Kansas Geological Survey, Public Information Circular (PIC) 25
A complete text of this file is available as a pdf document .
Radon (boldface terms in glossary), an invisible, odorless, and tasteless gas, comes from the breakdown of naturally occurring uranium found within soil, rocks, and ground water. Radon is classified as a Class A carcinogen by the U.S. Environmental Protection Agency (EPA), meaning that scientific research has established that radon exposure causes cancer in humans, a health issue that many Kansas homeowners unknowingly face. This report summarizes information necessary for Kansas homeowners about radon indoor-air issues and the geology related to radon's occurrence, and provides direction for further information or assistance regarding radon.
Knowledge of the detrimental human-health effect associated with radon is not new. Studies have long linked radon exposure to lung cancer within the mining industry. Protective residential exposure guidelines were estimated from these studies; however, a direct link to residential radon exposure and lung cancer was not firmly established by scientific research until recently. Two extensive health studies, the North American Residential Radon Studies and European Residential Radon Studies, conclusively linked lung cancer and radon in residential homes. Residential radon exposure at or even below the 4.0 picoCuries per liter (pCi/L) action level set by the EPA is a public health risk (McCray, 2005).
A National Health Advisory issued by the U.S. Surgeon General in January 2005 states that, after smoking, radon gas is the second-leading cause of lung cancer in the United States and breathing it over prolonged periods poses a serious health risk. According to the EPA, approximately 21,000 people die every year from radon-induced lung cancer, exceeding the annual death toll from drunken-driving accidents and residential house fires combined. Potential for radon occurrence arises from a combination of geology, soils, background uranium concentrations, and other factors (fig. 1).
Figure 1—Generalized geologic radon potential map of the United States, depicting geology, soils, and radioactivity that help, in part, control the radon potential in each state. The map cannot be used for characterization or predicting radon levels in specific areas or locations. Site-specific testing must be performed to determine the radon levels in any given residence or building (modified from USGS, 1995).
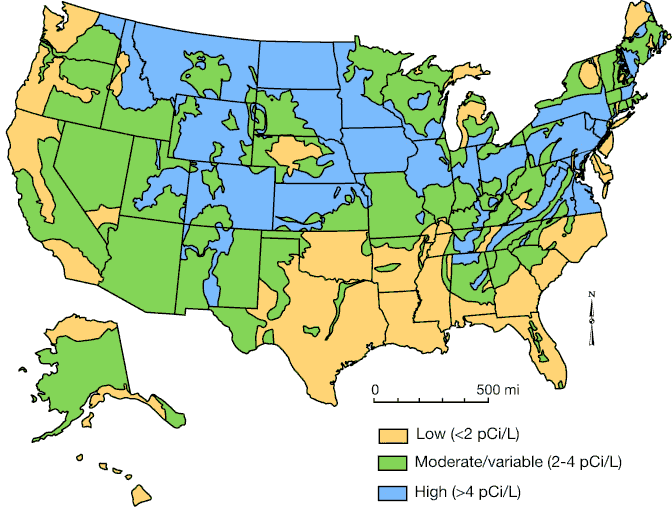
Elevated radon levels are found throughout Kansas. The EPA estimates that one in every 15 homes nationwide has an elevated radon level at or above the 4 pCi/L action level. In Kansas that number may be even higher. According to the Kansas Radon Program, approximately one in four Kansas homes may have elevated radon levels.
Radon levels cannot be predicted and often vary locally from house to house. While some geographical areas of Kansas tend to have radon levels below the EPA action level, every home in Kansas has potential for elevated radon. Average regional values or test results from a neighbor are not adequate substitutes for site-specific radon testing.
The origin of radon is linked to the decay and occurrence of the element uranium. Uranium, the ultimate parent product of radon, is a solid, radioactive element. Radioactivity in the element is caused by a natural energy imbalance within the nucleus of the atoms that form uranium. Radioactive elements inherently seek a more stable energy state through a process known as radioactive decay. Through decay, atoms release energy as subatomic particles or rays to form new elements at a lower energy state (i.e., daughter products). The particle or ray energy released during decay is called radiation.
Uranium is the first in a long chain of radioactive elements that decay until the stable element lead is formed. Important daughter products within the uranium decay chain are radium, radon, and polonium (fig. 2). Radium is the immediate precursor and source of radon. Radon and its polonium daughter products are linked to human lung cancer. The physical state of these daughter products ranges from a gas (e.g., radon) to extremely small solid particles (e.g., polonium).
Figure 2—The uranium decay series with daughter products and half-lives (modified from Zapecza and Szabo, 1988).
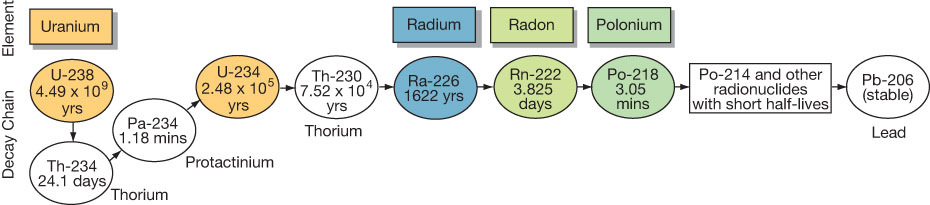
Radon and its immediate parent radium largely occur where uranium is present in rocks, soil, or ground water (Felmlee and Cadigan, 1979). So to understand radon occurrence, it is necessary to first understand the geology of its parent uranium.
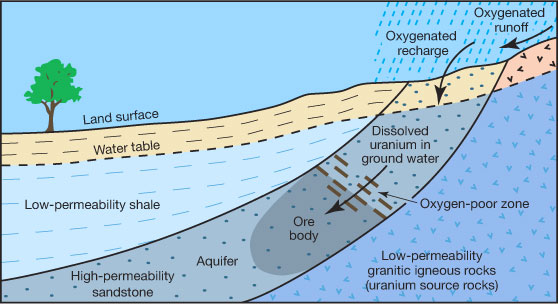
All rocks contain some uranium (average crustal abundance is 2.5 parts per million [ppm]). Uranium can also be present as a solid attached to mineral coatings on sand and silt (fig. 3). Uranium is very soluble and easily weathered into solution—similar to dissolved salt or sugar—by oxygen-rich water (i.e., oxidizing) running over or through rocks with a background concentration of uranium. Water can carry uranium, in solution or as a solid, long distances over millions of years before it is enriched, usually in oxygen-poor zones (i.e., reducing), within other rocks. Over time, if enough uranium accumulates, it can form an economically valuable rock formation, called an ore, which can be mined (fig. 4).
Figure 3—Uranium and radium adsorb to mineral coatings on sand and silt particles, which may be transported by flowing water (modified from Wirt, 1994).
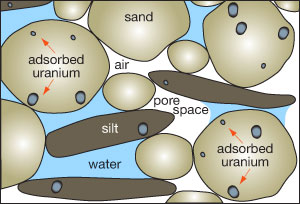
Figure 4—Oxygenated water carries dissolved uranium until it reaches an oxygen-poor zone where it is deposited, enriching the host rock and forming an ore body over time (modified from Wirt, 1994).
Varying depositional mechanisms help distribute uranium throughout the environment. Some typical uranium-enriched rocks include light-colored, silica-rich igneous or volcanic rocks such as granites or volcanic ash, sandstones containing organic material or silica cement, black shales with high organic contents, and coal. In the ocean, deep, anoxic marine waters are saturated with phosphate, which naturally combines with and concentrates uranium. Phosphate-bearing sediment accumulates with seafloor clay leading to the formation of uranium-enriched phosphate nodules or layers within black shale layers (Plant et al., 1999). If sea currents carry away the clay, phosphate ore is formed and uranium is often extracted as a byproduct of phosphate mining. Younger, unconsolidated uranium deposits may accumulate in bogs or peat deposits where uranium-rich water flows into these reducing environments and accumulates (Guilbert and Park, 1986). Uranium also is known to accumulate along structural features such as faults that convey deep water upwards into an environment containing uranium. Uranium accumulates along the front where the deep, oxygen-poor and oxygen-rich water meet.
Wherever uranium is found, its decay leads to the formation of radon's immediate parent radium (fig. 2). Because radium is not very mobile, it is usually found where uranium occurs in rocks, soil, or ground water. However, in some instances radium is found in soil or ground water away from its uranium parent. These include soils derived from carbonate rocks, such as limestone, which is typically not enriched in uranium (Tanner, 1986). During the soil-forming process, carbonate in the limestone is leached away leaving clay behind that is enriched with residual materials, which include uranium (Schumann, 1993). Also, in contrast to uranium, radium is soluble in acidic or chloride-rich, reducing water (Felmlee and Cadigan, 1978; Gundersen and Szabo, 1995). The reducing water can carry dissolved radium away, much like oxygenated water can carry uranium in solution away from a source area. Examples of radium-containing water include acid mine wastewater or brine waters associated with the extraction of oil, gas, and coal-bed methane (Gundersen and Szabo, 1995). Additionally, structural features such as faults can convey upwards deep, reducing water that is high in dissolved radium.
Uranium occurrence within Kansas was assessed by the Kansas Geological Survey (KGS) and the U.S. Geological Survey to evaluate the radon potential in Kansas (Schumann, 1993).
In Kansas, the apparent absence of a significant oxygen-reducing depositional environment, generalized in figure 4, suggests that commercial ore-grade uranium deposits are not likely present. However, above-average uranium concentrations are noted in some depositional environments that have phosphate, organic, or silica enrichment mechanisms, as well as volcanic ash deposits.
In general, above-average uranium concentrations are found in a number of Kansas rock formations (fig. 5). In eastern Kansas uranium is found in the thin, black shales that are a component of the alternating Pennsylvanian limestone/shale formations typical of this area. These shales are thought to represent deposition in the deepest part of an ocean. High organic content and deep marine waters saturated with phosphate led to localized uranium accumulation in these units. Uranium concentrations range from about 15 ppm to 100 ppm in the shales and up to 350 ppm in phosphate nodules within the shales.
Figure 5—The generalized geology of Kansas.
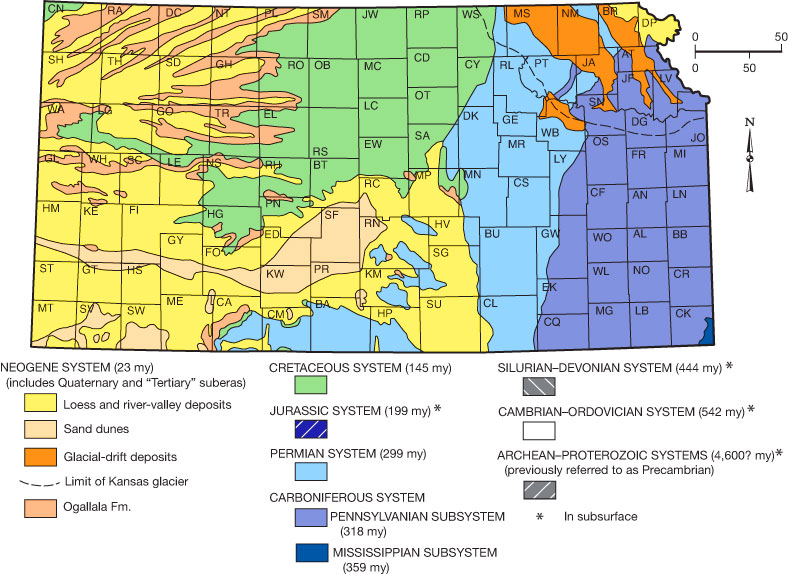
Scattered outcrops of Cretaceous Pierre Shale in the very northwestern corner of Cheyenne County and along the Smoky Hill River drainage basin in Wallace and Logan counties sometimes contain above-average uranium concentrations. Converse to the deep-water Pennsylvanian shales, the Cretaceous black shales are shallow, near-shore units, but were deposited under reducing conditions with an abundance of clay minerals that form weak bonds with metals, including uranium. Uranium concentrations range from about 10 to 40 ppm.
Uranium is associated with silica-cemented layers in the Ogallala Formation at some locations in western Kansas. The uranium tends to correlate with the amount of silica cementation in the rocks and is about 125 ppm in the most intensely silicified layers. In particular, a 10-mile-long and 1.5-mile-wide continuously silicified area is located in Meade and Clark counties. The source of the uranium and silica is thought to be volcanic ash that is mixed in with the Ogallala or alluvial sediments.
Four recognizable volcanic ash beds occur in Kansas. Three ash beds occur within the Ogallala. One ash bed, the Pearlette bed, is distributed through much of the state within unconsolidated Neogene deposits, although most significant deposits occur in central and western Kansas. Volcanic ash deposits are variable and range from a few yards to more than a mile wide and a few inches to more than 30 feet thick. Volcanic ash samples analyzed for uranium ranged from 3.9 to 9.1 ppm.
Anomalous uranium concentrations sometimes occur in the alluvium within some major drainages in central and western Kansas, presumably weathered from sandstones, shales, or volcanic ash deposits that contain uranium.
Uranium and radium also are associated with residual clay soils derived from eastern Kansas Pennsylvanian and Permian limestones. These soils lie directly on their limestone parent and are characteristically red or red-orange from iron oxides present in the soil (fig. 6).
Other uranium occurrences have been noted in the Cretaceous Dakota Formation in Ellsworth County and the Cretaceous Smoky Hill Chalk Member in Gove County. In general, aerial radiometric surveys and geophysical well logs indicate moderate radioactivity in some Cretaceous rocks in northern and west-central Kansas.
Some ground water in Kansas is known to have naturally occurring uranium and radium (Schumann, 1993; Zapecza and Szabo, 1988). Generalized areas include southeastern, central, and extreme western Kansas.
Ground water in southeastern Kansas is generally low in uranium but contains elevated concentrations of radium. Some uranium in ground water is noted in extreme southeastern Cherokee County (Macfarlane, 1981).
In central Kansas, uranium is present in some wells producing from the Permian Nippewalla Group, Cretaceous Kiowa Formation and Cheyenne Sandstone, and Neogene alluvium (Berendsen et al., 1988). In western Kansas, uranium is found in some wells producing from the Ogallala aquifer and Arkansas River alluvium (Berendsen and Hathaway, 1981).
Radon gas comes from radium decay within rocks, ground water, and soil. Because of radon's short 3.8-day half-life, radon is found close to its radium parent.
The bedrock setting may be important if the rock is highly fractured or contains solution cavities, which enhance radon transport and accumulation. Also, near-surface uranium-enriched bedrock may lead to locally high radon concentrations.
Radon enters water as tiny bubbles from radium decay next to or within water. These bubbles exist much like carbonation in a can of soda and easily escape when agitated (Wirt, 1994). Rivers, streams, and reservoirs have low radon concentrations due to agitation, mixing, and long storage time. As a consequence, most radon is found within ground water rather than surface water.
Soil is generally considered the greatest source of radon gas and therefore poses the largest health risk. The average radon content in soil air is about 200 to 2,000 pCi/L and may exceed 100,000 pCi/L (Gundersen et al., 1993; Otton, 1992).
Not all radium decay actually results in radon that can move into a home. In the soil, radon recoils away from its radium parent and is propelled into either adjacent solid particles or into small pores that contain water and air. Solids and water limit radon travel distance, allowing it to decay harmlessly due to its short half-life. In general, only radon that reaches air-filled pore space can eventually enter a structure. On average, only 10 to 50 percent of produced radon enters air-filled soil pore space (Otton, 1992).
Radon accumulation and movement in soil also depends on the radium content, the soil's physical properties, and the mechanical flow of air through the soil. In general, soils with higher radium concentrations are more likely to accumulate radon (Tanner, 1986). Greater porosity and permeability also promote radon accumulation and movement, while pore water retards radon movement. In general, clay soils limit and sandy soils promote radon movement. However, clay soils are susceptible to seasonal moisture changes that cause cracking or swelling, which could open or close avenues of radon transport.
After entering soil pore space, radon, either aided or impeded by the soil's physical properties, moves with the flow of air through the soil (Tanner, 1986). Radon diffuses from high to low concentrations, and radon flows in response to differences in pressure between the atmosphere, soil, and buildings. In effect the soil "breathes" from concentration differences, seasonal moisture changes, barometric pressure, and temperature.
Because this movement is somewhat complex, elevated radon values can be encountered in nearly any setting. For example, homes built on dry, sandy soils may have higher radon concentrations because the high permeability, porosity, and low moisture content promote radon travel and accumulation from long distances, even in low radon areas (Otton, 1992). Structural features such as faults or caves also promote radon accumulation. Conversely, homes built on moist, clayey soils also may have radon concentrations because certain soils, such as red-orange residual clays, can be high in radium leading to locally high radon values. The clay soil prevents migration and diffusion to the atmosphere, concentrating radon very near a structure. Low pressure in an adjacent basement induces high concentration radon flow over a short distance into the home. In either scenario, radon can also fluctuate with soil-moisture changes in response to seasonal precipitation, further complicating accumulation and movement.
In the end, because uranium is present nearly everywhere, so is radon. While general trends may be discerned from the geology and maps that compile radon testing results, elevated values above 4.0 pCi/L are present nearly everywhere in Kansas.
Radon gas is capable of seeping through pores in the soil, into the atmosphere, or the interior of a building. Radon gas entering the atmosphere quickly becomes diluted, but the confined space of a structure (e.g., the basement) allows it to concentrate.
Radon enters a home through sump pumps, cracks, joints, and pipes that penetrate the walls and floors of a home (fig. 6). Structures have a slight negative air pressure or suction relative to the soil, known as a stack effect, which sucks radon into a structure. Newer homes and windows tend to be more air tight and can exacerbate radon accumulation. Also, depending on the type of furnace, radon may circulate throughout the home, increasing exposure beyond the lower levels in contact with the soil.
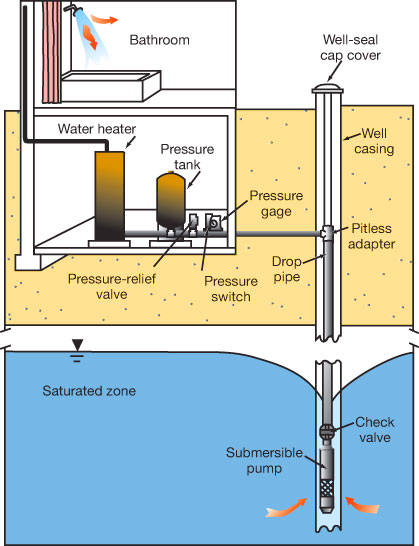
Radon also can enter a home if a water supply contains dissolved radon (fig. 7). Private water wells drawing from water with dissolved uranium or radium are most likely to have potential radon-associated health risks. Private wells usually do not have treatment systems and the short transit time between the pump and the home does not allow enough time for radon decay. According to the EPA, most risk is still from inhalation rather than ingestion because household activities such as showering and laundry release radon to the indoor air. Approximately 10,000 pCi/L of radon in water contributes about 1 pCi/L of radon to indoor air (Zapecza and Szabo, 1988).
Radon is usually not a problem in large public water supplies because water is generally obtained from multiple sources or surface water. Mixing, treatment aeration, and longer residence times within the treatment system promote dilution, off-gassing, and decay of radon (Zapecza and Szabo, 1988).
Figure 6—Radon home entry points. In some areas, residual clay soil derived from Pennsylvanian and Permian limestone and phosphatic black shale may contribute to localized areas with elevated radon levels.
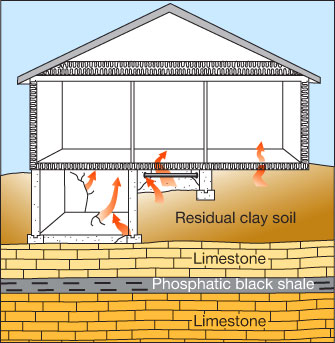
Figure 7—Radon home entry from ground water. In areas where radon is present in ground water, small private wells may contribute to the indoor-air radon concentration. In general, water with 10,000 pCi/L of radon contributes 1 pCi/L to indoor air (modified from Otton, 1992).
After radon seeps into a home, it continues to decay into polonium daughter products, which attach to dust particulates in the air. Radon gas and polonium are easily inhaled and emit alpha particles within the lungs and throat. Alpha particles are ejected during radiation decay like a subatomic bullet that penetrates the delicate internal cells of the respiratory tract causing changes or mutations to the cellular DNA. Over time, this cell damage is believed to begin the process of carcinogenesis.
Additional information on radon is available from the EPA, the Kansas Department of Health Environment, and the Kansas Radon Program. The EPA and the Kansas Radon Program maintain publications and fact sheets to help homeowners with frequently asked questions, test methods, real estate transactions, and mitigation techniques for both new and existing homes.
Kansas Radon Program, Engineering Extension
133 Ward Hall, Kansas State University
Manhattan, KS 66506
Phone 785-532-6026
Fax 785-532-6952
Kansas Department of Health and Environment Radon Information
Phone 800-693-5343
Alpha Particle—A positively charged particle made up of two neutrons and two protons emitted by certain radioactive nuclei. Alpha particles can be stopped by thin layers of light materials, such as a sheet of paper, and pose no direct or external radiation threat; however, they can pose a serious health threat if ingested or inhaled.
Daughter products—An atom formed by disintegration of a radioactive precursor (i.e., parent product). Different forms of atoms very specifically characterized by the number of protons, neutrons, and energy state are known as a nuclide.
Element—A substance composed of atoms having an identical number of protons in each nucleus. Elements cannot be reduced to simpler substances by normal chemical means. All matter fundamentally consists of elements. The smallest particle of an element is an atom, which consists of electrons centered about a nucleus of protons and neutrons.
Half-life—The rate of an element's radioactive decay is defined by its half-life; the time in which one-half of an original amount of a radioactive element decays to daughter products.
Parent product—An unstable atom that undergoes radioactive decay. Different forms of atoms are very specifically characterized by the number of protons, neutrons, and their energy state; if they are unstable, they are known as a radionuclide.
Permeability—A measure of the relative ease with which a fluid will move through a porous material. It is measured either as intrinsic permeability, which accounts for the physical properties of the media (e.g., grain diameter) only or, as the hydraulic conductivity, which accounts for the physical properties of both the media and the fluid (e.g., density and viscosity).
Polonium—Polonium-218, 214, and 210 are radioactive products of radon decay. Electrically charged polonium can attach to particulates that can be inhaled and attached to the internal cells of the respiratory tract. Polonium daughters are alpha particle emitters, which can potentially cause physical cellular damage.
Porosity—The percentage of rock or sediment that is a void or pore space. It is the ratio of the volume of voids to the unit volume of rock or sediment. Pore space may or may not be interconnected.
Radiation—Energy given off as either particles or rays from the unstable nucleus of an atom as a result of nuclear decay. The most common types of radiation are called alpha, beta, and gamma radiation.
Radioactivity—Spontaneous transformation of the nucleus of an atom; this results in a new element, generally with the emission of alpha or beta particles often accompanied by gamma rays.
Radioactive decay—The process in which an unstable (radioactive) nucleus emits radiation and changes to a more stable isotope or element. A number of different particles can be emitted by decay; the most typical are alpha or beta particles.
Radium—A naturally occurring radioactive metal. Radium is a radionuclide formed by the decay of uranium and thorium in the environment. It occurs at low levels in virtually all rock, soil, water, plants, and animals. Radon is a decay product of radium.
Radon—A naturally occurring radioactive gas found in soils, rock, and water. Radon causes lung cancer and is a threat to health because it tends to collect in homes, sometimes in very high concentrations. As a result, radon is the largest source of exposure to naturally occurring radiation.
Uranium—A naturally occurring radioactive element whose principal isotopes are Uranium-238 and Uranium-235. Natural uranium is a hard silvery-white shiny metallic ore that contains a minute amount of Uranium-234.
Berendsen, P., and Hathaway, L. R., 1981, Uranium in unconsolidated aquifers of western Kansas: Kansas Geological Survey, Mineral Resources Series 9, p. 43. [available online]
Berendsen, P., Hathaway, L. R., and Macfarlane, P. A., 1988, Radionuclide distributions in the natural environment in Kansas; a review of existing data; in, Geologic Causes of Natural Radionuclide Anomalies: Proceedings of GEORAD conference, St. Louis, Missouri, April 21-22, 1987; Rolla, Missouri, Missouri Department of Natural Resources, Special Publication 4, p. 65-74.
Felmlee, J. K., and Cadigan, R. A., 1978, Determination of radium in source rocks by using radium in Crystal Springs, Great Salt Lake area, Utah: U.S. Geological Survey, Open-file Report 78-102, p. 29. [available online]
Felmlee, J. K., and Cadigan, R. A., 1979, Radium and uranium concentrations and associated hydrogeochemistry in ground water in southwestern Pueblo County, Colorado: U.S. Geological Survey, Open-file Report 79-974, p. 54. [available online]
Guilbert, J. M., and Park, C. F., Jr., 1986, The geology of ore deposits: New York, W. H. Freeman and Co., 985 p.
Gundersen, L. C. S., Schumann, R. R., Otton, J. K., and Szari, S. L., 1993, The USGS/EPA radon potential assessments: An introduction; in, Geologic Radon Potential of EPA Region 7, R. R. Schumann, ed.: U.S. Geological Survey, Open-file Report 93-292-G, p. 1-18. [available online]
Gundersen, L. C. S., and Szabo, Z., 1995, Natural radionuclides in earth, air, and water, and the effect on human health; in, Energy and the Environment—Application of Geosciences to Decision-making, L. M. H. Carter, ed.: U.S. Geological Survey, Circular 1108, p. 22-24. [available online]
Macfarlane, P. A., 1981, Distribution of radium-226 in the lower Paleozoic aquifers of southeast Kansas and adjacent areas; in, Trace Substances in Environmental Health-XIV, a Symposium, D. D. Hemphill, ed.: University of Missouri, Columbia, p. 78-85.
McCray, L. K., 2005, Radon, what you can't see can hurt you: Spectator, v. 39, no. 1.
Otton, J. K., 1992, The geology of radon: U.S. Geological Survey, General Interest Publication, p. 28.
Plant, J. A., Simpson, P. R., Smith, B., Windley, B. F., Watson, J. V., and Plant, J., 1999, Uranium ore deposits; products of the radioactive earth regional geochemistry of uranium as a guide to deposit formation: Reviews in Mineralogy and Geochemistry, v. 38, no. 1, p. 255-319.
Schumann, R. R., 1993, Preliminary geologic radon potential assessment of Kansas; in, Geologic Radon Potential of EPA Region 7, R. R. Schumann, ed.: U.S. Geological Survey, Open-file Report 93-292-G, p. 71-94. [available online]
Tanner, A. B., 1986, Indoor radon and its sources in the ground: U.S. Geological Survey, Open-file Report 86-222, p. 5. [available online]
Wirt, L., 1994, Radioactivity in the environment; a case study of the Puerco and Little Colorado River basins, Arizona and New Mexico: U.S. Geological Survey, Water-Resources Investigation Report 94-4192, p. 23. [available online]
Zapecza, O. S., and Szabo, Z., 1988, Natural radioactivity in ground water-a review; in, National Water Summary 1986—Hydrologic Events and Ground-water Quality, D. W. Moody, Jerry Carr, E. B. Chase, and R. W. Paulson, eds.: U.S. Geological Survey, Water-Supply Paper 2325, p. 50-57. [available online]
Kansas Geological Survey, Public Outreach
webadmin@kgs.ku.edu
Web version March 2007
http://www.kgs.ku.edu/Publications/PIC/pic25.html