Kansas Geological Survey, Public Information Circular (PIC) 17
Prev. Page--Start of the Report || Next Page--Environmental Consequences, Sources
![]()
![]()
![]()
![]()
Kansas Geological Survey, Public Information Circular (PIC) 17
Prev. Page--Start of the Report ||
Next Page--Environmental Consequences, Sources
The first commercial ore discovery in the Tri-State mining district was made in southwestern Missouri around 1838. By the start of the Civil War, these mines were producing so much lead that both the North and South fought to control the mining area to secure a source of lead for bullets. The fighting closed the mines for most of the war.
Production from the Tri-State peaked between 1918 and 1941. During the 1920's, more than 11,000 miners worked in the area, and perhaps three times as many were involved in support work and industries.
Although zinc was much more common than lead throughout the Tri-State mining district, production up to 1869 was confined to lead, which could be easily smelted in homemade furnaces. Zinc production took off in the early 1870's, following the completion of railroad lines and the construction in 1873 of a coal-fired zinc smelter at Weir City, Kansas (fueled by coal from nearby mines). In 1878 another smelter was built at Pittsburg, Kansas. In the early 1900's, smelting costs were reduced by the discovery of a shallow gas field in southeastern Kansas. Using this cheap fuel source, new gas-fired smelters were built in southeastern Kansas, displacing the coal-fired smelters.
In much of the Tri-State, mining was done underground, using room-and-pillar methods, in which room-shaped areas are mined and similarly shaped areas are left for roof support, resulting in a checkboard-like arrangement of alternating rooms and pillars. Underground rooms had walls 25 to 100 feet high and pillars 20 to 50 feet thick. In the eastern part of the district, however, the ore was closer to the surface, and the shallow mining could be done using hand tools and a simple hoisting device that was either man- or animal-powered. Galena, Kansas, became known as a poor man's mining district because small claims could be worked by a few miners.
Many of the rock layers that were mined for ore also were aquifers--that is, water-bearing formations. Thus, water flowed into the mines through these rock layers. To keep the mines from filling with water, as many as 63 pumping plants operated 24 hours a day. In 1947, for example, more than 36 million gallons of water were pumped from the mines every day (enough to cover one acre of ground with water 110 feet deep).
After World War II, production in the Tri-State mining district gradually declined. In 1970 the last active mine, located 2 miles west of Baxter Springs, Kansas, shut down due to environmental and economic problems, bringing to an end a century of lead and zinc mining in the Tri-State.
During the life of the district, more than 4,000 mines produced 23 million tons of zinc concentrates and 4 million tons of lead concentrates. The Kansas part of the Tri-State district produced more than 2.9 million tons of zinc, with an estimated value of $436 million, and 650,000 tons of lead worth nearly $91 million.
Lead and zinc ores in the Tri-State area occur in Mississippian cherty limestones. After these cherty limestones were deposited at the bottom of an inland sea, they were exposed at the surface and subjected to erosion. Over time, the softer limestone was leached from the beds, while the more resistant chert remained. Caves developed in some places, but in many places the removal of the limestone caused the beds to collapse. These collapsed beds contained mostly broken pieces of chert and were very porous and permeable. Later these beds became sites of ore deposition.
Following this period of erosion, the seas returned during the Pennsylvanian Period (323 to 290 million years ago), and the shales of the Cherokee Group were deposited on top of the Mississippian rocks. This set the stage for the ores to be deposited, probably millions of years later.
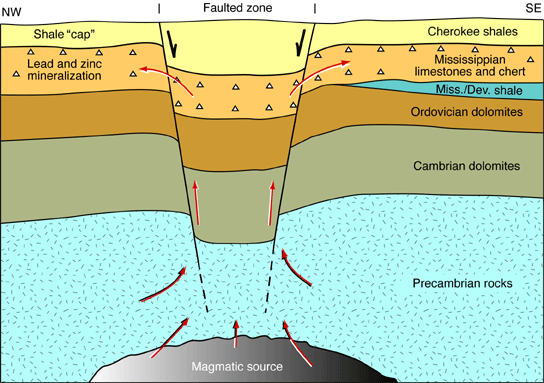
The lead and zinc ores found in the Tri-State district are believed to have formed from hot, metal-bearing solutions that originated deep within the earth. These solutions probably rose along major faults and fractures until they came to the Mississippian beds (fig. 2). The Cherokee Group shales acted as an impermeable barrier, or cap, to the rising metal-bearing solutions and forced them to migrate laterally. These solutions spread through the broken beds of chert and other porous and permeable layers in the Mississippian limestones, depositing the galena, sphalerite, and other associated materials.
Figure 2--Red arrows show origin, movement, and deposition of ore-bearing solutions.
Only two minerals, galena and sphalerite (figs. 3, 4), were commercially important in the Tri-State district. Sphalerite (zinc sulfide, ZnS) is five times more abundant than galena (lead sulfide, PbS). Sphalerite and galena can occur as crystals lining cavities, as cement that fills the spaces between broken chert fragments, or as finely disseminated grains.
Chalcopyrite, a copper mineral, also occurs in the region, but not in economic amounts. Other non-commercial minerals and rocks associated with galena and sphalerite include pyrite, marcasite, dolomite, calcite, quartz, and jasperoid.
Figure 3--Galena (lead sulfide) from Cherokee County, Kansas.

Figure 4--Sphalerite (zinc sulfide) from Cherokee County, Kansas.

Prev. Page--Start of the Report || Next. Page--Environmental Consequences, Sources
Kansas Geological Survey, Public Outreach
Comments to webadmin@kgs.ku.edu
Web version October 2001
http://www.kgs.ku.edu/Publications/pic17/pic17_2.html