Prev Page--Agricultural Use || Next Page--Paleozoic and Cretaceous
Ground Water, continued
Quality of Water
The general chemical character of the ground waters in Ford County is shown by the analyses of water from 69 representative wells and one spring, distributed as uniformly as practicable within the county and among the principal water-bearing formations (table 14). The samples of water were analyzed by Robert H. Hess, chemist, in the water and sewage laboratory of the Kansas State Board of Health. The constituents given were determined by the methods used by the U.S. Geological Survey.
Chemical Constituents in Relation to Use
The following discussion of the chemical constituents of ground water in relation to use has been adapted from publications of the United States Geological Survey.
Total dissolved solids--When water is evaporated the residue that is left consists mainly of the mineral constituents listed below and generally includes a small quantity of organic material and a little water of crystallization. Waters with less than 500 parts per million of dissolved solids are generally entirely satisfactory for domestic use, except for the difficulties resulting from their hardness or occasional excessive content of iron. The waters containing more than 1,000 parts per million are likely to include enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respects.
The ground waters from most of the Ford County wells that were analyzed contain less than 400 parts per million of dissolved mineral matter, and are entirely satisfactory for most ordinary purposes. The waters from three of the wells (32, 65, and 98) contained between 400 and 500 parts per million of dissolved solids, the waters from five other wells (51, 55, 202, 322, and 352) contained between 500 and 800 parts, the waters from three wells (176, 229, and 316) contained between 900 and 1,500 parts, and the waters from two wells (201, 357) contained 2,828 and 2,811 parts, respectively.
Hardness--Hardness of a water is commonly recognized by the increased amount of soap needed to produce a lather, and by the curdy precipitate that forms before a permanent lather is obtained. Calcium and magnesium are constituents that cause practically all the hardness of ordinary waters and they are also the active agents in the formation of the greater part of all the scale formed in steam boilers and in other vessels in which water is heated or evaporated.
In addition to the total hardness the table of analyses shows the carbonate hardness and the noncarbonate hardness. The carbonate hardness is that caused by calcium and magnesium equivalent to the bicarbonates of the water. It is largely removed by boiling. In some reports this type of hardness is called temporary hardness. The noncarbonate hardness is due to calcium and magnesium equivalent to sulphates or chlorides of calcium and magnesium. It cannot be removed by boiling and has sometimes been called permanent hardness. With reference to use with soaps, there is no difference between the carbonate and noncarbonate hardness. In general, the noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness of less than 50 parts per million is generally rated as soft, and its treatment for the removal of hardness is rarely justified. Hardness between 50 and 150 parts per million does not, seriously interfere with the use of water for most purposes, but it does slightly increase the consumption of soap, and its removal by a softening process is profitable for laundries or other industries that use large quantities of soap. Treatment for the prevention of scale is necessary for the successful operation of steam boilers using water in the upper part of this range of hardness. Hardness of more than 150 parts per million is noticed by anyone, and where the hardness is 200 or 300 parts per million it is common practice to soften water for household use or to install cisterns to collect soft rain water. Where public supplies are softened, an attempt is generally made to reduce the hardness to from 60 to 80 parts per million. The additional improvement from further softening of a whole public supply is not deemed worth the additional cost.
The ground waters of Ford County are all hard, most of the samples ranging in hardness from 170 to 300 parts per million. Seven samples contained between 403 and 854 parts per million of hardness (analyses 51, 55, 176, 202, 229, 316, and 322), and the samples from wells 201 and 357 contained 1,274 and 1,577 parts, respectively.
There are two processes in general use for softening water, the lime and soda process and the exchange silicate or so-called "zeolite" process. Both of these methods also effectively remove undesirable amounts of iron. None of the public water supplies in the county are softened or otherwise treated; but the water is treated, principally to soften it, for the railroads, several industrial plants, and a laundry.
Iron--Next to hardness, iron is the constituent of natural waters that in general receives the most attention. The quantity of iron in ground waters may differ greatly from place to place, even in waters from the same formation. If a water contains much more than 0.1 part per million of iron the excess may separate out after exposure to the air and settle as a reddish sediment. Iron, which may be present in sufficient quantity to give a disagreeable taste and to stain cooking utensils, may be removed from most waters by simple aeration and filtration, but a few waters require the addition of lime or some other substance.
Iron is found in objectionable amounts in some of the ground waters of Ford County, as indicated in table 14. All but 14 of the 70 samples of water from Ford County contained less than 1 part per million of iron. The samples from 4 (43, 65, 352, and 445) contained between 4 and 5 parts per million of iron. The sample of water from well 357, an irrigation well in the alluvium in the Arkansas valley, contained 26 parts per million of iron - an amount sufficient to give the water a disagreeable taste. The irrigation ditches leading from the well and much of the irrigated land was coated with a reddish sediment that had settled out after exposure to the air because of the large amount of iron in the water. The samples from 9 wells (58, 79, 131, 185, 324, 441, 464, 480, and 489) contained between 1.0 and 2.2 parts per million of iron.
Fluoride--Although determinable quantities of fluoride are not so common as fairly large quantities of the other constituents of natural waters it is desirable to know the amount of fluoride present in waters that are likely to be used by children. Fluoride in water has been shown to be associated with the dental effect known as mottled enamel which may appear on the teeth of children who drink water containing fluoride during the period of formation of the permanent teeth. It has been stated that waters containing 1 part per million or more of fluoride are likely to produce mottled enamel, although the effect of 1 part per million is not usually very serious (Dean, 1935, pp. 1269-1272). If the water contains as much as 4 parts per million of fluoride, 90 percent of the children exposed are likely to have mottled enamel and 35 percent or more of the cases will be classified as moderate or worse.
Of the 70 samples of water collected in Ford County, 26 contained 1.0 part per million or more of fluoride. Fifteen of these contained from 1.0 part to 2.0 parts; 10 contained between 2 and 3 parts; and one well (58), at a school, contained 3.6 parts. The following other school wells yielded waters having more than 1 part per million of fluoride: well 43, 2.6 parts per million; well 65, 2.2 parts; well 79, 2.9 parts; and well 185, 1.8 parts. A composite sample of water from the city wells of Spearville (36) contained 1.5 parts per million of fluoride. 44 samples contained less than 1 part per million of fluoride.
Water for irrigation--The suitability of water for use in irrigation is commonly held to depend on the quantity and chemical character of the dissolved salts and on the rainfall, the nature of the land, the crops, the manner of use, and the drainage. In a discussion of the interpretation of analyses with reference to irrigation in southern California, Scofield (1933, pp. 23-24) gives some limits for different factors as they have been found to hold in southern California.
All of the samples of water collected in Ford County would be classed as safe for use in irrigation according to the principles discussed by Scofield.
Sanitary Considerations
The analyses of water given in the tables show only the amounts of dissolved mineral matter in the water and do not indicate the sanitary quality of the water.
Dug wells and springs are more likely to become contaminated than are properly-constructed drilled wells, but great care should be taken to protect from pollution every well and spring used for domestic or public supply. It is important that the top of the casing be sealed in such a manner as to prevent surface water from entering the wells, and, where pump pits are used, the top of the casing should extend above the floor of the pit so that surface water cannot drain into the well. In constructing wells equipped with ordinary lift or force pumps, it is a good plan to allow the casing to extend several inches above the platform so that the pump base will fit down over the top of the casing, thus effecting a tight seal. If the casing is left flush with the top of the platform opportunity is afforded for drainage into the well and for possible contamination. Wells should not be located where there are possible sources of contamination such as drainage from the vicinity of buildings, privies, or cesspools. The type and condition of casing used for domestic wells also warrants careful consideration. During the course of the present investigation, several wells were visited in which the casings had become obstructed on the inside by the roots of trees and vines, whose presence indicated that there were openings in the casing, probably near joints.
Quality in relation to Water-bearing Formations
The Ogallala formation, by far the most important source of groundwater in Ford County, yields calcium bicarbonate waters of moderate mineral content. The forty Ogallala waters that were analyzed were remarkably uniform in total mineral content and in their content of the different, major mineral constituents. For example, 38 of the 40 waters analyzed had between 190 and 335 parts per million of total dissolved mineral matter, between 200 and 290 parts per million of bicarbonate, and between 170 and 280 parts per million of hardness. The waters generally had less than 35 parts per million of sulfate and of chloride. Analyses of typical Ogallala waters are shown graphically in figure 20. Number 36 is an Ogallala water of average mineral content; number 423 is one of the softer, less mineralized waters; and number 9 is one of the harder, more highly mineralized waters.
Figure 20--Analyses of typical waters from the Ogallala formation, the Dakota formation, and the alluvium in Ford County.
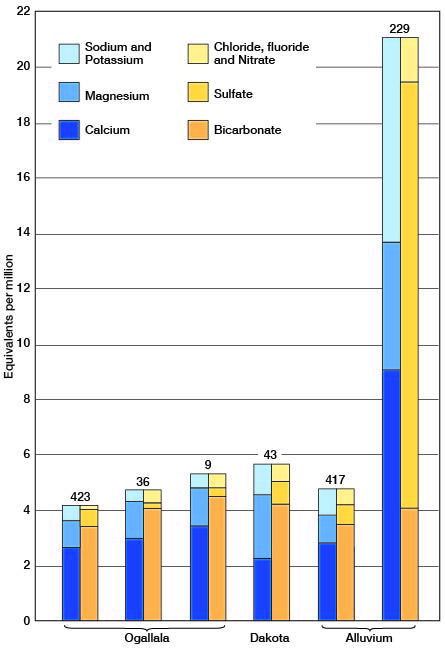
One water, presumably from the Ogallala, had 1,442 parts per million of sulfate. The very high nitrate content of this water (128 parts per million) suggests that the water has been contaminated by surface drainage and that it is not a true Ogallala water.
About half of the Ogallala waters that were analyzed had more than 10 parts of nitrate, suggesting that these waters, too, have been contaminated to some extent.
Generally the Ogallala waters appear to be low in iron. Only five of those analyzed had more than 0.5 part per million of iron and the highest of these was 2.2 parts.
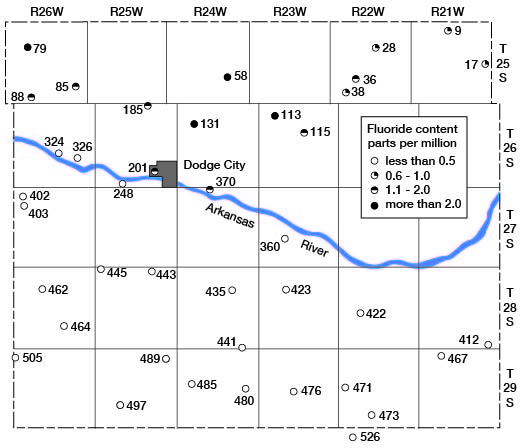
Ogallala waters north of Arkansas River were higher in fluoride content than were those south of the river. The areal distribution of fluoride in Ogallala waters is shown in figure 21. With but one exception all the analyses of Ogallala waters north of the river showed more than 0.5 part per million of fluoride and more than half of these analyses showed more than 1 part, whereas all the analyses of Ogallala waters south of the river showed less than 0.5 part of fluoride.
Figure 21--Map showing the fluoride content of waters in the Ogallala formation in Ford County.
Only five of the samples that were analyzed are definitely correlated as having come from the Dakota formation. These waters had from 239 to 464 parts per million of total dissolved mineral matter, 208 to 312 parts of bicarbonate and 170 to 239 parts of hardness. Only one water had more than 40 parts of sulfate and all had less than 35 parts of chloride. The analysis of a typical Dakota water (No. 43) is shown graphically in figure 20. Although this water is slightly less mineralized than the average water from the Dakota formation it is fairly typical of 31 samples of water collected for analysis from wells in the Dakota formation in Morton, Stanton, Hamilton, Gray, and Ford counties. The analyses of water from Morton and Stanton counties have been published (McLaughlin, 1942, pp. 59-62; Latta, 1941, pp. 57-59), and those from Hamilton and Gray counties will be published in forthcoming bulletins of the State Geological Survey of Kansas.
The information available indicates that waters in the Dakota formation are similar in mineral content and in chemical character to waters in the Ogallala formation. Fluoride was relatively high in the Dakota waters analyzed - four of the waters having more than 2 parts per million. The fifth water had 0.1 part per million of fluoride. One Dakota water (No. 350) had 58 parts per million of nitrate, suggesting contamination by surface drainage.
The Greenhorn limestone is relatively unimportant as a source of groundwater in Ford County and only two analyses were made of water from the formation. The two waters analyzed differed considerably in total mineral content, in sulfate content, and in hardness (Nos. 352 and 353, table 14). One was moderately high in sulfate, iron and fluoride and only moderately hard (No. 352), the other was low in sulfate, iron, and fluoride, and very hard (No. 353). These scanty data permit no generalizations as to the kind of water likely to be obtained from the Greenhorn limestone.
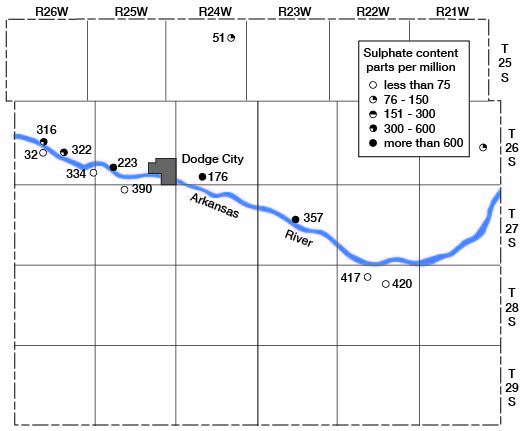
Eleven analyses indicate that two distinct types of waters may be obtained from the alluvium of Arkansas River. These two types are represented graphically in figure 20. The waters from alluvium on the south side of the river were typical calcium bicarbonate waters of moderate mineral content. They were very similar in chemical character and in mineral content to waters from the Ogallala formation. All these waters had less than 75 parts per million of sulfate. On the other hand waters from alluvium on the north side of the river had, with only one exception (No. 98), more than 300 parts per million of sulfate. One water (No. 357) had 1,740 parts of sulfate. These relations are shown in figure 22. The sulfate waters from the alluvium are the hardest waters found in the county. The waters in the alluvium north of the river also differed from those south of the river in fluoride content. Those north of the river had from 0.9 to 1.9 parts per million of fluoride, whereas those south of the river had 0.5 part or less. This relationship with respect to fluoride content is analogous to that found in the Ogallala waters. With but one exception the waters in the alluvium had no more than 0.1 part per million of iron. The exception, No. 357, which had 26 parts of iron, also has the highest sulfate content and is by far the hardest water found in the county.
Figure 22--Map showing the sulphate content of waters in the alluvium in Ford County.
Prev Page--Agricultural Use || Next Page--Paleozoic and Cretaceous
Kansas Geological Survey, Ford County Geohydrology
Web version April 2002. Original publication date Dec. 1942.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Ford/05_gw8.html