Prev Page--Introduction || Next Page--Emission Spectroscopy
Mineralogy
Introduction
The objectives of this section are twofold--to determine the mineralogical nature of the Upper Pennsylvanian and Lower Permian shales of Kansas, and to observe and account for the stratigraphic distribution of the major mineral components. Using the X-ray diffraction techniques developed in Appendix 1 and discussed in Cubitt (1975a, 1975b), it is possible both to identify the minerals and to quantify the major components present in the shales. The resulting stratigraphic distribution of minerals throughout the Upper Pennsylvanian and Lower Permian shales can be plotted, analyzed, and interpreted in terms of changing tectonic and sedimentary environments. Similarly, any periodic elements detected in the mineral distributions can be examined and tested by statistical analysis.
Analytical Equipment and Technique
The equipment employed in the X-ray diffraction analysis consisted of a Norelco X-ray Diffractometer, wide-range goniometer, AMR3-202 LiF curved crystal monochromator, proportional counter, and pulse-height analyzer.
All shale samples were ground to less than 60 microns and prepared for X-ray diffraction analysis using the sample mounting procedure described in Appendix 1. Individual samples were then placed in the sample cavity of the Norelco Diffractometer and examined at 2°2θ/min. over a period of 30 minutes under standard conditions (Cubitt, 1975b). All major peaks were recorded within the range 2°2θ to 60°2θ and minerals present were identified.
X-ray Identification and Characteristics of Minerals
The identification of minerals in the X-ray diffraction traces was primarily achieved by examining standard d-spacings in the ASTM Powder Data File. However, several clay minerals are not easily identified using this method and tests on the shale samples were required to establish the species present. The standard identification procedure for clay minerals (Carroll, 1970; Griffin, 1971) was therefore adopted.
- Position of main peaks--This is of primary importance in the identification of minerals as each has a unique and characteristic set of peak positions.
- Effects of glycolation on peak positions--Normally montmorillonite, illite, and the interstratified clay minerals are indistinguishable as major peak positions overlap, and minor peaks are inevitably lost in background noise. However, they may be differentiated by their swelling reactions in an atmosphere of ethylene glycol; illite does not respond to the organic reagent but swelling clays such as montmorillonite absorb the molecules, producing an expansion of the crystal lattice and a characteristic shift in major peak positions on the diffraction trace.
- Effects of heating on peak positions--Heating clay minerals results in an initial loss of water from the crystal lattice, especially in the case of illite, and at higher temperatures volatilization of organic molecules from the interlayer sites. Other notable features include the breakdown of kaolinite to an amorphous state at 500-600° C and chlorite at 800° C. Each of these events is marked by a drop in height of the X-ray peaks on the diffraction trace. Table 2 illustrates the important role these effects play in the identification of clay minerals in shale samples.
Table 2--Effects of test treatments on peak position of major minerals in the shale samples.
| Mineral | Basal Spacings (001 Unless Stated Otherwise) |
2θ Transformation on Main Peak |
Effect of Glycolation on Peak Position |
Effect of Heating on Peak Position |
|
|---|---|---|---|---|---|
| Quartz | 3.34Å | (101); | 26.66 | none | none |
| 4.26Å | (100) | 20.85 | |||
| Feldspar | 3.30Å | 27.70 | none | none | |
| Calcite | 3.03Å | (104) | 29.47 | none | none |
| Dolomite | 2.88Å | (104) | 30.05 | none | none |
| Chlorite | 14.1Å + integral series of basal spacings |
6.27 | none | (001) increases in intensity; at 800° C shows wt. loss (Mg form)/collapse (Fe form) |
|
| Kaolinite | 7.15Å; | 12.56 | none | Becomes amorphous at 550-600° C | |
| 3.75Å | (002) | ||||
| Illite | 10Å | (002) broad | 8.70 | none | (001) more intense as H2O removed from structure |
| N.B. Disordered kaolinite shows broadening of X-ray peaks. | |||||
The major mineral constituents of the shales were identified as quartz, calcite, dolomite, feldspar, illite, chlorite, and kaolinite. The following secondary minerals were also recorded: gypsum, jarosite, pyrite, mixed layered illite-montmorillonite, montmorillonite, and swelling chlorite. The feldspar minerals detected could not be individually identified although considerable effort was spent attempting to implement the X-ray and optical techniques of Goodyear and Duffin (1954), Smith (1956), Smith and Yoder (1956), and Wright (1968). These complicated and laborious procedures were discovered to be excessively time-consuming in sediment analysis and are considered appropriate only for igneous rock studies where considerable feldspar is often available. ASTM identification of feldspars also proved fruitless because it is essentially based not on the main peak positions, which occur within a very narrow range on the diffraction trace, but on secondary peaks. In all the shale samples studied, no secondary peaks were detected, presumably because the feldspar concentration is too low. Attempts at separating the feldspar content from the other minerals were also inconclusive. Dr. McCaleb of Sun Oil Company kindly examined a number of shale samples with high peak area measurements of feldspar (e.g., samples 96 and 170). On the basis of automated X-ray diffractometer readings, they were found to contain approximately 20 percent orthoclase and little or no plagioclase feldspar. This result seems dubious in the light of petrological investigations of the shales, where distinct albite-twinned plagioclase laths were recognized. In this study, a decision was therefore made to employ the general term feldspar for the X-ray measurement taken.
X-ray Measurements Made on Shale Samples
The following X-ray measurements were taken on samples prepared using the technique described in Appendix 1. First, peak height readings were taken of the quartz (100) peak at 26.66°2θ, the calcite (104) peak at 29.47°2θ, the feldspar peak at 27.7°2θ, the dolomite (104) peak at 30.05°2θ, the kaolinite (001) peak at 12.36°2θ, the illite (001) peak at 8.70°2θ, and the chlorite (001) peak at 6.87°2θ. Then peak areas were calculated for the quartz (101) peak at 20.85°2θ, the calcite (104) peak, the dolomite (104) peak, and the feldspar peak at 27.7°2θ. Interference from other mineral peaks was negligible, therefore avoiding inconsistent peak area measurements. Finally, using the calibration charts for quartz, calcite, and dolomite developed by Cubitt (1975a), percentages were estimated. A feldspar calibration curve could not be established because of the identification problem mentioned previously, and no curves could be set up for clay minerals as variable crystallinity prevented exact matching of sample clay minerals and "spikes." The results of the X-ray diffraction analyses are presented in Table 3.
Stratigraphic Distribution of Minerals
X-ray measurements described previously were taken on the major components of 127 clastic rock samples from the Upper Pennsylvanian and Lower Permian of Kansas (Table 3). The stratigraphic positions of these samples can be seen in Figure 1 and their geographic position will be found in Appendix 3. The results can be summarized as a statistical table (Table 4) and as a series of graphs (Figure 9) showing the mathematical distribution each mineral describes.
Table 3--Results of X-ray diffraction analyses of Pennsylvanian and Lower Permian shales of Kansas.
| X-ray Diffraction Measurements | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Number |
Quartz | Calcite | Feldspar | Dolomite | Kaolinite | Illite | Chlorite | |||||||
| Peak Area |
Peak Height |
Percent | Peak Area |
Peak Height |
Percent | Peak Area |
Peak Height |
Peak Area |
Peak Height |
Percent | Peak Height |
Peak Height |
Peak Height |
|
| 16 | 10833.00 | 100.00 | 57.00 | 2387.00 | 7.00 | 3.00 | 6119.00 | 23.00 | 2547.00 | 7.00 | 5.00 | 21.00 | 32.00 | 22.00 |
| 19 | 7393.00 | 100.00 | 41.00 | 2941.00 | 7.00 | 4.00 | 4310.00 | 15.00 | 2811.00 | 5.00 | 6.00 | 28.00 | 33.00 | 21.00 |
| 20 | 8684.00 | 100.00 | 47.00 | 5475.00 | 21.00 | 8.00 | 4962.00 | 21.00 | 5135.00 | 18.00 | 11.00 | 15.00 | 22.00 | 18.00 |
| 21 | 4227.00 | 53.00 | 24.00 | 16629.00 | 64.00 | 23.00 | 2790.00 | 9.00 | 7786.00 | 36.00 | 17.00 | 10.00 | 14.00 | 15.00 |
| 23 | 4019.00 | 44.00 | 22.00 | 15358.00 | 48.00 | 21.00 | 2979.00 | 8.00 | 7551.00 | 28.00 | 16.00 | 7.00 | 13.00 | 14.00 |
| 24 | 7208.00 | 76.00 | 40.00 | 4043.00 | 7.00 | 6.00 | 4306.00 | 12.00 | 3405.00 | 11.00 | 7.00 | 10.00 | 18.00 | 15.00 |
| 25 | 6806.00 | 96.00 | 38.00 | 3387.00 | 7.00 | 5.00 | 4534.00 | 17.00 | 3248.00 | 7.00 | 7.00 | 17.00 | 33.00 | 16.00 |
| 26 | 8200.00 | 100.00 | 46.00 | 4036.00 | 12.00 | 6.00 | 5377.00 | 19.00 | 2758.00 | 5.00 | 6.00 | 12.00 | 31.00 | 21.00 |
| 27 | 4273.00 | 53.00 | 24.00 | 20557.00 | 85.00 | 28.00 | 2355.00 | 9.00 | 1532.00 | 4.00 | 2.00 | 7.00 | 11.00 | 15.00 |
| 28 | 7445.00 | 87.00 | 42.00 | 3743.00 | 9.00 | 6.00 | 5866.00 | 10.00 | 3391.00 | 8.00 | 7.00 | 6.00 | 30.00 | 24.00 |
| 7 | 9564.00 | 100.00 | 50.00 | 3965.00 | 8.00 | 6.00 | 4127.00 | 10.00 | 4113.00 | 7.00 | 9.00 | 7.00 | 17.00 | 16.00 |
| 31 | 6152.00 | 91.00 | 35.00 | 3371.00 | 8.00 | 5.00 | 4650.00 | 16.00 | 3402.00 | 7.00 | 7.00 | 20.00 | 32.00 | 18.00 |
| 32 | 6754.00 | 90.00 | 37.00 | 3692.00 | 8.00 | 6.00 | 6291.00 | 18.00 | 4071.00 | 8.00 | 8.00 | 14.00 | 27.00 | 17.00 |
| 34 | 6456.00 | 86.00 | 36.00 | 7012.00 | 10.00 | 10.00 | 4938.00 | 15.00 | 5283.00 | 7.00 | 11.00 | 16.00 | 28.00 | 17.00 |
| 36 | 5688.00 | 66.00 | 32.00 | 15487.00 | 65.00 | 21.00 | 4015.00 | 12.00 | 2194.00 | 10.00 | 4.00 | 14.00 | 17.00 | 13.00 |
| 35 | 2709.00 | 31.00 | 17.00 | 21779.00 | 89.00 | 29.00 | 1980.00 | 8.00 | 15858.00 | 85.00 | 35.00 | 4.00 | 9.00 | 11.00 |
| 38 | 6003.00 | 97.00 | 34.00 | 3950.00 | 10.00 | 6.00 | 4872.00 | 14.00 | 2889.00 | 7.00 | 6.00 | 17.00 | 32.00 | 20.00 |
| 40 | 6543.00 | 97.00 | 37.00 | 3154.00 | 7.00 | 4.00 | 4583.00 | 15.00 | 2884.00 | 5.00 | 6.00 | 25.00 | 44.00 | 27.00 |
| 41 | 4176.00 | 68.00 | 23.00 | 13700.00 | 71.00 | 19.00 | 3498.00 | 18.00 | 4197.00 | 10.00 | 9.00 | 18.00 | 24.00 | 17.00 |
| 43 | 5875.00 | 74.00 | 33.00 | 3498.00 | 6.00 | 5.00 | 4951.00 | 14.00 | 3544.00 | 6.00 | 7.00 | 12.00 | 26.00 | 18.00 |
| 46 | 6240.00 | 86.00 | 35.00 | 4619.00 | 11.00 | 7.00 | 5399.00 | 14.00 | 3664.00 | 10.00 | 8.00 | 19.00 | 30.00 | 19.00 |
| 47 | 6659.00 | 99.00 | 37.00 | 4771.00 | 13.00 | 7.00 | 4662.00 | 15.00 | 3672.00 | 6.00 | 8.00 | 19.00 | 33.00 | 20.00 |
| 48 | 7402.00 | 100.00 | 42.00 | 4319.00 | 12.00 | 7.00 | 4650.00 | 15.00 | 4707.00 | 12.00 | 10.00 | 17.00 | 42.00 | 19.00 |
| 54 | 7465.00 | 100.00 | 42.00 | 12815.00 | 65.00 | 18.00 | 3862.00 | 34.00 | 2314.00 | 5.00 | 5.00 | 11.00 | 23.00 | 11.00 |
| 50 | 4998.00 | 69.00 | 28.00 | 7128.00 | 25.00 | 11.00 | 4687.00 | 16.00 | 2992.00 | 4.00 | 6.00 | 6.00 | 16.00 | 19.00 |
| 53 | 4087.00 | 46.00 | 23.00 | 2408.00 | 7.00 | 3.00 | 6119.00 | 7.00 | 2037.00 | 4.00 | 4.00 | 8.00 | 15.00 | 16.00 |
| 69 | 6721.00 | 100.00 | 38.00 | 3097.00 | 8.00 | 4.00 | 3457.00 | 16.00 | 3402.00 | 7.00 | 7.00 | 36.00 | 37.00 | 20.00 |
| 70 | 8113.00 | 100.00 | 44.00 | 3856.00 | 7.00 | 6.00 | 6071.00 | 17.00 | 3083.00 | 5.00 | 6.00 | 36.00 | 32.00 | 20.00 |
| 71 | 8926.00 | 100.00 | 48.00 | 3547.00 | 12.00 | 6.00 | 6044.00 | 29.00 | 2544.00 | 7.00 | 5.00 | 23.00 | 36.00 | 25.00 |
| 160 | 5971.00 | 82.00 | 33.00 | 6066.00 | 28.00 | 9.00 | 4956.00 | 13.00 | 3098.00 | 7.00 | 6.00 | 17.00 | 26.00 | 22.00 |
| 67 | 6915.00 | 100.00 | 38.00 | 2528.00 | 6.00 | 4.00 | 4807.00 | 19.00 | 2465.00 | 8.00 | 5.00 | 49.00 | 60.00 | 28.00 |
| 61A | 6259.00 | 96.00 | 35.00 | 7250.00 | 21.00 | 11.00 | 3201.00 | 15.00 | 2633.00 | 5.00 | 5.00 | 28.00 | 45.00 | 20.00 |
| 63 | 7075.00 | 100.00 | 39.00 | 2846.00 | 8.00 | 4.00 | 5380.00 | 12.00 | 2498.00 | 5.00 | 5.00 | 45.00 | 50.00 | 24.00 |
| 12 | 8009.00 | 100.00 | 44.00 | 3449.00 | 9.00 | 5.00 | 6140.00 | 31.00 | 3564.00 | 9.00 | 7.00 | 25.00 | 22.00 | 19.00 |
| 66 | 3043.00 | 27.00 | 18.00 | 3411.00 | 13.00 | 5.00 | 3411.00 | 4.00 | 21040.00 | 100.00 | 48.00 | 4.00 | 7.00 | 10.00 |
| 15 | 5257.00 | 66.00 | 29.00 | 13054.00 | 54.00 | 18.00 | 3201.00 | 10.00 | 2258.00 | 5.00 | 4.00 | 13.00 | 20.00 | 27.00 |
| 3 | 5598.00 | 83.00 | 31.00 | 2962.00 | 7.00 | 4.00 | 4098.00 | 10.00 | 2796.00 | 6.00 | 6.00 | 30.00 | 36.00 | 17.00 |
| 157 | 7387.00 | 88.00 | 41.00 | 2878.00 | 6.00 | 4.00 | 5270.00 | 15.00 | 2533.00 | 6.00 | 5.00 | 25.00 | 34.00 | 21.00 |
| 161 | 7398.00 | 92.00 | 41.00 | 4687.00 | 12.00 | 7.00 | 4773.00 | 16.00 | 2987.00 | 7.00 | 6.00 | 8.00 | 24.00 | 16.00 |
| 296 | 7748.00 | 99.00 | 43.00 | 4754.00 | 15.00 | 7.00 | 4754.00 | 15.00 | 2764.00 | 7.00 | 6.00 | 24.00 | 23.00 | 18.00 |
| 294 | 14173.00 | 100.00 | 75.00 | 2362.00 | 3.00 | 3.00 | 4047.00 | 21 00 | 2665.00 | 3.00 | 5.00 | 9.00 | 11.00 | 11.00 |
| 290 | 16877.00 | 100.00 | 85.00 | 2554.00 | 6.00 | 4.00 | 4648.00 | 17.00 | 2309.00 | 4.00 | 5.00 | 16.00 | 21.00 | 13.00 |
| 284 | 6662.00 | 100.00 | 37.00 | 3894.00 | 7.00 | 6.00 | 4884.00 | 11.00 | 2653.00 | 6.00 | 5.00 | 25.00 | 27.00 | 18.00 |
| 287 | 7243.00 | 100.00 | 40.00 | 5447.00 | 16.00 | 8.00 | 7716.00 | 13.00 | 2378.00 | 5.00 | 5.00 | 20.00 | 28.00 | 15.00 |
| 281 | 10365.00 | 100.00 | 54.00 | 4128.00 | 14.00 | 7.00 | 5636.00 | 15.00 | 7719.00 | 22.00 | 17.00 | 17.00 | 21.00 | 13.00 |
| 125 | 13387.00 | 100.00 | 72.00 | 2521.00 | 5.00 | 4.00 | 5297.00 | 18.00 | 2312.00 | 4.00 | 5.00 | 25.00 | 33.00 | 20.00 |
| 116 | 6296.00 | 83.00 | 35.00 | 8079.00 | 20.00 | 12.00 | 4709.00 | 12.00 | 3378.00 | 8.00 | 7.00 | 22.00 | 20.00 | 21.00 |
| 110 | 6531.00 | 69.00 | 37.00 | 7709.00 | 23.00 | 11.00 | 2569.00 | 13.00 | 2906.00 | 4.00 | 6.00 | 9.00 | 12.00 | 17.00 |
| 112 | 11034.00 | 100.00 | 58.00 | 2449.00 | 6.00 | 4.00 | 4132.00 | 15.00 | 2300.00 | 5.00 | 5.00 | 27.00 | 29.00 | 19.00 |
| 128 | 6916.00 | 87.00 | 39.00 | 4201.00 | 8.00 | 7.00 | 4201.00 | 11.00 | 4522.00 | 10.00 | 9.00 | 15.00 | 27.00 | 20.00 |
| 130 | 4689.00 | 53.00 | 25.00 | 7536.00 | 23.00 | 11.00 | 2205.00 | 6.00 | 21035.00 | 100.00 | 46.00 | 7.00 | 15.00 | 12.00 |
| 79 | 4584.00 | 62.00 | 25.00 | 11418.00 | 47.00 | 16.00 | 4807.00 | 9.00 | 4767.00 | 13.00 | 10.00 | 15.00 | 24.00 | 18.00 |
| 151 | 6428.00 | 98.00 | 37.00 | 3359.00 | 7.00 | 5.00 | 4684.00 | 19.00 | 3713.00 | 7.00 | 8.00 | 38.00 | 48.00 | 23.00 |
| 82 | 6738.00 | 98.00 | 38.00 | 3691.00 | 7.00 | 6.00 | 4396.00 | 11.00 | 2658.00 | 6.00 | 5.00 | 20.00 | 23.00 | 22.00 |
| 80 | 6497.00 | 85.00 | 36.00 | 3675.00 | 7.00 | 6.00 | 4204.00 | 13.00 | 3744.00 | 6.00 | 8.00 | 24.00 | 36.00 | 27.00 |
| 81 | 5811.00 | 77.00 | 33.00 | 3252.00 | 7.00 | 9.00 | 4124.00 | 11.00 | 2976.00 | 8.00 | 6.00 | 22.00 | 31.00 | 28.00 |
| 85 | 11761.00 | 100.00 | 63.00 | 2265.00 | 6.00 | 3.00 | 9967.00 | 35.00 | 3224.00 | 4.00 | 7.00 | 13.00 | 26.00 | 12.00 |
| 135 | 3041.00 | 30.00 | 18.00 | 11092.00 | 42.00 | 16.00 | 2098.00 | 4.00 | 15074.00 | 57.00 | 33.00 | 7.00 | 12.00 | 13.00 |
| 121 | 18913.00 | 100.00 | 96.00 | 1982.00 | 6.00 | 3.00 | 1884.00 | 9.00 | 2225.00 | 4.00 | 4.00 | 9.00 | 10.00 | 16.00 |
| 138 | 5141.00 | 57.00 | 28.00 | 8205.00 | 26.00 | 12.00 | 4314.00 | 10.00 | 3965.00 | 8.00 | 8.00 | 8.00 | 24.00 | 17.00 |
| 136 | 6398.00 | 100.00 | 36.00 | 6203.00 | 20.00 | 9.00 | 4535.00 | 16.00 | 6232.00 | 24.00 | 13.00 | 14.00 | 29.00 | 20.00 |
| 137 | 4293.00 | 50.00 | 24.00 | 22244.00 | 74.00 | 30.00 | 4962.00 | 8.00 | 2253.00 | 7.00 | 4.00 | 9.00 | 16.00 | 14.00 |
| 149 | 8859.00 | 100.00 | 48.00 | 6228.00 | 17.00 | 9.00 | 5475.00 | 17.00 | 2939.00 | 6.00 | 6.00 | 13.00 | 21.00 | 14.00 |
| 150 | 8987.00 | 100.00 | 48.00 | 3375.00 | 7.00 | 5.00 | 6272.00 | 17.00 | 3236.00 | 7.00 | 7.00 | 9.00 | 20.00 | 25.00 |
| 152 | 7697.00 | 100.00 | 42.00 | 3417.00 | 7.00 | 5.00 | 4664.00 | 17.00 | 2846.00 | 6.00 | 6.00 | 12.00 | 28.00 | 20.00 |
| 153 | 7105.00 | 100.00 | 40.00 | 2680.00 | 7.00 | 4.00 | 5417.00 | 19.00 | 2525.00 | 7.00 | 5.00 | 54.00 | 62.00 | 26.00 |
| 155 | 9678.00 | 100.00 | 51.00 | 3356.00 | 5.00 | 5.00 | 5765.00 | 19.00 | 2639.00 | 5.00 | 5.00 | 17.00 | 33.00 | 20.00 |
| 105 | 7453.00 | 92.00 | 41.00 | 12790.00 | 61.00 | 18.00 | 1383.00 | 11.00 | 2268.00 | 4.00 | 4.00 | 15.00 | 14.00 | 13.00 |
| 107 | 7152.00 | 81.00 | 40.00 | 2988.00 | 7.00 | 4.00 | 4354.00 | 13.00 | 2761.00 | 12.00 | 6.00 | 20.00 | 25.00 | 15.00 |
| 109 | 2872.00 | 28.00 | 16.00 | 25017.00 | 94.00 | 34.00 | 1383.00 | 8.00 | 1564.00 | 4.00 | 2.00 | 7.00 | 12.00 | 11.00 |
| 94 | 4640.00 | 17.00 | 25.00 | 14627.00 | 100.00 | 20.00 | 2586.00 | 4.00 | 3332.00 | 21.00 | 7.00 | 6.00 | 10.00 | 9.00 |
| 96 | 9900.00 | 100.00 | 52.00 | 2218.00 | 6.00 | 3.00 | 8381.00 | 39.00 | 2431.00 | 7.00 | 5.00 | 19.00 | 40.00 | 20.00 |
| 97 | 6387.00 | 77.00 | 36.00 | 4265.00 | 8.00 | 7.00 | 4526.00 | 11.00 | 3037.00 | 7.00 | 6.00 | 25.00 | 33.00 | 28.00 |
| 144 | 8097.00 | 96.00 | 45.00 | 2903.00 | 5.00 | 4.00 | 5758.00 | 18.00 | 6417.00 | 20.00 | 14.00 | 33.00 | 39.00 | 22.00 |
| 101 | 7558.00 | 100.00 | 42.00 | 3851.00 | 13.00 | 6.00 | 4687.00 | 18.00 | 2734.00 | 7.00 | 5.00 | 33.00 | 40.00 | 33.00 |
| 122 | 4013.00 | 47.00 | 22.00 | 20978.00 | 89.00 | 28.00 | 5010.00 | 8.00 | 2023.00 | 4.00 | 4.00 | 10.00 | 18.00 | 14.00 |
| 97B | 9859.00 | 100.00 | 51.00 | 5601.00 | 18.00 | 8.00 | 6164.00 | 23.00 | 2738.00 | 5.00 | 5.00 | 11.00 | 19.00 | 18.00 |
| 148 | 6906.00 | 76.00 | 38.00 | 3546.00 | 6.00 | 5.00 | 4534.00 | 15.00 | 3437.00 | 7.00 | 7.00 | 18.00 | 26.00 | 20.00 |
| 162 | 8976.00 | 100.00 | 48.00 | 2439.00 | 7.00 | 4.00 | 5974.00 | 27.00 | 2369.00 | 6.00 | 5.00 | 14.00 | 30.00 | 21.00 |
| 163 | 6131.00 | 39.00 | 35.00 | 3194.00 | 15.00 | 4.00 | 3020.00 | 6.00 | 2250.00 | 5.00 | 4.00 | 29.00 | 15.00 | 10.00 |
| 171 | 6364.00 | 100.00 | 36.00 | 2613.00 | 7.00 | 4.00 | 5244.00 | 16.00 | 2754.00 | 6.00 | 6.00 | 26.00 | 38.00 | 20.00 |
| 173 | 6566.00 | 99.00 | 37.00 | 3471.00 | 6.00 | 5.00 | 4875.00 | 14.00 | 2689.00 | 7.00 | 5.00 | 25.00 | 38.00 | 22.00 |
| 168 | 9848.00 | 100.00 | 52.00 | 2142.00 | 5.00 | 3.00 | 5926.00 | 38.00 | 2225.00 | 4.00 | 4.00 | 19.00 | 29.00 | 15.00 |
| 192 | 5964.00 | 77.00 | 34.00 | 6960.00 | 27.00 | 10.00 | 3543.00 | 18.00 | 17802.00 | 82.00 | 39.00 | 11.00 | 17.00 | 15.00 |
| 194 | 6576.00 | 95.00 | 37.00 | 6106.00 | 6.00 | 9.00 | 5073.00 | 22.00 | 3146.00 | 4.00 | 6.00 | 13.00 | 15.00 | 15.00 |
| 176 | 4883.00 | 69.00 | 26.00 | 3093.00 | 7.00 | 4.00 | 4171.00 | 11.00 | 3315.00 | 6.00 | 7.00 | 31.00 | 31.00 | 23.00 |
| 180 | 7337.00 | 100.00 | 41.00 | 2865.00 | 7.00 | 4.00 | 4289.00 | 18.00 | 2531.00 | 7.00 | 5.00 | 28.00 | 43.00 | 20.00 |
| 181 | 8449.00 | 100.00 | 46.00 | 4470.00 | 14.00 | 7.00 | 5387.00 | 14.00 | 2900.00 | 6.00 | 6.00 | 15.00 | 18.00 | 22.00 |
| 184 | 9291.00 | 100.00 | 49.00 | 2378,00 | 8.00 | 3.00 | 7654.00 | 31.00 | 3003.00 | 6.00 | 6.00 | 28.00 | 32.00 | 18.00 |
| 185 | 8818.00 | 100.00 | 48.00 | 4376.00 | 21.00 | 7.00 | 6300.00 | 16.00 | 3350.00 | 7.00 | 7.00 | 14.00 | 16.00 | 14.00 |
| 186 | 6206.00 | 74.00 | 35.00 | 5102.00 | 14.00 | 8.00 | 6725.00 | 11.00 | 3308.00 | 5.00 | 7.00 | 9.00 | 13.00 | 22.00 |
| 187 | 3862.00 | 44.00 | 21.00 | 13433.00 | 44.00 | 19.00 | 2831.00 | 9.00 | 3925.00 | 10.00 | 8.00 | 7.00 | 19.00 | 14.00 |
| 197 | 5161.00 | 69.00 | 29.00 | 8779.00 | 29.00 | 12.00 | 3179.00 | 12.00 | 9916.00 | 38.00 | 22.00 | 11.00 | 19.00 | 15.00 |
| 200 | 6768.00 | 86.00 | 37.00 | 5293.00 | 12.00 | 8.00 | 5010.00 | 17.00 | 3134.00 | 5.00 | 6.00 | 13.00 | 22.00 | 20.00 |
| 205 | 7287.00 | 98.00 | 40.00 | 3470.00 | 8.00 | 5.00 | 5037.00 | 15.00 | 2823.00 | 7.00 | 6.00 | 24.00 | 35.00 | 22.00 |
| 206 | 3550.00 | 39.00 | 20.00 | 23486.00 | 88.00 | 32.00 | 1692.00 | 4.00 | 2019.00 | 4.00 | 4.00 | 7.00 | 11.00 | 11.00 |
| 208 | 2876.00 | 40.00 | 17.00 | 23180.00 | 96.00 | 31.00 | 6044.00 | 6.00 | 1771.00 | 4.00 | 3.00 | 8.00 | 13.00 | 13.00 |
| 209 | 3100.00 | 34.00 | 18.00 | 20590.00 | 88.00 | 28.00 | 2233.00 | 6.00 | 3692.00 | 14.00 | 8.00 | 9.00 | 16.00 | 14.00 |
| 261 | 3032.00 | 41.00 | 18.00 | 18205.00 | 66.00 | 25.00 | 2032.00 | 6.00 | 2503.00 | 8.00 | 5.00 | 10.00 | 13.00 | 14.00 |
| 262 | 4686.00 | 46.00 | 25.00 | 13612.00 | 56.00 | 19.00 | 3002.00 | 9.00 | 2637.00 | 6.00 | 5.00 | 8.00 | 13.00 | 16.00 |
| 212 | 4594.00 | 61.00 | 25.00 | 18711.00 | 100.00 | 25.00 | 2569.00 | 8.00 | 1776.00 | 4.00 | 3.00 | 7.00 | 20.00 | 15.00 |
| 213 | 3079.00 | 34.00 | 18.00 | 22979.00 | 98.00 | 31.00 | 5380.00 | 6.00 | 1581.00 | 4.00 | 2.00 | 7.00 | 13.00 | 12.00 |
| 264 | 4779.00 | 46.00 | 26.00 | 10649.00 | 35.00 | 15.00 | 3438.00 | 10.00 | 6009.00 | 17.00 | 13.00 | 8.00 | 13.00 | 14.00 |
| 237 | 2888.00 | 30.00 | 17.00 | 30646.00 | 100.00 | 41.00 | 1572.00 | 5.00 | 1546.00 | 5.00 | 2.00 | 5.00 | 11.00 | 11.00 |
| 235 | 3929.00 | 41.00 | 22.00 | 12965.00 | 43.00 | 18.00 | 2406.00 | 7.00 | 2110.00 | 4.00 | 4.00 | 6.00 | 15.00 | 13.00 |
| 238 | 7137.00 | 77.00 | 40.00 | 5783.00 | 19.00 | 9.00 | 4511.00 | 13.00 | 2908.00 | 4.00 | 6.00 | 6.00 | 21.00 | 17.00 |
| 249 | 3528.00 | 43.00 | 20.00 | 7200.00 | 29.00 | 11.00 | 1888.00 | 6.00 | 20020.00 | 100.00 | 44.00 | 6.00 | 12.00 | 13.00 |
| 248 | 5169.00 | 49.00 | 29.00 | 6163.00 | 24.00 | 9.00 | 3089.00 | 8.00 | 14905.00 | 54.00 | 33.00 | 6.00 | 12.00 | 14.00 |
| 234 | 2874.00 | 20.00 | 17.00 | 15325.00 | 70.00 | 21.00 | 1987.00 | 6.00 | 2148.00 | 4.00 | 4.00 | 9.00 | 11.00 | 20.00 |
| 230 | 5727.00 | 65.00 | 33.00 | 6515.00 | 18.00 | 10.00 | 4620.00 | 10.00 | 3077.00 | 6.00 | 6.00 | 5.00 | 20.00 | 18.00 |
| 226 | 5685.00 | 69.00 | 31.00 | 8599.00 | 32.00 | 12.00 | 3230.00 | 9.00 | 2626.00 | 5.00 | 5.00 | 5.00 | 16.00 | 13.00 |
| 227 | 8007.00 | 97.00 | 44.00 | 3179.00 | 6.00 | 4.00 | 3975.00 | 11.00 | 3013.00 | 5.00 | 6.00 | 5.00 | 20.00 | 17.00 |
| 221 | 6599.00 | 67.00 | 37.00 | 11286.00 | 40.00 | 16.00 | 3842.00 | 8.00 | 3363.00 | 7.00 | 7.00 | 5.00 | 16.00 | 13.00 |
| 222 | 2107.00 | 19.00 | 14.00 | 27611.00 | 100.00 | 37.00 | 1368.00 | 3.00 | 1341.00 | 4.00 | 0.00 | 4.00 | 8.00 | 10.00 |
| 243 | 5292.00 | 61.00 | 29.00 | 6564.00 | 22.00 | 10.00 | 3910.00 | 9.00 | 2999.00 | 6.00 | 6.00 | 8.00 | 23.00 | 31.00 |
| 244 | 5249.00 | 87.00 | 29.00 | 4849.00 | 9.00 | 7.00 | 6824.00 | 11.00 | 3907.00 | 8.00 | 8.00 | 6.00 | 30.00 | 22.00 |
| 273 | 4549.00 | 51.00 | 25.00 | 3597.00 | 6.00 | 6.00 | 2616.00 | 10.00 | 14651.00 | 62.00 | 32.00 | 7.00 | 12.00 | 16.00 |
| 253 | 3100.00 | 38.00 | 18.00 | 19456.00 | 73.00 | 26.00 | 1763.00 | 7.00 | 2795.00 | 10.00 | 6.00 | 8.00 | 15.00 | 12.00 |
| 251 | 4106.00 | 41.00 | 24.00 | 11884.00 | 37.00 | 17.00 | 2633.00 | 6.00 | 7477.00 | 21.00 | 16.00 | 8.00 | 16.00 | 13.00 |
| 256 | 7697.00 | 92.00 | 42.00 | 7854.00 | 24.00 | 11.00 | 3449.00 | 13.00 | 6091.00 | 26.00 | 13.00 | 9.00 | 15.00 | 12.00 |
| 265 | 3545.00 | 39.00 | 20.00 | 19237.00 | 66.00 | 17.00 | 2222.00 | 7.00 | 3521.00 | 11.00 | 7.00 | 10.00 | 16.00 | 16.00 |
| 268 | 6293.00 | 78.00 | 35.00 | 2830.00 | 7.00 | 4.00 | 3381.00 | 13.00 | 3895.00 | 14.00 | 8.00 | 11.00 | 29.00 | 17.00 |
| 270 | 7606.00 | 96.00 | 42.00 | 3524.00 | 11.00 | 6.00 | 4006.00 | 12.00 | 5358.00 | 21.00 | 11.00 | 9.00 | 19.00 | 15.00 |
| 272 | 4152.00 | 49.00 | 23.00 | 16211.00 | 59.00 | 22.00 | 2948.00 | 7.00 | 2192.00 | 4.00 | 4.00 | 7.00 | 15.00 | 19.00 |
| 298 | 8917.00 | 100.00 | 48.00 | 3439.00 | 7.00 | 5.00 | 4528.00 | 16.00 | 7104.00 | 28.00 | 15.00 | 9.00 | 15.00 | 16.00 |
| 300 | 4844.00 | 68.00 | 27.00 | 2465.00 | 5.00 | 5.00 | 3039.00 | 9.00 | 23333.00 | 100.00 | 52.00 | 6.00 | 16.00 | 16.00 |
Table 4--Summary statistics f X-ray diffraction measurements.
| X-ray Measurements | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minerals | Quartz | Calcite | Feldspar | Dolomite | Kaolinite | Illite | Chlorite | |||||||
| Statistics | Peak Height |
Peak Area |
Percent | Peak Height |
Peak Area |
Percent | Peak Height |
Peak Area |
Peak Height |
Peak Area |
Percent | Peak Height |
Peak Height |
Peak Height |
| Mean | 76.8 | 6611.0 | 36.2 | 26.9 | 7648.0 | 10.9 | 13.6 | 4332.0 | 13.7 | 4351.0 | 9.1 | 15.5 | 28.8 | 17.6 |
| Standard Deviation |
25.1 | 2673.0 | 13.4 | 28.5 | 6546.0 | 8.6 | 6.8 | 1537.0 | 20.7 | 4148.0 | 9.4 | 10.0 | 10.9 | 4.7 |
| Median | 86.0 | 6476.5 | 36.5 | 12.5 | 5694.0 | 7.0 | 13.0 | 4251.0 | 7.0 | 3067.0 | 6.0 | 16.0 | 21.5 | 17.0 |
| Minimum | 17.0 | 2107.0 | 14.0 | 3.0 | 1982.0 | 3.0 | 3.0 | 1368.0 | 3.0 | 1341.0 | 0.0 | 4.0 | 7.0 | 9.0 |
| Maximum | 100.0 | 18913.0 | 96.0 | 100.0 | 30646.0 | 41.0 | 39.0 | 9967.0 | 100.0 | 23333.0 | 52.0 | 54.0 | 62.0 | 33.0 |
Figure 9--Histograms showing the variation and distribution of the major mineral components measured by X-ray diffraction (total samples 128); peak areas for (a) quartz, (b) calcite, (c) feldspar, (d) dolomite; peak heights for (e) kaolinite (f) illite, and (g) chlorite. Vertical scale is 0 to maximum value in 10 divisions.
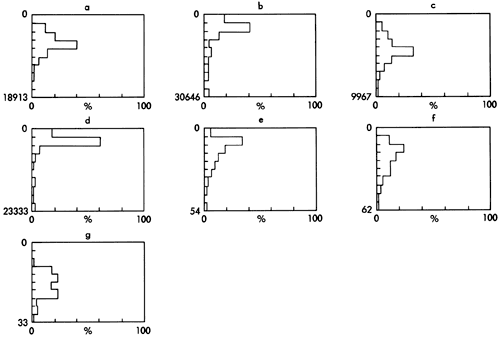
Quartz forms the major mineral component of most shales and varies from 14 to 96 percent as a unimodal population with a slightly skewed distribution. The tail towards the higher value end of the histogram is produced by a number of sandstones and siltstones with high quartz values.
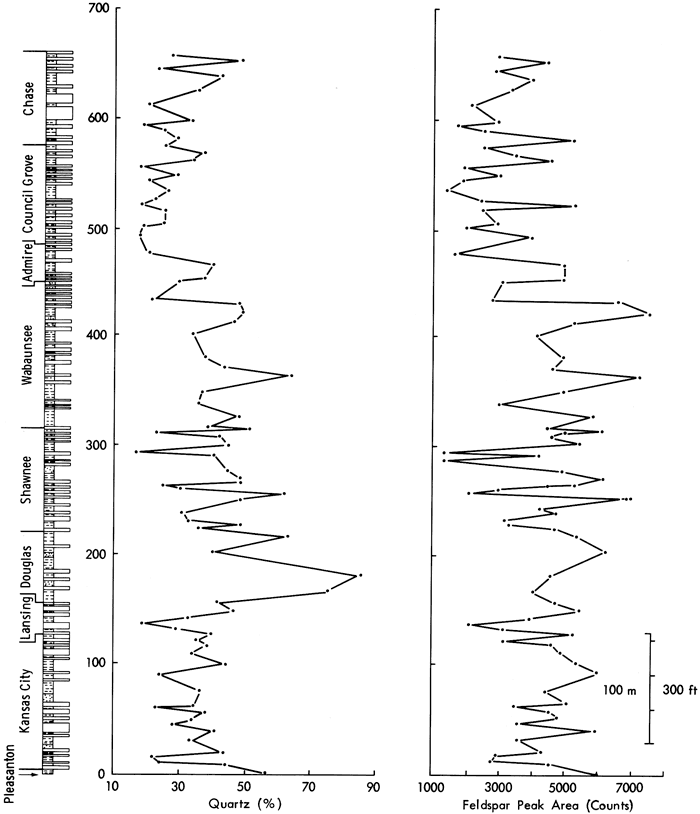
Stratigraphically, the distribution of quartz in Upper Pennsylvanian and Lower Permian shales (Figure 10) can be interpreted in terms of five zones. [Note: Note that the stratigraphic distributions illustrated, with the exception of Figures 21, 36, 44, 47, and 48 are calculated by averaging, for individual stratigraphic members, all included sample values. By carefully examining the mineralogical and geochemical results, most of the data variation was found to be between members rather than within members, allowing a certain degree of artistic license in illustrating the stratigraphic distributions. It is primarily for simplicity of illustration therefore that sample averages are employed. However, it should also be noted that interpretation of these distributions takes the generalization into account, and that the figures considered of major importance to the conclusions drawn in each chapter contain all sample values (Figures 21, 36, 44, 47, and 48).] First the Pleasanton, Kansas City, and Lansing Groups are distinguished by samples with relatively low concentrations of quartz (from 17 to 57 percent) and large fluctuations in concentration between samples. Second, the Douglas Group clastics are rich in quartz (up to 85 percent) as they consist mainly of coarse silts and sands, whereas the succeeding Shawnee Group shales are characterized by variable quartz concentrations (16 to 96 percent). The Wabaunsee shales have more stable quartz values (21 to 52 percent) indicating consistent environmental conditions, and a general tendency for smaller concentrations in the younger beds. This is reversed in the Lower Permian Groups where increased values are recorded in Chase Group samples. Nevertheless, the percentages determined are still low (14 to 48 percent).
Feldspar measurements of peak area (Figure 10) and peak height stratigraphically vary in a similar manner to the quartz distribution, indicating a detrital association between the minerals. The graphs correspond within the Pleasanton, Kansas City, Lansing, Shawnee, and Lower Permian Groups, but the high quartz values in the Douglas samples are not present in the feldspar measurements.
Figure 10--Distribution of quartz (%) and feldspar (peak area) in shales from the Upper Pennsylvanian and Lower Permian of Kansas.
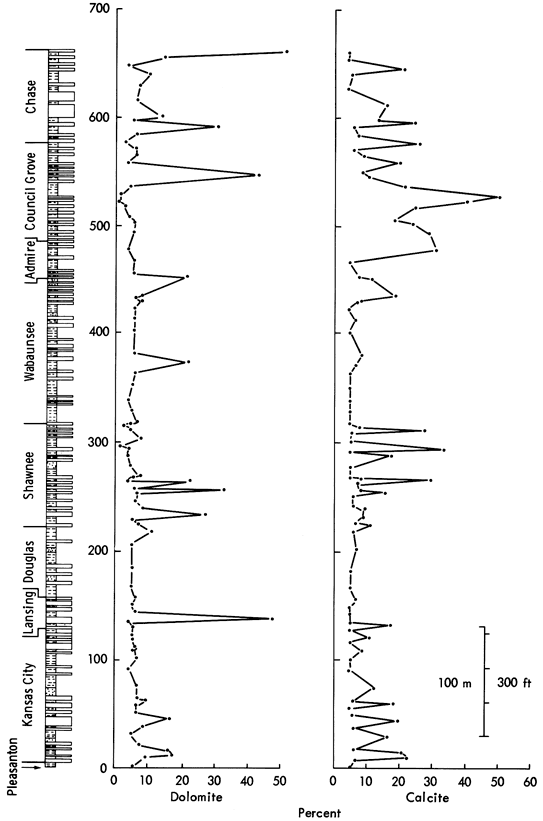
Periods in which low quartz values are recorded such as the Shawnee, Lower Permian, Pleasanton, Kansas City, and Lansing Groups are marked by intensive carbonate generation. Both calcite and dolomite distributions (Figure 11) show low values in the Douglas and Wabaunsee Groups (averaging 5 to 10 percent carbonate), whereas calcite shows high concentrations in the Lower Permian, Upper Shawnee, and Kansas City Groups and dolomite has high values in the Lansing, Council Grove, and Chase Groups (Figure 11). Differences in the peak area histograms of these minerals (Figure 9) indicate a possible cause for the variation between carbonate stratigraphic distributions. The calcite histogram approximates to a "normal" curve (averaging 15 to 25 percent), whereas dolomite shows a bimodal histogram with one group of samples with low values (5 to 10 percent dolomite) and another with exceptionally high dolomite (50 percent average), indicating that the dolomite present may be of secondary diagenetic origin as opposed to the primary precipitated calcite. Petrological evidence supports this conclusion.
Figure 11--Variation of calcite and dolomite in Upper Pennsylvanian and Lower Permian shales of Kansas. Vertical scale matches that in Figure 10 allowing stratigraphic comparisons.
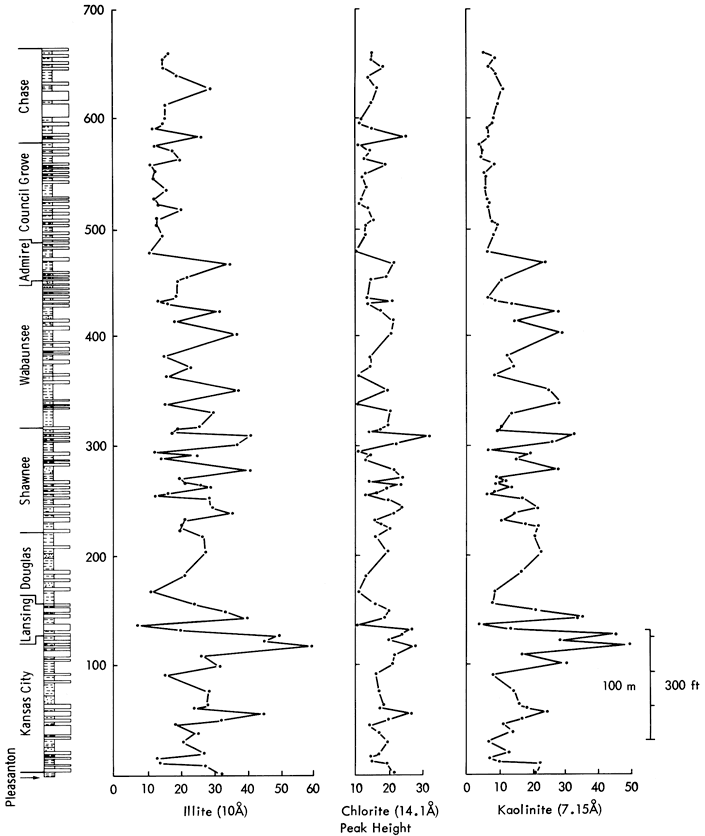
The three major clay mineral components have skewed (kaolinite and illite) or irregular bimodal histograms (chlorite). There is little evidence for stratigraphic control of clay mineral distributions (Figure 12), although in the Upper Kansas City Group, a series of high peak height values are recorded for all three clay minerals. One can also, by inspection of the data, note a similarity between the clay mineral distributions and a lack of similarity to other mineral distributions.
Figure 12--Variation in illite, chlorite, and kaolinite clay mineral peak heights from Upper Pennsylvanian and Lower Permian shales of Kansas. Vertical scale is identical to Figure 10 and 11 allowing stratigraphic comparisons.
This association of minerals is supported statistically, in the similarity matrix (Table 5). Here we can see that quartz, feldspar, and clay minerals have high positive correlations and form one combination of minerals, whereas the carbonates have negative correlations with respect to the other minerals. High correlations are recorded between quartz and calcite; kaolinite, chlorite, and illite; and between illite and calcite. These are produced primarily by the inverse relationship between mineral groups characteristic of clastic and calcareous deposits, and by a close environmental relationship between the clays.
Table 5--Similarity matrix for the mineral distributions employing correlation coefficients. Note that although peak area measurements were used for quartz, calcite, feldspar, and dolomite, and peak heights for kaolinite, illite, and chlorite, the correlation coefficient is a unitless measure and as such the original measurement units have no influence on the similarities recorded. Only the lower half of the correlation matrix is reproduced as this is a mirror image of the upper half. Correlations significant at the 95 percent level are indicated by * and at the 99 percent level by **.
| Quartz | 1.00 | ||||||
|---|---|---|---|---|---|---|---|
| Feldspar | 0.26** | 1.00 | |||||
| Calcite | -0.62** | -0.43** | 1.00 | ||||
| Dolomite | -0.20* | -0.21* | -0.03 | 1.00 | |||
| Kaolinite | 0.20* | 0.10 | -0.32** | -0.24** | 1.00 | ||
| Illite | 0.26** | 0.23** | -0.52** | -0.24** | 0.73** | 1.00 | |
| Chlorite | 0.16 | 0.14 | -0.45** | -0.25** | 0.48** | 0.69** | 1.00 |
| Mineral | Quartz | Feldspar | Calcite | Dolomite | Kaolinite | Illite | Chlorite |
On examining the mineral distributions by eye, it is apparent that quartz and calcite show repetitive variability in a stratigraphic sense, i.e., there is a stratigraphic oscillation of peaks and troughs in the distribution of quartz and calcite in Upper Pennsylvanian and Lower Permian shales. This we can interpret in terms of changing sedimentary environments. For example, shales in the Pleasanton, Kansas City, and Lansing beds oscillate between calcite- and quartz-dominated mineralogies. This pattern is repeated in the Shawnee and to a minor extent in the Lower Permian. Between these oscillatory stratigraphic zones, there are beds of relatively quartz-rich shales, namely the Douglas and Wabaunsee Group clastics. It appears, therefore, that there are two cycles of events being recorded in the quartz and calcite mineral distributions, first a short-term, bed-by-bed oscillatory variation, and second a long-term group-by-group repetition.
The observed mineralogical periodicity in the sediments can be tested using a statistical procedure designed originally for wave-form analysis in electrical engineering, Fourier analysis (Preston and Henderson, 1964; Harbaugh and Merriam, 1968; Davis, 1973; Dunn, 1974). From this the natural cycles can be recognized. A series of computations have to be performed prior to the Fourier analysis, however, to transform the data into a suitable form for entry into the computer program. Initially, using a linear interpolation procedure (Davis, 1973), the data is changed from sample values with irregular depth intervals to a pattern of values taken at a constant depth interval. Ten feet (3.05 m) was chosen as the most practical equal spacing interval, as Schwarzacher (1967) has shown that the smallest lithological cycle occurs at intervals of 45 feet, and any larger interval would obscure these cycles. The total number of data points is, in this way, increased from 128 to 218. In the case of the quartz distribution shown in Figure 13A, it was noted that, although major peaks correspond to those in the raw data plot (Figure 10), it was possible that through this interpolation process there may have been a significant alteration of the data variability and trend. A linear regression analysis showed that, although the trend and goodness of fit measures had changed, the differences were acceptable, i.e., goodness of fit increased from .06 to .13 and the correlation coefficient from .26 to .37. The equal spaced data were then run through a Fourier analysis and the raw power spectrum calculated (Figure 14). It can be seen that the two main peaks corresponding to the third and seventh harmonics (multiples of the fundamental wavelength chosen, i.e., 10 feet), are indistinguishable from surrounding points. Noise in the equal spaced data may have produced this power spectrum fuzziness. An 11-term smoothing operation on the raw data, however, removed the short-term noise and revealed the power spectrum shown in Figure 14 (dashed lines). Features retained in the smoothed power spectrum include two main peaks at the third and seventh harmonics and subsidiary peaks at the fifth, 11th, 13th, and 14th. These indicate fundamental periodic elements in the quartz distribution with 30, 50, 70, 110, 130, and 140 foot intervals that may be explained in terms of oscillations in the quartz values of Pleasanton, Kansas City, Lansing, Shawnee, and Lower Permian shales.
Figure 13--Distribution of quartz (in percent) after (a) equal spacing by a linear interpolation procedure, (b) equal spacing and smoothing (using an 11-term moving average equation). For a stratigraphical comparison, see figure 10.
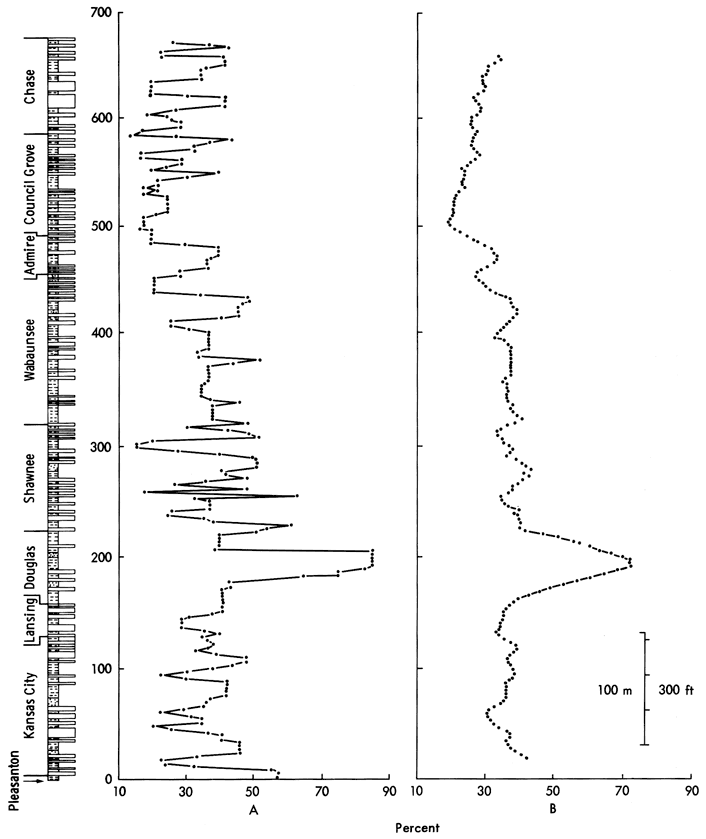
Figure 14--Power spectrum of quartz in the Upper Pennsylvanian and Lower Permian shales of Kansas. The fundamental wavelength chosen is 10 ft.
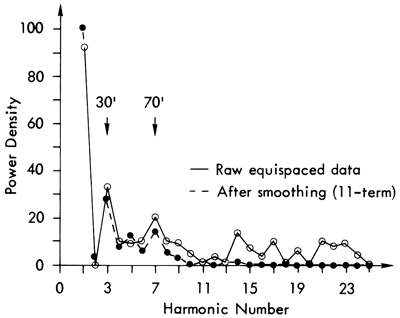
Although these features were recognized initially in the quartz distribution, no evidence for these conclusions could be found in the power spectra of dolomite, feldspar, illite, chlorite, or kaolinite. In calcite's spectrum, on the other hand, a peak was developed at the seventh harmonic and a plateau at the third. However, the negative association of calcite with quartz in all these results may only be a reflection of the closed data set under examination (Vistelius and Sarmanov, 1961; Krumbein and Graybill, 1965; Chayes, 1971; Davis, 1973).
Multivariate Statistical Analysis of the Mineralogical Distribution
In the previous section we have noted how some minerals vary stratigraphically in relation to each other, and how some show periodic elements in their distributions. However, it is very rare in the earth sciences to find a single factor such as quartz percentage controlling the development of sedimentary environments. It is normally a combination of variables that produces the overall sedimentological effects, e.g., in Kansas an assemblage of mineral distributions may relate more approximately to the periodicity of shale deposition than to one mineral phase.
Multivariate classification techniques enable the investigator to quantify the relationships between variables and classify shale samples into groups dependent on the variable relationships. The distribution of individuals within the groups matched against depth can then be used to indicate stratigraphic divisions not previously noted and any repetition in the shale group associations.
With this in mind, an R-mode principal components analysis (Davis, 1973; Joreskog et al., 1976) of the peak area measurements of quartz, calcite, feldspar, and dolomite and the peak heights of kaolinite, illite, and chlorite was performed. Three components were found to be significant (i.e., eigenvalues greater than one). The principal axis component loadings (Figures 15 and 16a) reveal similar relationships to the simple correlation coefficients. On the first component, the carbonate minerals have high positive loadings, whereas illite, chlorite, kaolinite, quartz, and feldspar have high negative loadings. This component can therefore be considered as an indicator of elastic or carbonate conditions of sedimentary deposition. Similarly, the second component's high positive loadings for quartz and feldspar and high negative loadings of calcite and clay minerals reflect the detrital versus nondetrital origin of most of the shales. Quartz and feldspar are commonly associated with detrital sediments, and calcite is normally a nondetrital mineral. It would seem at first glance unusual to note a connection between clay minerals and calcite, but it must be remembered that many of the thin shales occurring between limestones in the Kansas City and Shawnee Groups have high values for both calcite and clay minerals. Alternatively, this component may reflect the high quartz and feldspar content of some coarse shales, silts, and sandstones and corresponding lack of calcite and clay minerals in these samples. The high negative loading of dolomite on the final significant component represents the influence of the irregular dolomite distribution and may be an indicator of primary or secondary mineral origin. As noted previously, the irregular distribution of dolomitic shales indicates a diagenetic origin for the dolomite.
Figure 15--Principal axis loadings of the significant components (i.e., components with eigenvalues > 1.0).
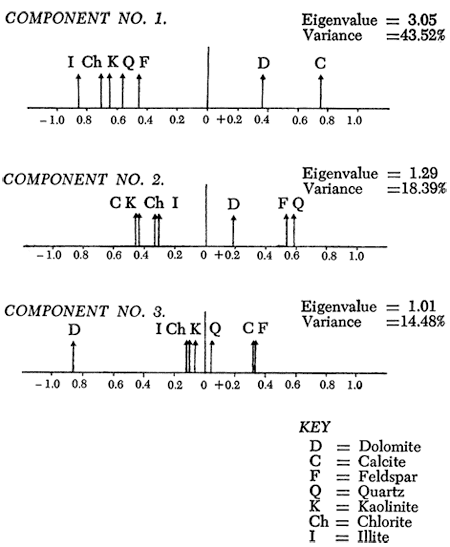
Figure 16--Plot of loadings on two components extracted from raw mineralogy data. Variables plotted are (1) quartz, (2) feldspars, (3) calcite, (4) dolomite, (5) kaolinite, (6) illite, (7) chlorite. Note that promax axes are not orthogonal (correlation = -0.0308) but, for simplicity, are drawn so.
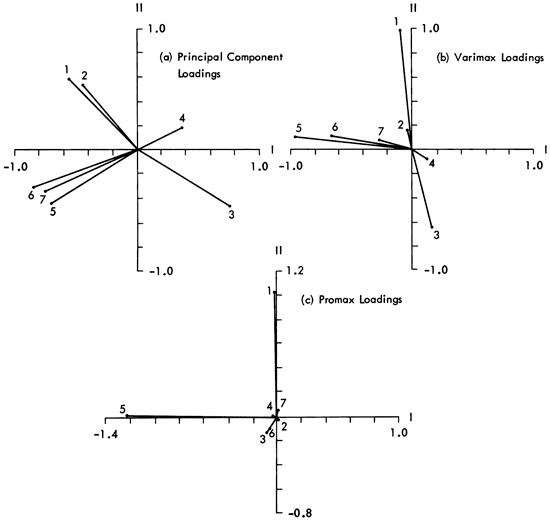
We can now express the variation of mineralogy in the Kansas shales in terms of three geologically interpretable components. An attempt can, however, be made to clarify the geological interpretation of the components by emphasizing the loadings of the influential variables using varimax and promax rotation procedures (Imbrie and Purdy, 1962; Harman, 1967). With the component loadings matrix, we find that on varimax rotation there is an accentuation of the clay mineral loadings (Figure 16b) on Factor 1, and the quartz/calcite antipathy is weighted more strongly on Factor 2. The highlighting of known information has proved valuable but, with promax rotation (an oblique factor rotation) of the mineralogical data (Figure 16C), interpretation becomes a major problem. The results of promax rotation will therefore only be included for comparative purposes.
Having established the variable relationships, scores of samples on the components are calculated by Harman's short regression and square root method (Harman, 1967, pp. 362-369) and form the basis of the sample classification now undertaken. A Q-mode cluster analysis (Davis, 1973; Cubitt, 1975b), employing an agglomerative, polythetic unweighted pair-group algorithm with distance coefficients, arranges the samples on the basis of their scores on component axes into the hierarchical structure (dendrogram) shown in Figure 17. The dendrogram can be divided into eight clusters (labeled A to H) that represent natural subdivisions of the sample classification. However, before placing faith in these results, it is necessary to ensure that the groups of samples are not just a product of the clustering procedure but are unique subdivisions of the sample population. A multiple discriminant analysis (a procedure for statistically developing functions that maximize between group variation) of the raw mineralogical data, arranged in the same manner as the dendrogram, produces a distribution of groups shown in Figures 19 and 20. The mineralogical nature of the groups can be determined by superimposing the relative contribution of each variable to each axis (Figure 18) on the discriminant plot. H and G are, therefore, recognized as groups of highly dolomitic shales and D as more argillaceous than the others. On the other hand, quartz predominates in group C samples and calcite in E. A, B, and F appear to be intermediate groups in both Figures 19 and 20. Examining the raw mineralogical data, it is noticeable that samples in F are more akin to group E than to B or A. This is emphasized on the plot of first and third discriminant axes (Figure 20). For simplicity therefore E and F are combined.
Figure 17--A dendrogram produced by a Q-mode cluster analysis of mineralogical data. Clusters produced are outlined on the left of the diagram. The reverse linkage is caused by the pair group method of clustering.
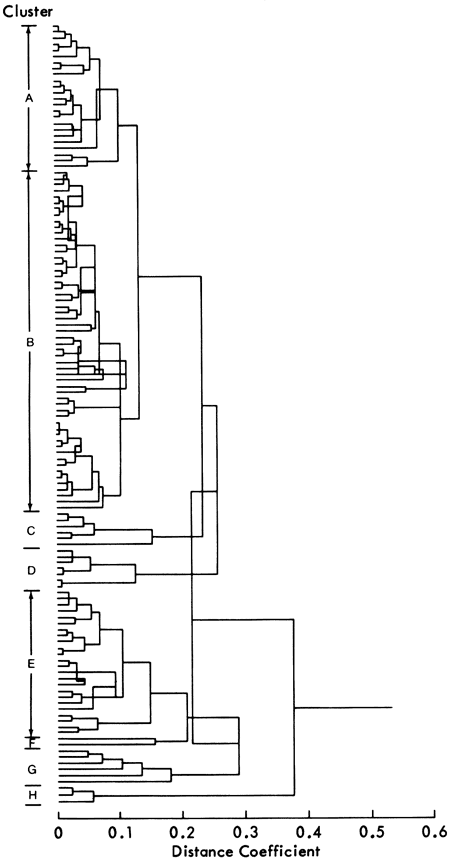
Figure 18--Loadings of variables on significant discriminant axes (i.e., eigenvalue > 1.0)--D = dolomite, C = calcite, Q = quartz, K = kaolinite, F = feldspar, Ch = chlorite, I = illite.
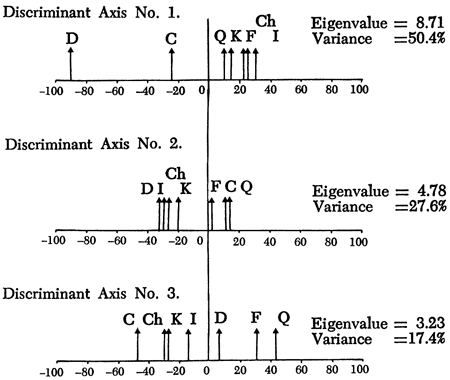
Figure 19--Distribution of cluster analysis groups on the first two discriminant axes. Primary mineralogical variation is superimposed for reference. Diamonds represent the mean of each group at 1 standard deviation of each axis.
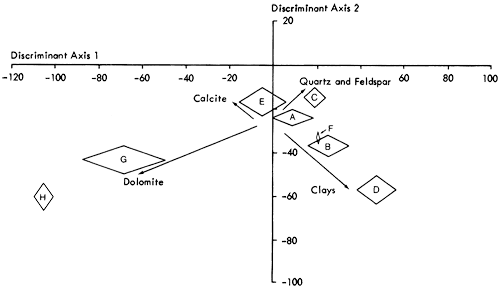
Figure 20--Distribution of cluster analysis groups on the first and third discriminant axes. Primary mineralogical variation is superimposed for reference. Diamonds represent the mean of each group ± 1 standard deviation on each axis.
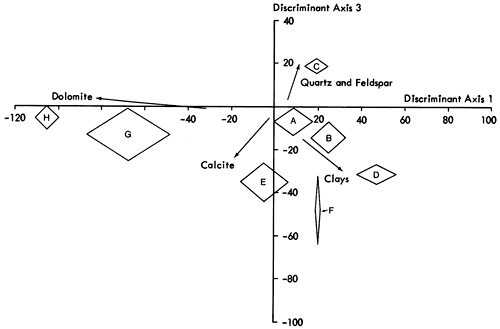
A number of individuals from groups whose boundaries are closely related (i.e., A, B, and E) are also misplaced by the clustering algorithm. Manual examination of sample scores on the first three discriminant axes indicate that samples 281, 298, 270, 256, 79, 41, 136, and 197 should be attached to group E, while sample 20 is more naturally associated with group A. On reallocation we can conclude that the seven groups established are now mineralogically distinct.
A plot of samples arranged according to their cluster analysis group against stratigraphic position (Figure 21) reveals seven zones in the Upper Pennsylvanian and Lower Permian stratigraphic column:
- Pleasanton and Lower Kansas City Groups;
- Upper Kansas City Group;
- Lansing Group;
- Douglas Group;
- Shawnee Group (including the Upper Lawrence Formation of the Douglas Group);
- Wabaunsee and Lower Admire Groups; and
- Upper Admire, Council Grove, and Chase Groups.
Figure 21--Stratigraphic distribution of samples arranged on a horizontal scale according to their cluster analysis class (Figure 17). The order of classes along the horizontal scale is arbitrary but may be interpreted as calcareous classes on the right and non-calcareous on the left.
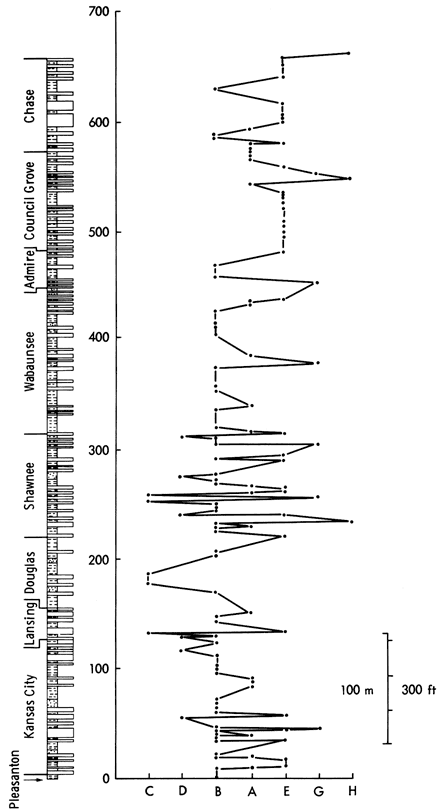
Each division is characterized by differing mineralogical and sedimentological conditions. The Pleasanton and Lower Kansas City Groups, Lansing Group, and Shawnee Group zones show an alternation of calcite- (normally associated with limestones) and quartz-rich shales (predominantly clusters B, E, and A) indicating oscillating calcareous and noncalcareous environments. The noncalcareous sediments could have been derived from either the lowlands to the north and east of Kansas or from the Ouachita orogenic belt to the south. Source areas for the shales may be distinguished by comparing their geochemical and mineralogical results. On the other hand, the calcareous sediments were deposited at a time of predominantly limestone deposition and could represent either normal marine shales or the equivalent of limestone insoluble residues.
The Douglas and Wabaunsee Groups are dominated by shales that have quartz as the primary mineral component (clusters B and C). The Douglas Group shows the most extreme values of quartz content as the sediments are largely coarse siltstones and sandstones. The Wabaunsee also has coarse units but no samples are recorded in cluster C, unlike the Douglas or even the Shawnee Group.
In the Upper Admire, Council Grove, and Chase Groups, considerable periods of carbonate deposition (cluster E) are broken by an occasional quartz-rich shale (cluster B) or intermediate shale (cluster A), indicating an overall change in the sedimentary environment. The increase in carbonate formations is a product of the gradual restriction of the Kansas epeiric sea during Early Permian times (Heckel, 1972a) and is followed by a succession of evaporitic and calcareous beds in the Middle Permian. After establishing the nature of mineralogical variation with respect to stratigraphy, we can postulate a group-by-group control of sedimentary environments by local or regional tectonic events.
Prev Page--Introduction || Next Page--Emission Spectroscopy
Kansas Geological Survey, Geology
Placed on web May 6, 2009; originally published December 1979.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/217/03_miner.html