Prev Page--Kansas Rocks || Next Page--Sedimentary Structures
Minerals
All rocks are composed of one or more minerals. Because most of the rocks at the surface of Kansas are sedimentary in origin, so are most of the minerals. Salt, a common mineral, was deposited at the bottom of an ancient sea. So was calcite, the mineral that is the primary component of limestone.
What is a mineral? A mineral is a natural, inorganic substance with a characteristic chemical composition and definite physical properties. Natural means that it occurs in nature and is not manmade. Inorganic means that the minerals have not been formed directly by any kind of life, either plant or animal. A characteristic chemical composition means that no matter where a mineral is found, it contains the same types and quantities of chemical elements. Quartz, for instance, is always composed of the two elements silicon and oxygen which combine in the proportion of one atom of silicon to two of oxygen to form silicon dioxide, SiO2. Fool's gold, or pyrite, always consists of iron and sulfur together, forming iron sulfide, FeS2, and sphalerite is always zinc sulfide, ZnS.
The definite physical properties of a mineral depend on its chemical composition and its molecular arrangement. Describing a mineral without describing its physical properties is difficult. The following are terms commonly used to describe minerals.
Color, the easiest physical property to describe, is one of the easiest means of identifying certain pure minerals. For example, gold is always yellow; turquoise, always light blue. Impurities, however, often change the color of minerals.
Luster concerns the character of the light that is reflected by the mineral. Metallic, glassy, earthy, pearly, silky, and similar terms are used to describe luster.
When a mineral is rubbed on a piece of unglazed porcelain, it leaves a streak of finely powdered material. This streak may have a different color from that of the mineral itself and is an excellent check in identifying many minerals. Hematite, the common iron ore, invariably has a red-brown streak.
Some minerals are very soft; others very hard. The degree of hardness is an aid in identifying the minerals. Diamonds are harder than quartz and will therefore scratch quartz, quartz will scratch calcite, calcite will scratch gypsum, and so on. To help identify minerals, geologists have assigned numbers to the hardness of several minerals. In this hardness scale, the softer minerals are assigned a low number and the harder minerals a higher number.
Rankings on Mohs' scale of hardness:
- Talc
- Gypsum
- Calcite
- Fluorite
- Apatite
- Orthoclase
- Quartz
- Topaz
- Corundum
- Diamond
In the field, an easy way of estimating the hardness of a mineral is by trying to scratch it with common objects such as a fingernail with a hardness of 2.5, or a pocketknife blade, hardness 5.5. Glass has a hardness of slightly less than 6 and will scratch most minerals. To test a mineral for hardness, try to scratch it with one of these common objects. Minerals with a hardness of 6 or more will easily scratch a piece of glass. A sample such as calcite is too soft to scratch glass but is hard enough to scratch a fingernail. Therefore it has a hardness between 6 and 2.5. Hardness is another clue in identifying minerals, and in this book the hardness for each mineral is listed alongside its name.
Transparency is when light passes through a small piece of a mineral so that objects can be seen through it. If no light passes through and nothing can be seen through a small piece, the mineral is called opaque. Minerals that are neither opaque nor transparent, those through which light passes but through which no objects can be seen, are said to be translucent.
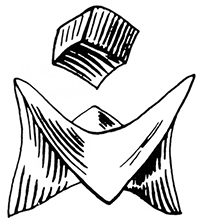
Minerals always take on definite shapes, geometric forms, which may not be visible to the naked eye. The crystal forms depend on the arrangement of the atoms and molecules of the mineral. Unfortunately, large and visible crystals only form sporadically, mainly because growing crystals compete for space and crowd each other. Perfect crystals generally are found where they project or grow slowly in open space, such as in fractures or veins. When good crystals are found, they are extremely valuable in identifying minerals. Some crystals are flat and are called tabular; some are long and thin like needles or fibers and are described as either needlelike or fibrous; still others are shaped like pyramids, prisms, cubes, and many other forms or combinations of forms. If the crystal outlines cannot be distinguished and the mineral is composed of compact material with indefinite form, the structure is called massive. Crystals may be grouped into a globular shape that looks like a cluster of grapes. A few minerals seemingly have no crystal structure at all.
Quartz Crystals.

When struck with a hard blow, some minerals break only along certain planes. Other minerals break just as easily in one direction as in another. When a mineral has a tendency to break along certain planes, it is said to have cleavage, resulting from the arrangement of the bonds between different molecules and atoms. Minerals may have only one plane of weakness or cleavage or they may have two, three, or more. The second type of breaking, that not determined by any arrangement of molecules, is called fracture, and this also varies among different minerals. Various types of fracture are described as smooth, uneven, ragged, and conchoidal or shelllike.
Obsidian, an Igneous Rock from New Mexico, Illustrates Conchoidal Fracturing.

The following descriptions of minerals have been divided into several groups based primarily on the chemical composition of the mineral. For example, any mineral that is found in nature in its native state-that is, not combined with any other elementis called a native element. While gold and silver are two well-known native minerals, the only native mineral commonly found in Kansas is sulfur.
All other minerals are combinations of elements. Sulfide minerals, which are fairly common in Kansas, are formed by the direct union of an element with sulfur. The combination of zinc and sulfur, for example, produces the sulfide mineral called sphalerite (ZnS). Another common type of mineral in Kansas is the silicates, complex compounds composed of silicon, oxygen, and one or more metals. One form of mica, the mineral muscovite, is a silicate that is composed of silicon, oxygen, aluminum, and potassium ions.
All the common minerals in Kansas fall into seven classes of minerals. The following descriptions of minerals show their classification, where they are found, what they look like, and other information.

Native elements
As mentioned before, minerals that are found in nature in their native state-not combined with other minerals-are called native elements. Only about 20 of these native minerals exist, and sulfur is the only one common in Kansas.
Sulfur (hardness 1 1/2-2 1/2)
Sulfur (S) occurs as irregular masses, as earthy coatings on other substances, and as fine crystals. It is bright yellow, so soft that it can be scratched by a fingernail, and burns with a blue flame.
Sulfur in Kansas has been reported on the surfaces of coal dumps as slender, needle-shaped crystals resulting from the decomposition of pyrite. Small quantities of the earthy variety are present in many Kansas rocks that contain pyrite. Most of it is impure and mixed with clay and limonite.
Sulfides
Nearly all sulfide minerals are formed by the direct union of atoms of an element with sulfur atoms. For example, the combination of lead and sulfur forms a sulfide mineral called galena. Many of the sulfide minerals are valuable ores, such as galena and sphalerite, the sulfide minerals that produce lead and zinc ore. In Kansas, many of the sulfide minerals are found in the southeastern corner of the state.
Galena (hardness 2 1/2)
Galena, the principal ore of lead, is composed of lead sulfide (PbS). It is found in metallic to lead-gray, cube-shaped crystals that break into cubic, rightangled fragments. Some galena crystals are very large. Galena is heavy, has a metallic luster on fresh surfaces, has a gray-black streak, and is so soft that it will mark on paper.
Galena was once mined in southeastern Kansas in the Tri-State district, which was the most important lead- and zinc-producing area in the world in the early part of this century. In the late 1800's, hundreds of small lead and zinc mines operated in Cherokee County. Today these mines are closed. However, galena is still found with sphalerite, chalcopyrite, cerussite, dolomite, calcite, quartz, barite, and other minerals, especially at old mine dump sites. Galena is also found near Pleasanton, Linn County, and has been reported from Chautauqua, Douglas, Elk, and Sumner counties and in rock fragments brought to the surface during drilling for oil in many other counties.
Galena from Tri-State District of Southeastern Kansas.

Sphalerite (hardness 3 1/2-4)
Sphalerite, also called zinc blende, blende, blackjack, and mock lead, is composed of zinc sulfide (ZnS) and is the most important ore of zinc. Pure sphalerite is nearly colorless, but it is commonly brown, yellow, black, or dark red because of impurities. It has a white to dark-brown streak, always much lighter than the color of the specimen. As a rule the mineral crystals are shaped like triangular pyramids, with three sides and a base. Because it has good cleavage in six directions, sphalerite will break into 12-sided blocks. It has a brilliant resinous or almost metallic luster, and it can be scratched by a knife.
Some sphalerite is found as massive deposits varying from coarse to fine grained. In warm hydrochloric acid, powdered sphalerite breaks down and forms hydrogen sulfide, which has a decidedly unpleasant odor, something like the smell of a rotten egg. Sphalerite is easily identified by its cleavage and its resinous luster.
The best specimens of sphalerite found in Kansas are from the lead and zinc mines of Cherokee County. Sphalerite is also found as small crystals in clay-ironstone concretions in the Pennsylvanian shales of eastern Kansas.
Chalcopyrite (hardness 3 1/2-4)
An important are of copper where it occurs in abundance, chalcopyrite is a sulfide of copper and iron (CuFeS2). It is a brassy yellow mineral that makes a greenish-black streak and has a metallic luster. It is brittle, may be tarnished, and can be scratched by a knife. It occurs normally as four-sided pyramidlike crystals, but the crystals are usually poorly formed when the mineral occurs as massive sulfide are. Chalcopyrite is very similar in appearance to pyrite, but they can be distinguished because each has a characteristic color and hardness.
Chalcopyrite occurs with lead and zinc ores in the Tri-State district in Cherokee County. Although chalcopyrite is mined throughout the world as a copper ore, commercial quantities of the mineral are not known in Kansas.
Greenockite (hardness 3-3 1/2)
Greenockite, a rare mineral, is composed of cadmium sulfide (CdS). It has a yellow color, resinous to earthy luster, and cannot be scratched by a fingernail. Thin films of greenockite sometimes coat sphalerite and other minerals in the lead and zinc mines of Cherokee County.
Pyrite (hardness 6-6 1/2)
Pyrite (iron sulfide, FeS2) is a pale, brass-yellow, opaque mineral that is brittle and has a metallic luster. It makes a black streak and is so hard that it can scratch glass. Most pyrite crystals are cubeshaped (like galena), but they also occur in other forms such as octahedrons. Pyrite is also found as granular masses, as cones and globules, and as nodules in shale, limestone, and sandstone. It is called "fool's gold" because its color is yellow like gold; however, pyrite is brittle, has a greenish tinge, and tarnishes, whereas gold is softer, leaves a yellow streak instead of a black one, and does not tarnish easily.
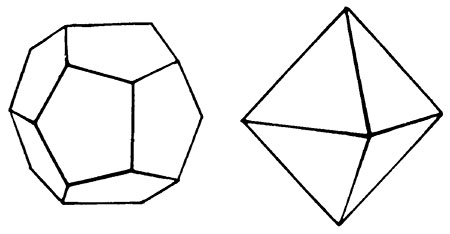
In Kansas, pyrite occurs in rocks of all ages, but it is especially abundant in coal deposits and in areas where lead and zinc occur in the Tri-State district. It is also found with gypsum in the dark shales. For a few years it was produced as a byproduct of coal at West Mineral southwest of Pittsburg and was used in making sulfuric acid.
Marcasite (hardness 6-6 1/2)
Marcasite, sometimes called white iron pyrite, is a mineral composed, like pyrite, of iron sulfide (FeS2). Marcasite is a secondary mineral-it forms by chemical alteration of a primary mineral such as chalcopyrite. On fresh surfaces it is pale yellow to almost white and has a bright metallic luster. It tarnishes to a yellowish or brownish color and gives a black streak. It is a brittle mineral that cannot be scratched by a knife. The thin, flat, tabular crystals, when joined in groups, are called "cockscombs." When combined into balls or nodules, or into more complicated groups, they are marcasite rosettes. The mineral can be distinguished from pyrite by its crystal form. Marcasite weathers readily to form secondary minerals such as limonite and melanterite.
In Kansas marcasite occurs as concretions in coal, shale, and limestone. Well-developed crystals have been taken from the lead and zinc mines of the TriState district in Cherokee County and can be found in all of the coal mines in southeastern Kansas.
Oxides
Oxide minerals are those natural compounds in which oxygen is combined with one or more metals. An example of an oxide mineral found in Kansas is hematite (Fe2O3), a combination of molecules of iron and oxygen. The oxide minerals usually are harder than any other class of mineral except for the silicates, and they are generally heavier than the other classes except for sulfides.
Hematite (hardness 5 1/2-6 1/2)
Hematite is a compound of iron and oxygen (Fe2O3) that may be either red and earthy or black with a dull or metallic luster. Both types have a redbrown or Indian-red streak by which the mineral is readily identified. The earthy variety marks paper easily.
Pure hematite is rare in the surface rocks of Kansas. Most Kansas hematite is of the red variety and is found scattered in clays and shales. It is the cementing material in red sandstones. Small patches of impure hematite mixed with beds of hematite sand are found in the Dakota Formation in eastern Russell County and in Lincoln County near Juniata.
Hematite was once the chief source of iron ore in other parts of the United States, such as northern Minnesota, Michigan, Wisconsin, Pennsylvania, and Alabama.
Ilmenite (hardness 5 1/2-6)
Ilmenite, named for the Ilmen Mountains in the Soviet Union, is an ironlike mineral composed of iron, titanium, and oxygen (FeTiO3). It makes a black to brownish-black streak and cannot be scratched by a knife. Most large specimens of ilmenite are dense, granular masses, but the mineral may occur as platy crystals and as grains in sand. Specimens of the massive variety of ilmenite have been found in the kimberlite near Stockdale in Riley County. In New York state, ilmenite is mined as an ore for its titanium content. Some of the largest deposits of ilmenite occur as beach sands in many parts of the world.
Pyrolusite (hardness 1-2) and psilomelane (hardness 5-6)
Pyrolusite and psilomelane are both oxides of manganese (MnO2), although psilomelane contains varying amounts of other elements. Pyrolusite is a black mineral that is so soft that it will easily make a black streak on paper. It usually occurs in radiating fibers or as treelike patterns (dendrites) on rock surfaces and in the moss agate of Wallace, Trego, and Logan counties. Psilomelane, also a black mineral that makes a very dark brown to black streak, is much harder than pyrolusite--it cannot be scratched by a knife. An earthy form of psilomelane, however, is known as wad, and is soft enough to soil the fingers. Wad forms the coating around pebbles in some gravel deposits, and it also occurs as soft black lumps in gravels and in some soils in southwestern Kansas. Pyrolusite and psilomelane are often found together in the same deposit. Both are sources of manganese ore.
Magnetite (hardness 6)
Magnetite (iron oxide, Fe3O4) is so named because it is readily attracted by a weak magnet and because some magnetite specimens called lodestones are in themselves magnets. The mineral is black, has metallic luster, and makes a black streak. It is so hard that it cannot be scratched with a knife. It is found as granular masses, but, especially in igneous rocks, it occurs as individual crystals, most of which have eight triangular faces and are called octahedrons. Magnetite is an important ore of iron.
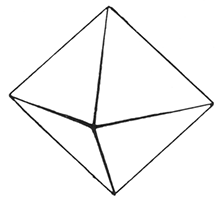
Kansas magnetite is found in the kimberlite near Bala in Riley County, where it occurs as tiny, black, shining octahedrons imbedded in the rock. Occasional grains of magnetite may be found in many river sands.
Limonite (hardness varies)
Limonite, a compound of iron, oxygen, and water [FeO(OH) · nH2O + Fe2O3 · nH2O], is a yellowbrown to dark-brown or black, seemingly noncrystalline mineral. It is formed by the alteration of other minerals that contain iron. Limonite has a characteristic yellow-brown streak, but its hardness depends on the form in which it occurs. The yellowbrown, earthy form of limonite, really a mixture of limonite and clay called yellow ochre, is so soft that it easily leaves a mark on paper. The dark-brown to black variety (bog iron ore) is so hard that it cannot be scratched by a knife. Small quantities of limonite give a yellowish or buff color to most sandstones and to many clays, shales, and limestones. As a scum on quiet water, it may be mistaken for oil. It was once an iron ore of minor importance in some states, but Kansas does not have commercial deposits.
Much of the limonite in Kansas is in the form of concretions (particularly in the Dakota Formation) and in the form of impurities in sedimentary rocks. Limonite which has taken the place of and which has kept the crystal form of pyrite (a pseudomorph after pyrite) has been reported from northeast of Lincolnville in Marion County.
Halides
Halides are compounds that are characterized by atoms of a chemical group called halogens. Halogens include the elements chlorine, iodine, bromine, and fluorine. Because of their chemical makeup, halides are usually soft minerals that have moderate to high boiling points. The only common halide mineral in Kansas is halite, known more commonly as salt.
Halite (hardness 2 1/2)
Halite, common table salt, is composed of sodium chloride (NaCl). Most of its crystals are transparent, colorless cubes, but various impurities in the salt may give halite a brilliant red, blue, or yellow color. Normally halite has three good cleavages at right angles to each other, so broken fragments also may be very nearly cube-shaped. Some red halite has a fibrous or columnar structure. Halite is easy to identify because it has a salty taste and because it dissolves rapidly in water.
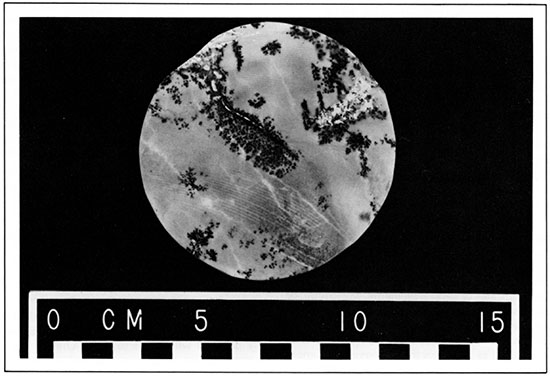
Salt in Kansas is found in thick beds in Permian rocks deep in the ground. It does not form outcrops because rain and ground water dissolve salt from the surface exposures. Most of the salt is found in the Hutchinson salt bed, which underlies approximately 37,000 square miles in central Kansas. The formation averages about 250 feet in thickness and contains a staggering 13 trillion tons of salt, enough to make a block measuring 10 miles on each side. It is mined at Hutchinson, Kanopolis, and Lyons and is used in chemical industries, in meat packing, for livestock, and as common table salt. Each year Kansas produces over a million tons of salt.
Salt from Central Kansas.

Carbonates
This group includes some of the most common minerals in Kansas, such as calcite and dolomite. The carbonate minerals are distinguished by a complex chemical makeup that includes an element combined with atoms of carbon and oxygen. Calcite, for example, is composed of atoms of calcium, carbon, and oxygen.
Calcite (hardness 3)
Calcite (calcium carbonate, CaCO3) is the primary constituent of limestone and is therefore one of the most common minerals in Kansas. Generally it is white or colorless, but it may be tinted gray, red, green, or blue. It occurs in many varieties of crystal forms (more than 300 have been described). It also may be granular, coarse to fine, or even so fine grained that it has an earthy appearance. Calcite can be scratched by a knife, but not by a fingernail, and it fizzes freely in cold dilute hydrochloric acid. If a large piece of calcite is shattered with a hammer, it breaks into small rhomb-shaped blocks because it has perfect cleavage in three directions.
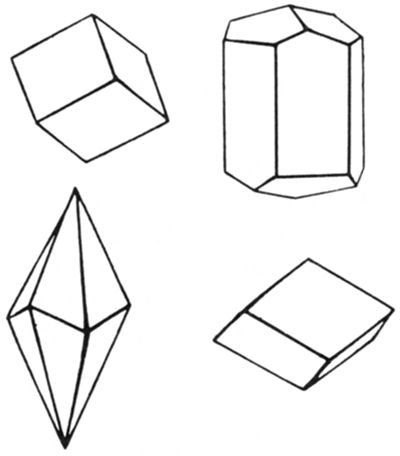
Besides being the mineral that forms limestone, calcite occurs as a common cementing material in many Kansas sandstones. It is found in calcareous shales and clays and as veins in the igneous rocks of Riley County. In the Cretaceous Niobrara Chalk and other Cretaceous rocks, it has formed fairly large veins. Calcite is an important part of many concretions. Brown calcite and colorless-to-yellow calcite crystals are common in the septarian concretions of the Pierre Shale in Wallace and other counties. Tiny calcite crystals form the lining of geodes in certain limestones and shales, and they coat the insides of many fossil shells. Among the finest calcite crystals in Kansas are those from the lead and zinc mines of Cherokee County, most of these being pale yellow and some of them very large. The most important use of calcite is in the manufacture of cements, limes for mortar, in the chemical industry, and in fertilizers.
Siderite (hardness 3 1/2-4)
Siderite is a common mineral composed of iron carbonate (FeCO3). It is light to dark brown, and some of it occurs as rhomb-shaped crystals with curved faces (like dolomite). Most siderite, however, is granular or earthy. The mineral can be scratched by a knife. It fizzes in hot hydrochloric acid but reacts more slowly in cold acid. Weathered surfaces change to limonite and turn dark brown.
Most siderite in Kansas is in the impure form called clay ironstone. This is a mixture of siderite with limonite, clay, and silt, forming small nodules or whole beds in clays, shales, and sandstones.
Limestone in Fort Hays Limestone in Jewell County.

Smithsonite (hardness 4-5)
Smithsonite (a secondary zinc carbonate, ZnCO3) is commonly brown in color, but it may be green, blue, pink, or white. Although it does occur as rough, curved, rhomb-shaped crystals, its occurrence as rounded, globular forms or as honeycomb masses is more common. Smithsonite is harder than most carbonate minerals, but it can be scratched by a knife. It fizzes in dilute cold hydrochloric acid.
Smithsonite is common in the near-surface parts of the zinc deposits in easternmost Cherokee County, where it has formed as the result of the action of carbonated water on sphalerite. Smithsonite is a zinc ore and was named in honor of the Englishman James Smithson, who also supplied funds for the founding of the Smithsonian Institution.
Dolomite (hardness 3 1/2-4)
The mineral dolomite is composed of calcium magnesium carbonate [CaMg(CO3)h] and is closely related to calcite. In large masses the mineral forms the rock called dolomite. It may be white, gray, greenish gray, brown, or pink, and it has a glassy to pearly luster. It occurs in coarse- to fine-grained granular masses and in crystals. Most dolomite crystals are rhomb-shaped like calcite cleavage blocks, but, unlike most other minerals, the crystal faces are typically curved. Dolomite is slightly harder than calcite, although it can easily be scratched by a knife. It will not fizz readily in dilute cold hydrochloric acid unless first ground to a powder.
Curved white crystals of dolomite are common in lead and zinc mines of Cherokee County, where they occur with sphalerite, chalcopyrite, galena, and several other minerals. Dolomite crystals also have been found in Pennsylvanian limestones in the Ross quarry near Ottawa in Franklin County, about two miles north of Williamsburg in Franklin County, and three miles north of Garnett in Anderson County. They also are found in the rock dolomite formations and certain red and green shales of McPherson, Rice, Reno, Kingman, and Clark counties.
Aragonite (hardness 3 1/2-4)
Aragonite has the same chemical composition as calcite (CaCO3), but it has poorer cleavage and a different crystal form than calcite. Aragonite crystals commonly occur as radiating groups of fibrous or needlelike shapes. Like calcite, aragonite fizzes and dissolves readily in cold dilute hydrochloric acid and can be scratched with a knife. This mineral is colorless to white, gray, yellow, green, brown, and violet and is ordinarily found as a vein mineral, in cave deposits, and as the pearly layer of many types of shells.
Aragonite is much less common than calcite because it changes easily to calcite without altering its external shape. It is difficult to identify in the field. The mineral has been reported from several areas in Kansas: as nodules in a clay deposit in northern McPherson County and in a sand pit two miles southeast of McPherson; as veinlets cutting country rock at Silver City in southern Woodson County; as small crystals in vugs, or cavities, in the limestone of the Ross quarry near Ottawa in Franklin County; and in many concretions in the Cretaceous shales of western Kansas. This mineral is named for the region of Aragon in Spain.
Cerussite (hardness 3-3 1/2)
Cerussite (lead carbonate, PbCO3) occurs as granular masses and as platy crystals which commonly cross each other to form a latticelike effect. Cerussite has a brilliant, glassy luster and is colorless or white. It fizzes slightly in cold dilute hydrochloric acid and is very heavy for a nonmetallic mineral.
In Kansas, small amounts of cerussite are occasionally found as a result of the chemical change of galena (lead sulfide) in the near-surface parts of lead deposits in easternmost Cherokee County.
Malachite (hardness 3 1/2-4)
Malachite, a copper ore, is a bright-green copper carbonate mineral having the composition Cu2CO3(OH)2. It has a dull to glassy luster and a light-green streak. The mineral fizzes in cold dilute hydrochloric acid and can be scratched by a knife.
Malachite occurs in Sedgwick, Sumner, and Harper counties, where it is associated with copper mineralization in the Permian shales and carbonate rocks. It occurs as tiny, brilliant-green specks in some thin dolomite beds near the top of the Wellington shale and in a few other Permian rocks. It may be found, among other places, at the bridge crossing the Ninnescah River two miles south of Milan, Sumner County. It also occurs in the Tri-State district in southeastern Kansas.
Sulfates
Another common group of Kansas minerals is the sulfates. These minerals consist of an element combined with atoms of sulfur and oxygen. One of the widespread sulfate minerals is gypsum, composed of atoms of calcium combined with atoms of sulfur and oxygen.
Barite (hardness 3-3 1/2)
Barite (barium sulfate, BaSO4) is a common mineral in Kansas, but it is not found in large quantities. Because of its high density, it is sometimes called "heavy spar." It occurs as flat, tabular crystals, either singly or in groups, and it also occurs in granular or earthy forms. The individual crystals are transparent to opaque, have a glassy luster, and have perfect cleavage in two directions. Barite is usually colorless or white but may be light shades of blue, yellow, or red. It can be scratched with a knife but not with a fingernail. In appearance, it resembles gypsum, calcite, or celestite. However, aside from its relatively heavy weight, barite can be distinguished from gypsum by its greater hardness and from calcite because it does not fizz in hydrochloric acid. A flame test is the best means of distinguishing between barite and celestite. If powdered barite is heated on a clean platinum wire in a Bunsen burner, the flame will become green, but celestite will turn the flame bright red.
Barite has been found in Kansas in some of the Pennsylvanian and Permian limestones, especially in Brown, Anderson, Franklin, and Chase counties; in septarian concretions of the Cretaceous Pierre Shale in Logan and Wallace counties; in petrified wood; and occasionally in the lead and zinc mines of Cherokee County. It occurs in veins a few millimeters thick in the Niobrara Chalk in north-central and northwestern Kansas. It also occurs as a cementing material between sand grains in peculiar roselike concretions called "desert roses" or "petrified walnuts" in certain sandstones of the Cretaceous Kiowa Shale. These barite "roses" and "walnuts" are found in an area near Bavaria in Saline County and are abundant near Horsethief Canyon in Ellsworth County.
Barite is used in paint pigments, as a filler in paper and cloth, in making glazes for pottery, and in the refining of sugar. It has not been found in commercial quantities in Kansas but has been mined in Missouri and Arkansas.
Barite Rose from Oklahoma.

Celestite (hardness 3-3 1/2)
Celestite (strontium sulfate, SrSO4) is similar to barite in appearance, in geologic occurrence, and in crystal form. It has a glassy luster and its crystals are colorless, white, or a faint blue or red. This mineral is found in Kansas as radiating pink fibers, as vein fillings, and as scattered particles. Celestite cannot be scratched by a fingernail. It differs from barite in its lighter weight and in its property of coloring a flame red.
Celestite has been found in solid blue or pink crystals and as pink to white radiating fibers at Kanopolis dam near the water's edge below the spillway outlet. It also has been found as pink crystals and as veins in Brown County north and west of Morrill, and in Chase and other counties. Celestite has been found in the weathered zone at the top of Permian rocks below Cretaceous sands and shales.
Anhydrite (hardness 3-3 1/2)
Anhydrite, composed of calcium sulfate (CaSO4), constitutes one of the three main evaporite deposits, the other two being gypsum and halite. It occurs commonly as light-gray, crystalline masses, although some anhydrite has a fibrous structure. It may occur as individual crystals in other rocks, particularly in dolomite. It has a glassy luster and is translucent. It is harder and heavier than gypsum; although it can be scratched easily with a knife, it cannot be scratched with a fingernail. Anhydrite may change into gypsum if water is added, a common occurrence in near-surface exposures. Fine-grained dolomite and anhydrite look somewhat similar but can be distinguished from one another because cold hydrochloric (muriatic) acid will not act on anhydrite.
Kansas anhydrite is found in Permian-age deposits associated with beds of gypsum, dolomite, and red silt. With gypsum, it caps many of the Red Hills of Barber and other counties.
Gypsum (hardness 2)
Gypsum is calcium sulfate containing water (CaSO4 · 2H2O). The same chemical compound without water is anhydrite, a quite different mineral. Gypsum is a common mineral that is widely distributed in the sedimentary rocks of Kansas in the form of thick beds, well-formed single crystals, and joint or crack fillings. It is colorless to white or light gray, rarely bright red, and is so soft that it can be scratched by a fingernail.
Three varieties of gypsum are recognized. The type of most interest to collectors is the coarsely crystalline, transparent variety called selenite. It consists of flat, diamond-shaped crystals having such perfect cleavage that they can be split into thin sheets. Selenite is common in dark shales such as the Kiowa, Carlile, and Pierre shales of Cretaceous age in western Kansas. Most selenite crystals found on weathered shale slopes have irregular or etched surfaces, but fresh, clear crystals can be uncovered by careful digging into the hillside. Occasionally one finds specimens consisting of two crystals grown together into what is known as a "fish-tail twin." In some places a network of selenite crystals is found in thin joint fillings, and some of the crystals may be grown together in a pattern called "gypsum flowers." Small quantities of bright-red selenite are found in some of the stream banks on the outskirts of Wichita in Sedgwick County.
Selenite from Red Hills of South-Central Kansas.

Satin spar is another variety of gypsum. It is white or pink, fibrous, and has a silky luster. It is found as thin layers in beds of rock gypsum and in certain shales. The third recognized variety is massive or rock gypsum. It is coarsely to finely granular, white to gray, and contains various amounts of impurities. A good outcrop of rock gypsum can be seen about 10 miles west of Medicine Lodge along Highway 160. Alabaster, which rarely occurs in Kansas, is a very fine grained type of massive gypsum. Gypsite, or gypsum dirt, is formed in the soil or in shallow lakes, and consequently it is a sandy or earthy deposit, although it contains a large amount of the mineral gypsum. It is found in Clay, Saline, Dickinson, Marion, Harvey, and Sedgwick counties.
Satin Spar from Barber County.

Large quantities of rock gypsum are mined in Barber and Marshall counties and large deposits also are found in Permian rocks in Saline, Dickinson, and Comanche counties. It is used in making plaster of Paris, Portland cement, various wall plasters and mortars, wallboard, and as a fertilizer.
Goslarite (hardness 2)
Goslarite (ZnSO4 · 7H2O) is zinc sulfate containing water and is formed by chemical action on sphalerite. It is found occasionally in the Tri-State area as long, slender, needlelike crystals. Goslarite not uncommonly develops on the mine walls. It has a white, reddish, or yellowish color.
Silicates
More than 90% of the rock-forming minerals are silicates, compounds containing silicon and oxygen as quartz or combined with one or more metals in more complex molecules. These minerals make up about 95% of the earth's crust and some silicates, such as quartz and feldspar, are especially common.
Garnet (hardness 6 1/2-7 1/2)
Garnets are a group of minerals whose crystals have many faces, all of about equal size. They have a glassy luster and are hard enough to scratch window glass. Most garnets are red to brown, but some are black, green, or colorless. In chemical composition they are silicates of calcium, magnesium, iron, manganese, aluminum, and chromium in various combinations, with the aluminum-silicate varieties predominating.
Small red and brown garnets occur in the kimberlite outcrop near Stockdale in Riley County, and they may be found in the bed of the small stream that cuts across this outcrop. Garnets also have been found in other Riley County kimberlites and in the streams flowing near the kimberlites.
Hemimorphite (hardness 4 1/2-5)
Hemimorphite, sometimes called calamine, is a silicate of zinc containing water. Its chemical formula can be written Zn4Si2O7(OH)2 · H2O. It is a white mineral found in radiating crystal groups and in globular forms. It can be scratched by a knife. Hemimorphite usually occurs with zinc ores, and in Kansas it is fairly common in the upper parts of the sphalerite deposits of the Tri-State area in Cherokee County. In some parts of the world, hemimorphite is mined for zinc ore, although not in Kansas.
Mica (hardness 1 1/2-3)
Mica is the name of a group of several minerals that are unusual because they split into thin, flat, flexible sheets. These minerals split this way because they have one perfect cleavage. They are composed of aluminum silicates of several elements. Muscovite, or common "white" mica, is transparent and colorless. In some igneous rocks (outside Kansas), it occurs in crystals several feet wide, large enough that they were once used as windows in coal stoves. In Kansas it is usually seen as tiny, flat, shining flakes in sandstones, siltstones, and shales, and as small crystals in boulders of metamorphic and igneous rocks. In some sands of the Ogallala Formation, muscovite has weathered to resemble gold flakes. Muscovite was so named because it was used as a substitute for glass in old Russia (Muscovy).
Biotite (black mica), rarer in Kansas than muscovite, may be seen in some of the Tertiary and Quaternary sands. The color of biotite is caused by iron and magnesium. Phlogopite and vermiculite mica are yellowish brown, have a copperlike luster on the cleavage surfaces, and often are mistaken for flakes of gold. Phlogopite is found in the kimberlites of Riley County and near Silver City in Woodson County.
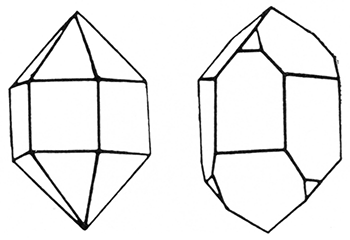
Quartz (hardness 7)
Quartz, the most common of all minerals, is composed of silicon and oxygen (silica, SiO2) and is found in many different varieties. When pure it is colorless, but it also assumes various shades of yellow, pink, purple, brown, green, blue, or gray. It has no good cleavage and has a glassy to greasy luster. One of the hardest of the common minerals, it will easily scratch window glass. In fact, quartz can be distinguished from calcite, another extremely common rock, by its hardness. The knife will scratch calcite but not quartz.
The two primary types of quartz are the coarsely crystalline and the fine or cryptocrystalline forms. The crystals of the first type are six-sided prisms with pyramids capping one or both ends. Well-formed, colorless quartz crystals of this type are found in geodes and as a lining on the inside of fossils in many parts of the state, particularly in the Lone Star quarry at Bonner Springs in Wyandotte County and in an area of eastern Chase County. Quartz crystals with a bluish cast are found in the igneous rock south of Yates Center in Woodson County. Nearly all sands and sandstones are composed of tiny, worn particles of crystalline quartz.
The second primary type of quartz is called cryptocrystalline because the crystals are so small they can only be seen with a microscope. One of the best-known varieties of this group is flint or chert, which is common in many Kansas limestones as nodules or beds. Chert is opaque and is dull gray, brown, or black in color. It breaks with a shell-like fracture, and the edges of the broken pieces are sharp. The famous "chat mountains" at the lead and zinc mines of Cherokee County consist almost entirely of chert fragments. Chalcedony is a cryptocrystalline quartz with a waxy luster that forms banded layers or globular masses. Agate is a many-colored, banded form of chalcedony that has been deposited in cavities or in veins and that is used for ornamental purposes. Beautiful agates, doubtless from the Lake Superior region, have been found in the glaciated district in Kansas.
Opal (hardness 5-6)
Opal consists of silicon dioxide, like quartz, plus an indefinite amount of water (SiO2 · nH2O). It never forms as crystals, but probably is deposited as a jellylike substance that later hardens. The mineral may be white, yellow, red, brown, green, gray, blue, or transparent and colorless. Precious opal, which is not found in Kansas, shows a beautiful play of colors and is highly prized as a gemstone. Opal cannot be scratched by a knife, but is slightly softer than quartz. It is found as a lining or filling in cavities in some rocks, as a deposit formed by hot springs, and as the petrifying material in much fossil wood.
A common Kansas mineral, opal is widespread in the Ogallala Formation in Clark, Ellis, Logan, Ness, and Rawlins counties. This Ogallala opal is colorless to white or gray and is found with a white, cherty, calcareous rock. Some of it is called "moss opal" because it contains an impurity, manganese oxide, that forms dark, branching deposits like small mosses in the opal. Moss opal (or moss agate) has been found in Trego and Wallace counties. Opalized fossil bones and shells of diatoms also are found in the Ogallala Formation, as is a green opal that acts as a cement in hard, erosion-resistant sandstones.
Moss Opal from Wallace County.
Feldspar (hardness 6)
The term feldspar applies to all members of a group of minerals composed of aluminum silicates carrying principally potassium, sodium, or calcium. The feldspars are light in color (pink, green, white, and gray), have a glassy or satiny luster, and have good cleavage in two directions, almost at right angles to each other. They cannot be scratched by a knife. Feldspars commonly occur in igneous rocks. Granite boulders found in Woodson County intrusives (mica-peridotite) are largely made up of white feldspar and quartz. The feldspar found in sedimentary rocks in Kansas was brought in as pebbles in streams and in boulders of igneous rocks in the glacial till or boulder clay. Feldspar pebbles may be distinguished from quartz pebbles by their good cleavage.

Prev Page--Kansas Rocks || Next Page--Sedimentary Structures
Kansas Geological Survey
Placed on web Feb. 28, 2017; originally revised and reprinted 1998.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/ED2/04_mine.html