Prev Page--Hydrologic Properties || Next Page--Formations
Quality of Ground Water
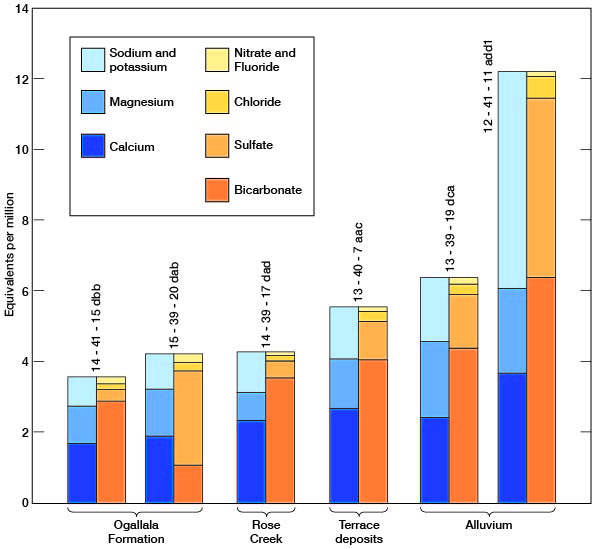
The chemical character of ground water in Wallace County is indicated by analyses of samples from wells deriving water from the principal aquifers (Table 7). The analyses of water were by Howard A. Stoltenberg, chemist, in the Sanitary Engineering Laboratory of the Kansas State Board of Health. The results of the analyses are given in parts per million. Factors for converting parts per million of mineral constituents to equivalents per million are given in Table 8. The analyses show only the dissolved mineral constituents and do not indicate the sanitary condition of the water. Representative analyses of ground water from the principal aquifers are shown in Figure 20.
Table 8--Factors for converting parts per million to equivalents per million.
| Mineral constituents | Chemical symbol | Factor |
|---|---|---|
| Calcium | Ca++ | 0.04990 |
| Magnesium | Mg++ | 0.08220 |
| Sodium | Na+ | 0.04350 |
| Potassium | K+ | 0.02558 |
| Carbonate | CO3-- | 0.03333 |
| Bicarbonate | HCO3- | 0.01639 |
| Sulfate | SO4-- | 0.02082 |
| Chloride | Cl- | 0.02820 |
| Fluoride | F- | 0.05263 |
| Nitrate | NO3- | 0.01613 |
Figure 20--Graphic of chemical constituents of samples of water from Wallace County.
Chemical Constituents in Relation to Use
The following discussion of the chemical constituents of ground water has been adapted in part from publications of the U. S. Geological Survey and the State Geological Survey of Kansas. (See Table 7 for chemical analyses of water from Wallace County.)
Dissolved Solids--The residue that is left after a sample of water has evaporated consists mainly of the dissolved minerals in the original sample, but may also include some organic material and water of crystallization. Water containing less than 500 ppm (parts per million) of dissolved solids generally is satisfactory for domestic and many industrial purposes. Water containing more than 1,000 ppm of dissolved solids is likely to contain enough of certain constituents to cause noticeable taste or to make the water unsuitable in other respects.
The dissolved solids in samples of water collected in Wallace County ranged from 193 to 4,420 ppm. Most of the samples were relatively low in dissolved solids; more than half contained less than 300 ppm. Only 5 samples contained more than 500 ppm, and only 1 sample contained more than 1,000 ppm.
Hardness--Hardness of water is recognized most commonly by the amount of soap needed to produce a lather or suds and by an insoluble scum that forms during washing processes. Calcium and magnesium cause almost all the hardness of water and may be deposited as constituents of scale in heat-exchange equipment.
The hardness of water is of two types--carbonate hardness and noncarbonate hardness. Carbonate hardness includes that portion of the calcium and magnesium that would combine with the bicarbonate and the small amount of carbonate that are present. Carbonate hardness can be virtually removed by boiling the water, which causes the magnesium and calcium to precipitate. Noncarbonate hardness is caused by that portion of calcium and magnesium that would combine with the sulfate, chloride, and nitrate ions that are present, plus the slight hardness of other minor constituents. Noncarbonate hardness cannot be removed by boiling.
Water that has a hardness of less than 50 ppm is considered soft. A hardness of 50 to 150 ppm is satisfactory for most purposes, but the amount of soap needed increases with hardness, and water in the upper part of this range will cause considerable scale in steam boilers. Hardness of more than 150 ppm is obviously noticeable, and water that has a hardness of 200 or 300 ppm is considered undesirable for household purposes until it is treated by a softening process. Where municipal water supplies are softened. the hardness is generally reduced to about 100 ppm.
Hardness of the water samples collected in Wallace County ranged from 124 to more than 1,000 ppm. Only 9 samples had a hardness of 150 ppm or less. Six samples had a hardness of more than 300 ppm and 1 sample had 1,480 ppm.
Nitrate--The nitrate content of natural water may vary greatly and frequently may, seem unrelated to any geologic formation. Although some nitrate may be derived from nitrate-bearing rocks and minerals in the water-bearing formation, strong concentrations of nitrate probably are from other sources. Nitrates are dissolved readily from soils that contain nitrate concentrations derived from plants, nitrate fertilizer, animal waste, or nitrifying action. High nitrate concentrations in water from a well may be due to direct flow of surface water into the aquifer. Because privies, cesspools, and barnyards are sources of organic nitrogen, a large amount of nitrate in well water may indicate harmful bacteria or pollution.
In the last two decades investigations of the effects of nitrate have shown that too much nitrate in water may cause cyanosis in infants (blue babies) when it is used for drinking or in the preparation of the formula for feeding. The Kansas State Board of Health, as well as the U. S. Public Health Service, regards 45 ppm as the safe limit of nitrate (as NO3-). This amount of nitrate is equivalent to 10 ppm of nitrogen. Water containing as much as 90 ppm of nitrate generally is considered very dangerous to infants, and water containing as much as 150 ppm may cause severe cyanosis. Moderate nitrate concentrations are seemingly not harmful to older children or adults. Nitrate cannot be removed from water by boiling.
The nitrate content of samples of water collected in Wallace County ranged from less than 1 to 84 ppm (Table 7). Only one sample contained more than the 45 ppm limit set by the State Board of Health.
Fluoride--Fluoride generally is present only in small amounts in ground water. However, the fluoride content of drinking water should be known, because if children drink water containing too much fluoride while their permanent teeth are forming, it may cause mottling of the tooth enamel. if the fluoride content is as much as 4 ppm, about 90 percent of the children using the water may have mottled tooth enamel (Dean, 1936). Although too little fluoride has a detrimental effect, a smaller amount in drinking water, about 1 ppm, lessens the incidence of tooth decay (Dean and others, 1941). The U. S. Public Health Service (1961) recommended standards for the content of mineral constituents in drinking water that is used on interstate carriers. The recommended maximum content of fluoride is 1.5 ppm.
Although the fluoride content of samples of water collected in Wallace County ranged from 0.2 to 2.6 ppm, most samples contained less than 1.5 ppm. (Table 7).
Chloride--Chloride is quite abundant in nature, and many rocks contain small to large amounts of chloride salts which may be dissolved by ground water. Chloride has little effect on the suitability of water for ordinary use unless present in such concentrations as to make the water unpotable or corrosive. Water that contains less than 150 ppm of chloride is satisfactory for most purposes. Water containing more than 250 ppm generally is objectionable for municipal supplies, and water containing more than 350 ppm is objectionable for most irrigation or industrial uses; water containing as much as 500 ppm has a disagreeable taste. However, animals can drink water with much greater chloride concentration; the upper limit in water consumed by cattle is believed to be as much as 4,000 to 5,000 ppm.
Most water samples collected in Wallace County were low in chloride content, which ranged from only 5.5 to 103 ppm. All but 3 samples contained less than 50 ppm.
Iron--Iron and manganese in quantities that exceed a few tenths of a part per million are undesirable, as they stain fabrics and plumbing fixtures and produce an objectionable coloration in the water. The limit generally specified is 0.3 ppm. Water in the ground may contain considerable iron in the ferrous state, but upon exposure to air most of the iron is oxidized and precipitated as reddish-brown ferric hydroxide. Iron can be removed from most water by aeration and filtration, but some water requires additional treatment. Drinking-water standards of the U. S. Public Health Service (1961) recommend that the iron content should not exceed 0.3 ppm and the manganese 0.05 ppm.
The iron content of water samples collected in Wallace County ranged from 0.2 to 2.3 ppm; eleven out of 35 samples contained more than 0.3 ppm.
Sulfate--Sulfate (SO4--) in ground water is derived principally from gypsum or anhydrite (calcium sulfate) and from the oxidation of pyrite (iron disulfide). Magnesium sulfate (Epsom salt) and sodium sulfate (Glauber's salt), if present in sufficient (quantities, will impart a bitter taste to the water, and the water may act as a laxative for people not accustomed to drinking it. More than 250 ppm of sulfate in drinking water generally is undesirable (U. S. Public Health Service, 1961). Most water samples collected in Wallace County were low in sulfate; only four samples exceeded 250 ppm, two of which exceeded 1,000 ppm.
Silica--Silica combined with oxygen in the form of SiO2, is called silica. Silica is a mineral constituent in most ground water. Except for the scale it may form, silica has little effect on the usefulness of water for most purposes. Silica may be deposited as scale with other incrustants, generally in the form of calcium or magnesium silicate. The silica content of water samples collected in Wallace County ranged from 17 to 50 ppm.
Bicarbonate--Bicarbonate, the predominant anion in ground water in Wallace County, and carbonate cause alkalinity of ground water. The concentration of bicarbonate in samples of water from wells in Wallace County ranged from 110 to 410 ppm.
Sodium--The sodium content of water to be used for irrigation is important because a large percentage (equivalents per million of sodium divided by equivalents per million of sodium, potassium,, calcium, and magnesium, expressed as a percentage) is undesirable. The effect of sodium in irrigation water is discussed on the following pages.
Suitability of Water for Irrigation
This discussion of the suitability of water for irrigation is based on Agriculture Handbook 60, U. S. Department of Agriculture (U. S. Salinity Laboratory Staff, 1954).
In areas of sufficient rainfall and ideal soil conditions, soluble salts in the soil or salts added to the soil with water are carried downward by percolation and ultimately reach the water table. Soil that was originally nonsaline and nonalkali may become unproductive if an excess of soluble salts or exchangeable sodium is allowed to accumulate as a result of improper irrigation and soil management. If the amount of water applied to the soil is not more than is needed by plants, water will not percolate downward below the root zone, and mineral matter will accumulate at that point. Likewise, impermeable soil zones near the surface can retard the downward movement of water and cause waterlogging of the soil and deposition of salts.
The characteristics that seem to be most important in determining the suitability of irrigation water are the dissolved-solids content and the concentration of sodium ions. For diagnosis and classification, the dissolved-solids content of irrigation water can be estimated from specific conductance, which is a measure of the capacity of the inorganic salts in solution to conduct an electrical current. The specific conductance can be measured accurately in the laboratory, or it can be approximated by multiplying the total equivalents per million of cations (calcium, magnesium, sodium, and potassium) by 100 or by dividing the dissolved-solids content in parts per million by 0.64.
Salt-sensitive crops such as strawberries, green beans, and red clover may be affected adversely by irrigation water having a specific conductance exceeding 250 micromhos per centimeter, but water having a specific conductance below 700 micromhos per centimeter is generally satisfactory for irrigation, insofar as salt content is concerned. Water in the range of 750 to 2,250 micromhos per centimeter is widely used, and satisfactory crop growth is obtained under good management and favorable drainage conditions. But saline conditions will develop if leaching and drainage are inadequate. Use of water having a conductivity of more than 2,250 micromhos per centimeter is the exception, and few cases can be cited where such water has been used successfully.
The sodium-adsorption ratio may be determined by the formula
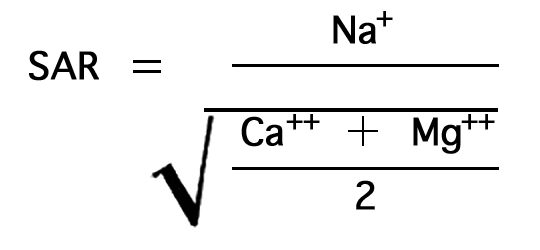
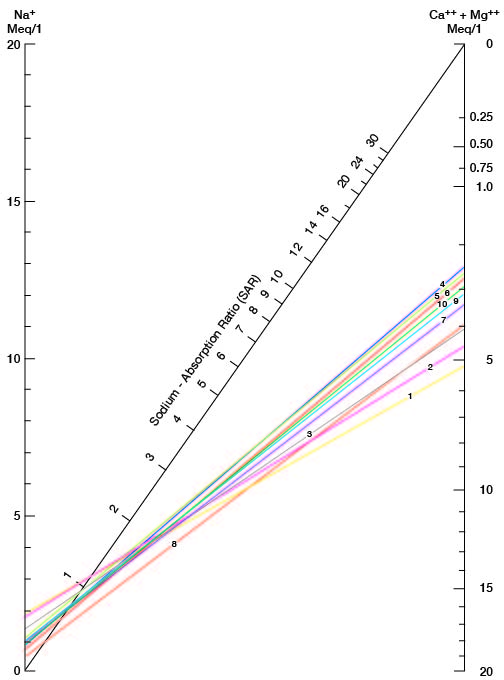
where the ionic concentrations are expressed in equivalents per million. It may be determined also by use of the nomogram shown in Figure 21. In using the nomogram to determine the sodium-adsorption ratio of a water sample, the concentration of sodium expressed in equivalents per million is plotted on the left scale (A), and the concentration of calcium plus magnesium expressed in equivalents per million is plotted on the right scale (B). (In this report the concentrations of sodium and potassium are given together as sodium, but the amount of potassium is considered negligible.) A line connecting these two points intersects the sodium-adsorption-ratio scale (C) at the sodium-adsorption ratio of the water. Table 9 gives the wells and index numbers of samples for which analyses are plotted in Figures 21 and 22; also given are sodium-adsorption ratios, approximate electrical conductivities, and values for sodium and for calcium plus magnesium.
Figure 21--Nomogram for determining the sodium-adsorption ratio of water.
When the sodium-adsorption ratio and the electrical conductivity of a water are known, the suitability of the water for irrigation can be determined graphically by plotting these values on the diagram shown in Figure 22. Low-sodium water (S1) can be used for irrigation on almost all soils with little danger that harmful levels of exchangeable sodium will develop. Medium-sodium water (S2) may be used safely on coarse-textured or organic soils having good permeability, but S2 water will present an appreciable sodium hazard in certain fine-textured soils, especially under poor leaching conditions. High-sodium water (S3) may produce harmful levels of exchangeable sodium in most soils and will require special soil management such as good drainage, leaching, and additions of organic matter. Very high-sodium water (S4) generally is unsatisfactory for irrigation unless special action is taken, such as addition of gypsum to the soil.
Figure 22--Classification of water being used for irrigation in Wallace.
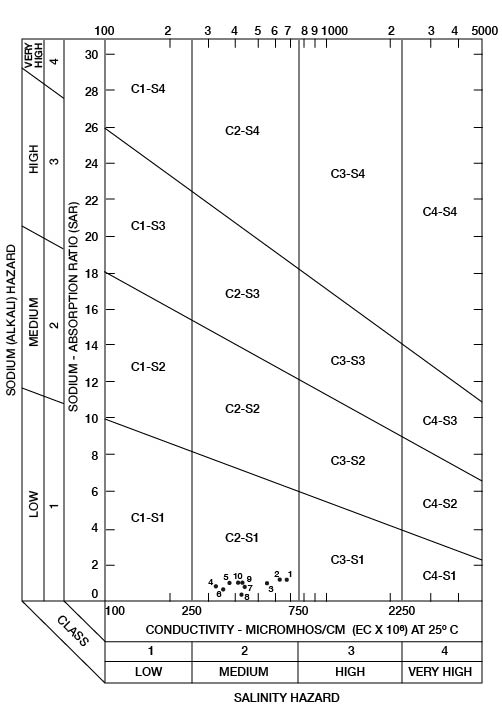
Low-salinity water (C1) can be used for irrigation of most crops on most soils with little likelihood that soil salinity will develop. Medium-salinity water (C2) can be used if a moderate amount of leaching occurs. Crops that tolerate moderate amounts of salt, such as potatoes, corn, wheat, oats, and alfalfa, can be irrigated with C2 water without special practices. High-salinity water (C3) cannot be used on soils having restricted drainage. Very high-salinity water (C4) can be used only on certain crops and then only if special practices are followed.
Ten representative chemical analyses of water samples from irrigation systems were selected for determining the suitability of water in Wallace County for irrigation (Table 9). Specific conductance ranged from 330 to 690 micromhos. In Figure 22, all the water samples were classified as low-sodium (S1) and medium-salinity (C2) water.
Table 9--Sodium-adsorption ratios (SAR), conductivities, sodium content, and calcium plus magnesium content of water samples from selected wells.
| Sample number in Figures 21 and 22 |
Well | Na (equivalents per million) |
Ca + Mg (equivalents per million) |
SAR | Conductivity (micromhos per centimeter at 25°C) |
|---|---|---|---|---|---|
| 1 | 11-38--5bbc | 1.92 | 5.19 | 1.15 | 690 |
| 2 | 13-39-19dca | 1.80 | 4.59 | 1.15 | 630 |
| 3 | 13-40-7aac | 1.45 | 4.09 | 1.00 | 550 |
| 4 | 13-43-36abb | .87 | 2.48 | .80 | 330 |
| 5 | 14-40-36dcc | 1.17 | 2.64 | 1.00 | 380 |
| 6 | 14-41-15dbb | .81 | 2.77 | .70 | 360 |
| 7 | 15-38-30ccb | 1.02 | 3.40 | .80 | 440 |
| 8 | 15-38-34cbc | .44 | 3.94 | .35 | 430 |
| 9 | 15-39--25bbb | 1.35 | 3.10 | 1.00 | 430 |
| 10 | 15-41-32aca | 1.26 | 2.95 | 1.0(0 | 40 |
Sanitary Considerations
The analyses of water in Table 7 give only the dissolved-solids content of the water and do not indicate the sanitary quality of the water, although a large amount of certain mineral constituents such as nitrate or chloride may indicate pollution. Water containing mineral matter that imparts an objectionable taste or odor may be free from harmful bacteria and safe for drinking. Conversely, water clear and pleasant to the taste may contain harmful bacteria. Great care should be taken to protect domestic and public water supplies from pollution. To guard against contamination, a well must be properly sealed to keep out dust, insects, vermin, debris, and surface water. Wells should not be placed where barnyards, privies, or cesspools are possible sources of pollution.
Prev Page--Hydrologic Properties || Next Page--Formations
Kansas Geological Survey, Geology
Placed on web July 9, 2007; originally published November 1963.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Wallace/07_qual.html