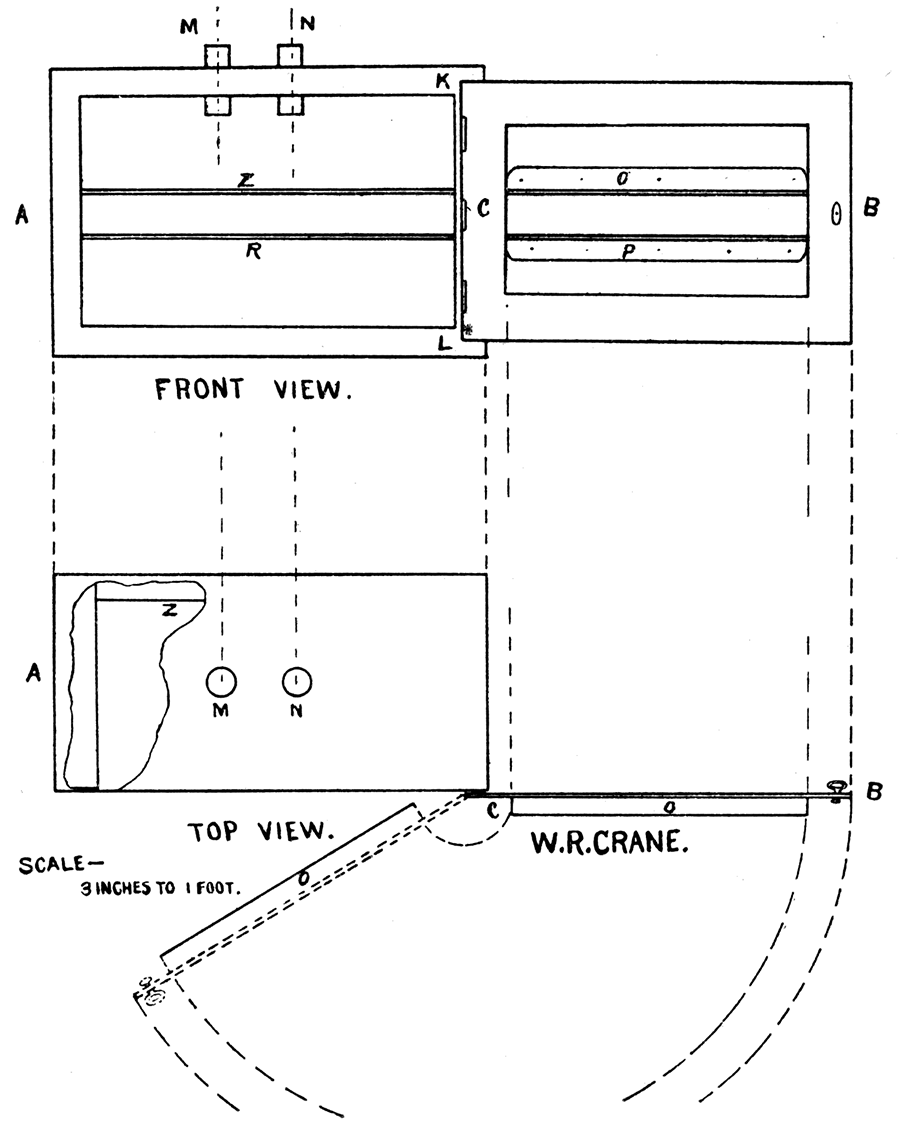
Method in Detail
Stand the oven upon a tripod and introduce a thermometer into the top of the oven, through a hole prepared for it, MN, Figure 55. Heat by means of a Bunsen burner. Place the piece to be tested in the above mentioned grooves, close the door, and by regulating the supply of gas, etc., raise to constant temperature. In the meantime fill the testing vessel with the same quantity of water as is used in actual tests with coal. Note the temperature of the water in the vessel. By this time the piece of apparatus should have reached a temperature common with the oven and contained air. Remove the thermometer from the oven, lift the oven from the tripod, and place directly above vessel of water, then strike the latch, which holds the oven door shut, against the edge of jar. This unlatches door, which will now be resting upon the edge of the vessel. By lifting the oven the door is opened, although still resting upon the edge of the vessel, whereupon the piece of apparatus will slide quickly and smoothly down the groove into water. Bya little practice the apparatus may be changed from the constant temperature of the oven to the water almost instantaneously and, what is of the most importance, without an appreciable loss of temperature, the temperature seldom falling more than 2 tenths of a degree in transit. The method is one very readily employed and extremely simple, while the results, as will be seen from the above table are quite constant.
The total loss of heat in the calorimetric method is about 15 percent, the oxygen mixture being responsible for 12.92 percent, while the remaining 2.08 percent is due to other causes mentioned above. A prominent error both in the experimental tests and the calculations of the calorific powers of fuels lies in the fact that the carbon of the fuel does not exist either as a solid or as a gas alone, but as both, the difficulty experienced being, that the percent of either the gas or solid is a decidedly variable quantity.
It will be seen from the above calculations that the correction of ten percent of the manufacturers of the instrument and that generally employed is small, also that the correction as determined by Professor Blake is large. As the calculations made heretofore, on Kansas coals, have been made by taking one or the other of these corrections, it might be well to give the results of the application of all three of them, which results will be found side by side in the table of tests.
Heat of Combustion by Calculation
The need of a simpler method of finding the calorific power of coal will be recognized by anyone who attempts the actual tests with any of the methods generally employed. There are several methods by means of which the heat of combustion of fuels, especially coals, may be determined by calculation. The two most important are, the method of M. Carnut, chief engineer to the Northern Steam Users' Association, of France, and Dulong's Laws. As M. Carnut's law gives us a means of arriving at results more approximately correct than Dulong's we will consider that law only. The formula by which the law is expressed is as follows:
Q = 8,080 C'+ 11,214 C" + 34,462 H.
Where Q = the total quantity of heat = the calorific power desired; C' = the fixed carbon; C" = the volatile carbon (hydrocarbons) found in coal; and H = the hydrogen.
The elucidation of this formula will not be undertaken here, as it requires demonstration too lengthy and complex for the intended scope of this work.
The law is quite simple and easily applied, one advantage being that liquid fuels may also have their calorific powers determined by similar calculations. The principal cause of error is the fact that the calorific value of the hydrocarbons (volatile carbon) is not distinguished according to the temperatures at which they are set free.
From the formula it will be seen that the quantity, H, must be known, which quantity was not determined in the chemical analyses of the coals under consideration. It will therefore be impossible to calculate the calorific power of the coals by this method.
In the following table will be found the results obtained by Prof. L. I. Blake, of the University of Kansas, who tested some of the coals from the same localities as those used in the tests made by this Survey. As will be seen on examining the following table, the percents (correction) are ten and thirty, which, have been taken into consideration in the above tests, so that a comparison of the two sets of tests may readily be made. See [previous].
From his results Professor Blake deduces the following facts: "The coals depreciate in their steam producing power from the southeastern part toward the north and west." This deduction corroborates in a general way our own conclusions, as already expressed.
Table VI—Showing physical tests of Kansas coals
| Number | Location | Temp. of room |
Temp. of water before combustion |
Temp. of water after combustion |
Rise in temp. of water |
Time of burning |
Color of gas |
Mine (see table IV) |
Number of parts of oxygen mixture |
Remarks | Pounds of water at 212° F. or 100° C. evaporated per pound of coal burned |
Time of day when the following data were taken |
Calories | Liters of water evaporated per kilogram of fuel |
Rate of radiation of heat |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correction on apparatus | ||||||||||||||||||
| 10% | 30% | 15% | Calculated calorific power |
|||||||||||||||
| 1 | Fleming | 27 9° C. | 73.2° F. | 85.1° F. | 11.9°F. | 75 sec. | Creamy | 22 | Violent burning | 13.09 | 15..4 | 13.6 | 11 to 12 a.m. | 6390.3 | 13.6 | 0.03 ° F. per min. | ||
| 2 | Cornell | 79.3° F. | 71.8° F. | 83.1° F. | 11.3° F. | 48 sec | Creamy | No. 21 | 22 | Violent burning | 12.43 | 14.69 | 13.0 | 1:00 p.m. | 6068.1 | 13.0 | 0.03 ° F. per min. | |
| 3 | Midway | 22.6° C. | 69.8° F. | 81.0° F. | 11.2° F. | 60 sec. | Creamy | No. 20 | 22 | 12.32 | 14.56 | 12.88 | 2:00 p.m. | 6014.4 | 12.88 | 0.03 ° F. per min. | ||
| 4 | Leavenworth | 22.9° C. | 70.9° F. | 80.7° F. | 9.8° F. | 140 sec. | Slightly creamy | No. 25 | 22 | Brownish residual | 10.78 | 12.74 | 11.27 | 10:00 a.m. | 5322.6 | 11.27 | 0.03 ° F. per min. | |
| 5 | Pittsburg | 23.3° C. | 71.0° F. | 82.7° F. | 11.7° F. | 55 sec. | Creamy | No. 18 | 22 | Violent burning | 12.87 | 15.21 | 13.45 | 10:30 a.m. | 6282.9 | 13.45 | 0.03 ° F. per min. | |
| 6 | Chicopee | 23.0° C. | 70.9° F. | 82.6° F. | 11.7° F. | 60 sec. | Creamy | No. 16 | 22 | 12.87 | 15.21 | 13.45 | 10:45 a.m. | 6282.9 | 13.45 | 0.03 ° F. per min. | ||
| 7 | Cherokee | 23.3° C. | 69.8° F. | 83.4° F. | 13.6° F. | 48 sec. | Very creamy | No. 3 | 22 | Violent burning | 14.96 | 17.68 | 15.45 | 11:00 a.m. | 7303.2 | 15.45 | 0.03 ° F. per min. | |
| 8 | Weir City | 23.4° C. | 69.8° F. | 81. 7° F. | 11.9° F. | 56 sec. | Creamy | No. 8 | 22 | Violent burning | 13.09 | 15.4 | 13.6 | 11:30 a.m. | 6390.3 | 13.6 | 0.03 ° F. per min. | |
| 9 | Pleasanton | 23.5° C. | 70.1° F. | 80.9° F. | 10.8° F. | 59 sec. | Creamy | No. 24 | 22 | Violent burning | 11.88 | 14.04 | 12.42 | 8:30 a.m. | 5799.6 | 12.42 | 0.03 ° F. per min. | |
| 10 | Weir City | 23.8° C. | 69.9° F. | 82.3° F. | 12.4° F. | 50 sec. | Dark cream colored | No. 9 | 22 | Violent burning | 13.64 | 16.12 | 14.26 | 8:45 a.m. | 6658.8 | 14.26 | 0.03 ° F. per min. | |
| 11 | Stippville | 23.6° C. | 70.5° F. | 83.8° F. | 13.3° F. | 50 sec. | Dark cream colored | No. 4 | 22 | Violent burning | 14.63 | 17.29 | 15.3 | 8:50 a.m. | 7142.1 | 15.3 | 0.03 ° F. per min. | |
| 12 | Weir City | 23.6° C. | 70.4° F. | 82.5° F. | 12.1° F. | 57 sec. | Creamy | No. 12 | 22 | Violent burning | 13.31 | 15.73 | 13.9 | 9:00 a.m. | 6497.7 | 13.9 | 0.03 ° F. per min. | |
| 13 | Pittsburg | 24.1° C. | 70.2° F. | 81.4° F. | 11.2° F. | 49 sec. | Creamy | No. 19 | 22 | Violent burning | 12.32 | 14.56 | 12.88 | 9:25 a.m. | 6014.4 | 12.88 | 0.03 ° F. per min. | |
| 14 | Scammon | 24.1° C. | 70.3° F. | 82.8° F. | 12.5° F. | 53 sec. | Creamy | No. 5 | 22 | 13.75 | 16.25 | 14.35 | 9:40 a.m. | 6712.5 | 14.35 | 0.03 ° F. per min. | ||
| 15 | Arcadia | 24.4° C. | 70.2° F. | 82.0° F. | 12.2° F. | 51 sec. | Creamy | No. 23 | 22 | Violent burning | 13.42 | 15.86 | 14.0 | 9:50 a.m. | 6551.4 | 14.0 | 0.02 ° F. per min. | |
| 16 | Weir City | 24.4° C. | 70.3° F. | 82.0° F. | 11.7° F. | 59 sec. | Creamy | No. 10 | 22 | Violent burning | 12.87 | 15.21 | 13.45 | 10:00 a.m. | 6282.9 | 13.45 | 0.02 ° F. per min. | |
| 17 | Weir City | 24.8° C. | 70.3° F. | 81.8° F. | 11.5° F. | 61 sec. | Creamy | No. 13 | 22 | 12.65 | 14.95 | 13.22 | 10:15 a.m. | 6175.5 | 13.22 | 0.025 ° F. per min. | ||
| 18 | Cherokee | 24.9° C. | 70.5° F. | 82.4° F. | 11.9° F. | 50 sec. | Creamy | No. 2 | 22 | Brown residue | 13.09 | 15.4 | 13.6 | 10:20 a.m. | 6390.3 | 13.6 | 0.100 ° F. per min. | |
| 19 | Weir City | 22.5° C. | 69.8° F. | 81.4° F. | 11.6° F. | 44 sec. | Very yellow | No. 15 | 22 | Violent burning | 12.76 | 15.08 | 13.34 | 10:40 a.m. | 6229.2 | 13.34 | 0.04 ° F. per min. | |
| 20 | Mineral City | 25.0° C. | 70.3° F. | 82.1° F. | 11.8° F. | 68 sec. | Creamy | No. 1 | 22 | 12.98 | 15.34 | 13.55 | 10:50 a.m. | 6336.6 | 13.55 | 0.02 ° F. per min. | ||
| 21 | Morganville. Mo. | 22.1° C. | 69.9° F. | 81.6° F. | 11.7° F. | 47 sec. | Quite creamy | No. 22 | 22 | Violent burning | 12.87 | 15.21 | 13.45 | 11:00 a.m. | 6282.9 | 13.45 | 0.01 ° F. per min. | |
| 22 | Scammon | 22.4° C. | 69.8° F. | 80.9° F. | 11.1° F. | 56 sec. | Cream colored | No. 6 | 22 | Violent burning | 12.21 | 14.43 | 12.76 | 11:10 a.m. | 5960.7 | 12.76 | 0.012 ° F. per min. | |
| 23 | Frontenac | 22.3° C. | 70.0° F. | 81.3° F. | 11.3° F. | 46 sec. | Cream colored | No. 17 | 22 | Violent | 12.43 | 14.69 | 13.0 | 11:25 a.m. | 6068.1 | 13.0 | 0.03 ° F. per min. | |
| 24 | Cherokee | 23.3° C. | 69.7° F. | 80.6° F. | 10.9° F. | 74 sec. | Cream colored | No. 7 | 22 | 11.96 | 14.17 | 12.53 | 11:38 a.m. | 5853.3 | 12.53 | 0.035 ° F. per min. | ||
| 25 | Weir City | 22.3° C. | 69.8° F. | 81.4° F. | 11.6° F. | 54 sec. | Cream colored | No. 14 | 22 | 12.76 | 15.08 | 13.34 | 11:45 a.m. | 6229.2 | 13.34 | 0.036 ° F. per min. | ||
| 26 | Neosho Rapids | 24.3° C. | 70.2° F. | 81.4° F. | 11.2° F. | 65 sec. | Not very cream colored | F. S. Kelley | 24 | 12.32 | 14.56 | 12.88 | 12:00 a.m. | 6014.4 | 12.88 | 0.024 ° F. per min. | ||
| 27 | Carbondale | 25.4° C. | 70.5° F. | 80.9° F. | 10.4° F. | 131 sec. | Bluish white | Sam. Watkinson | 24 | Brown residue | 11.44 | 13.52 | 11.96 | 2:00 p.m. | 5584.8 | 11.96 | 0.03 ° F. per min. | |
| 28 | Scranton | 25.4° C. | 70.7° F. | 81.0° F. | 10.3° F. | 242 sec. | Bluish white | Miller | 24 | Brown residue | 11.33 | 13.39 | 11.84 | 2:25 p.m. | 5531.1 | 11.84 | 0.03 ° F. per min. | |
| 29 | Osage | 23.4° C. | 69.5° F. | 78.4° F. | 8.9° F. | 226 sec. | Considerable ash; white | Miller | 24 | A large quantity of brown residue | 9.79 | 11.57 | 10.23 | 2:40 p.m. | 4979.3 | 10.23 | 0.03 ° F. per min. | |
| 30 | Osage | 23.5° C. | 69.8° F. | 80.0° F. | 10.2° F. | 127 sec. | White | Miller | 24 | 11.22 | 13.26 | 11.73 | 3:00 p.m. | 5476.4 | 11.73 | 0.031 ° F. per min. | ||
| 31 | Pleasanton | 23.6° C. | 70.2° F. | 81.6° F. | 11.4° F. | 130 sec. | White | Frank Seawright | 22 | Brown residue | 12.54 | 14.82 | 13.11 | 3:25 p.m. | 6121.8 | 13.11 | 0.03 ° F. per min. | |
| 32 | Pleasanton | 23.9° C. | 70.0° F. | 82.1° F. | 12.1° F. | 94 sec. | White | Mine Creek Coal Co. | 22 | 13.31 | 15.73 | 13.91 | 4:05 p.m. | 6497.7 | 13.91 | 0.025 ° F. per min. | ||
| 33 | Burlingame | 24.0° C. | 69.9° F. | 80.6° F. | 10.7° F. | 170 sec. | White | T. Chappell | 24 | Brown residue | 11.77 | 13.91 | 12.3 | 8:00 a.m. | 5745.9 | 12.3 | 0.01 ° F. per min. | |
| 34 | La Cygne | 24.2° C. | 70.4° F. | 81.0° F. | 10.6° F. | 166 sec. | White | Vanlyle | 22 | Brown residue | 11.66 | 13.78 | 12.19 | 8:40 a.m. | 5692.2 | 12.19 | 0.031 ° F. per min. | |
| 35 | Burlingame | 25.0° C. | 70.0° F. | 81.0° F. | 11.0° F. | 193 sec. | Bluish white | No. 3, Red Jacket | 24 | Black residue | 12.10 | 14.30 | 12.65 | 8:55 a.m. | 5907.0 | 12.65 | 0.033 ° F. per min. | |
| 36 | La Cygne | 24.8° C. | 70.3° F. | 82.8° F. | 12.5° F. | 53 sec. | White | Gage Coal Co. | 22 | 13.75 | 16.25 | 14.:17 | 9:40 a.m. | 6710.2 | 14.37 | 0.03 ° F. per min. | ||
| 37 | Unknown | 24.5° C. | 70.9° F. | 80.4° F. | 9.5° F. | 120 sec. | White | Unknown | 27 N-C | Brown residue | 10.45 | 12.35 | 10.92 | 11:00 a.m. | 5101.5 | 10.92 | 0.01 ° F. per min. | |
| 38 | Burlingame | 24.9° C. | 70.9° F. | 81.7° F. | 10.8° F. | 100 sec. | White | J. D. Jack | 27 N-C | Brown residue | 11.88 | 14.04 | 12.42 | 9:00 a.m. | 5799.6 | 12.42 | 0.02 ° F. per min. | |
| 39 | Burlingame | 25.9° C. | 70.3° F. | 80.4° F. | 10.1° F. | 242 sec. | White | Foster's | 27 N-C | Brown residue | 11.11 | 13.13 | 11.61 | 10:30 a.m. | 5423.7 | 11.61 | 0.028 ° F. per min. | |
| 40 | Osage City | 25.8° C. | 70.2° F. | 80.6° F. | 10.4° F. | 310 sec. | Bluish white | No. 4 | 22 | Quite red | 11.44 | 13.52 | 11.96 | 2:00 p.m. | 5584.8 | 11.96 | 0.029 ° F. per min. | |
| 41 | Osage City | 26.4° C. | 70.5° F. | 80.1° F. | 9.6° F. | 49 sec. | Slightly cream colored | Sunflower | 18 | Drab | 10.56 | 12.48 | 11.04 | 8:15 a.m. | 5155.2 | 11.04 | 0.03 ° F. per min. | |
| 42 | Osage City | 26.4° C. | 70.7° F. | 79.8° F. | 9.1° F. | 63 sec. | Dirty white | No. 24 | 18 | Dark red | 10.01 | 11.83 | 10.46 | 8:40 a.m. | 4886.7 | 10.46 | 0.03 ° F. per min. | |
| 43 | Fort Scott | 26.6° C. | 70.6° F. | 80.8° F. | 10.2° F. | 68 sec. | Cream colored | 22 | Red | 13.42 | 15.86 | 11.73 | 3:00 p.m. | 5551.4 | 11.73 | 0.036 ° F. per min. | ||
Table VII—Blake's physical tests of Kansas coals.
| Locality | Number | Pounds of water at 212° F. evaporated per pound of coal |
Duration of burning |
Remarks | |
|---|---|---|---|---|---|
| Correction on apparatus |
|||||
| 10% | 30% | ||||
| Cherokee coals | 1 | 13.53 | 15.97 | 40 seconds | |
| Cherokee coals | 2 | 13.64 | 16.10 | 45 seconds | |
| Cherokee coals | 3 | 13.01 | 15.35 | 45 seconds | |
| Cherokee coals | 4 | 13.31 | 15.71 | 60 seconds | |
| Cherokee coals | 5 | 13.31 | 15.71 | 60 seconds | Violent burning |
| Cherokee coals | 6 | 13.86 | 16.36 | 65 seconds | |
| Cherokee coals | 7 | 13.53 | 15.97 | 50 seconds | Violent burning |
| Cherokee coals | 8 | 13.94 | 16.45 | 75 seconds | |
| Cherokee coals | 9 | 13.42 | 15 84 | 90 seconds | |
| Cherokee coals (upper vein) Pittsburg | 1 | 12.43 | 14.67 | 55 seconds | |
| Cherokee coals | 2 | 12.76 | 15.06 | 45 seconds | |
| Cherokee coals | 3 | 13.31 | 15.7 | 60 seconds | |
| Fort Scott coals | 1 | 13.64 | 16.10 | 65 seconds | |
| Fort Scott coals | 2 | 13.09 | 15.45 | 60 seconds | |
| Fort Scott coals | 3 | 13.86 | 16.35 | 55 seconds | |
| Leavenworth coals | 1 | 13.36 | 15.76 | 75 seconds | |
| Leavenworth coals | 2 | 12.25 | 14.46 | 80 seconds | Brown residue |
| Leavenworth coals | 3 | 13.86 | 16.35 | 65 seconds | |
| Linn county coals | 1 | 12.76 | 15.C6 | 60 seconds | |
| Linn county coals | 2 | 12.76 | 15.06 | 70 seconds | |
| Linn county coals | 3 | 12.87 | 15.49 | 80 seconds | Brown residue |
| Linn county coals | 4 | 12.54 | 14.80 | 75 seconds | |
| Osage county | 1 | 11.88 | 14.02 | 110 seconds | Brown residue |
| Osage county | 2 | 10.96 | 12.93 | 90 seconds | Brown residue |
| Osage county | 3 | 12.98 | 15.32 | 120 seconds | Brown residue |
| Osage county | 4 | 11.66 | 13.76 | 120 seconds | Brown residue |
| Osage county | 5 | 12.43 | 14.67 | 120 seconds | Brown residue |
| Osage county | 6 | 11.99 | 14.15 | 165 seconds | Brown residue |
| Osage county | 7 | 11.66 | 13.76 | 120 seconds | Brown residue |
| Osage county | 8 | 11.88 | 14.02 | 120 seconds | Brown residue |
| Osage county | 9 | 11.55 | 13.63 | 70 seconds | Brown residue |
| Osage county | 10 | 11.44 | 13.50 | 80 seconds | Brown residue |
| Osage county | 11 | 11.66 | 13.76 | 105 seconds | Brown residue |
| Franklin county coals | 1 | 12.32 | 14.54 | 125 seconds | |
| Cloud county | 1 | 9.90 | 11.68 | 135 seconds | |
Prev Page--Mining Systems || Next Page--Value, Commerce
Kansas Geological Survey, Geology
Placed on web Sept. 12, 2018; originally published 1898.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/Vol3/53_chemical.html