
Kansas Geological Survey, Bulletin 215, originally published in 1978

Originally published in 1978 as Kansas Geological Survey Bulletin 215. This is, in general, the original text as published. The information has not been updated.
Rock salt, or halite, has been an important mineral resource in Kansas since the early days of territorial settlement. In more recent times, subsurface salt-bearing formations have been of importance for reasons other than the mining of salt. Shallow subsurface salt is a source of potential natural pollution of fresh ground water in some areas, and this dissolution of salt sometimes has the secondary effect of creating geologic hazards where the overlying formations are subject to collapse into caverns left when salt is removed. Salt interferes with the interpretation of information from seismic exploration methods used in the search for oil and gas, but it is useful for strategic storage of petroleum and natural gas because of its integrity as a leak-proof container where special cavities are dissolved into nearly pure salt beds. This quality of salt, to act as a container for sensitive materials in a remote underground environment away from the effects of ground water, was important in development of the idea of subsurface storage of radioactive wastes in the 1950s.
Although the idea of storing radioactive material in Kansas was proved to be inadvisable, in the course of studies which led to this conclusion several core samples of salt-bearing rock formations beneath the central and western part of the state were taken to determine the true, in-place character of the salt.
This study focuses on the composition and extent of the salt in two of these formations, the Blaine Formation and the Flower-pot Shale, and of the associated red shale and sandstone formations beneath the salt, in the area centering on Wichita County, Kansas. These salt formations are distinctly different than the evenly bedded, slightly older salt deposits in central Kansas, in that the Flower-pot and Blaine salts are coarsely crystalline halite in a matrix of red silty mudstone. Anhydrite is also an important component of these salt-bearing formations.
The concentration of bromine in these salts is very low. This chemical characteristic, when considered with the textural and stratigraphic evidence developed here, indicates that the salt was deposited in a shallow, continental basin, which was subject to occasional flooding by the sea. The salt was repeatedly dissolved and reprecipitated in place during deposition. Knowledge of this history has made possible maps showing the extent of the salt and suggesting how these deposits relate to other rocks in westernmost Kansas and adjacent states.
This study is an important contribution in the series of studies the Kansas Geological Survey is sponsoring to learn more about the potential usefulness of Kansas salt beds.
An unusual red bed-evaporite sequence of Permian Age was cored in western Kansas for the Atomic Energy Commission in 1972. The core extends upwards from the Harper and Salt Plain formations, through the Cedar Hills Sandstone, Flower-pot Shale and the Blaine Formation. In the core, the Harper and Salt Plain formations and the Cedar Hills Sandstone are red bed deposits, commonly cemented by halite. Overlying the Cedar Hills, the sediments corresponding to the Flower-pot Shale and the Blaine Formation are composed predominantly of fine to coarsely crystalline halite which is intimately associated with varying amounts of red silty mudstone.
The bromine concentration of the halite is very low, commonly less than 5 ppm, throughout the section. The textural and stratigraphic relationships of the sediments suggest that this is the result of repeated solution and reprecipitation of the halite in situ during deposition, and was not caused by widespread post-depositional recrystallization. The low bromine concentration of the halite, minor amount of carbonate in the sequence, and the intimate association of evaporites with red beds suggest that the deposition of these sediments took place in a shallow, continental basin, which was subject to occasional flooding by the sea.
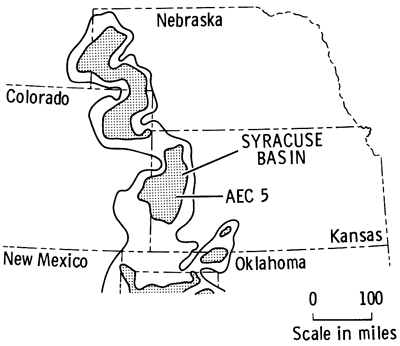
The existence of complex red bed-evaporite sequences in the Nippewalla Group, upper Leonardian, Permian, in the subsurface of western Kansas, has been known since the late 1800s. Much progress was made in the identification of the individual units during the 1920s and 1930s as a result of numerous wells drilled in exploration for oil and gas. Correlation and distribution of the evaporite units, mainly halite and anhydrite, have previously been established only on the basis of geophysical and sample logs. In 1972, a core was taken of the evaporite sequences in Wichita County, Kansas (Fig. 1), for the Atomic Energy Commission (A.E.C. Test Hole 5) to determine whether the thick halite beds were suitable for the storage of high-level radioactive wastes. The site was chosen in order to penetrate the salt beds in the area where they are thickest, as indicated by geophysical logs, and is located 150 ft. north and 150 ft. east of the center of sec. 22, T. 19 S., R. 37 W. Fresh water mud was used for drilling between 0-1159 ft. (0-353 m), and salt-saturated mud between 1159-2450 ft. (353-747 m). The total depth drilled was 2450 ft. (747 m). The top of the Permian was reached at 1185 ft. (361 m), and a 3.5 in. (89 mm) diameter core was drilled from 1540 to 2058 ft. (470-627 m). Only when the core had been drilled was it found that the salt is intimately associated with variable amounts of red anhydritic clay, and is quite unsuitable for the disposal of waste. The core penetrated 510 ft. (155 m) of sediments, extending upwards from the Harper and Salt Plain formations through the Cedar Hills Sandstone, Flower-pot Shale, and the Blaine Formation. Photographs of rocks illustrated here, Figs. 6-50, are all from sections of core from this well, A.E.C. Test Hole 5.
Figure 1--Occurrence of evaporite facies in the Nippewalla Group of the Midcontinent (after Rascoe, 1972) and location of drilling site for AEC Test Hole 5 in the Syracuse Basin.
Evaporites of equivalent age occur in the subsurface of southwestern Nebraska, and western Oklahoma and, as in Kansas, correlation of the deposits and interpretation of the environments in which they were deposited has been based upon studies of well logs and samples. The A.E.C. core appears to be the only core of the Nippewalla Group evaporite deposits in the western Midcontinent. Previously, no detailed petrographic study of the evaporites in the subsurface has been possible, because of the lack of cored material. Such a study is essential in order to interpret the depositional environment of the evaporitic sediments. This extremely valuable core serves as the basis for the present study.
The Permian System in Kansas was originally defined by Cragin in 1896. Later, Norton (1939) studied the Permian red beds in Kansas and Oklahoma, and made further stratigraphic subdivisions which, for the most part, are still in use today.
The stratigraphy of the Nippewalla Group in the subsurface in Kansas was studied by Merriam (1958), Malone (1962), Campbell (1963), and Schumaker (1966). More recently, regional stratigraphic correlations and lithofacies maps for the Permian of the Midcontinent were compiled by Rascoe (1968) and Rascoe and Baars (1972), and for the United States as a whole by McKee et al. (1967a; 1967b).
Several studies have been conducted on the Blaine in outcrop and subsurface in Kansas and Oklahoma, including those by Kulstad et al. (1956), Ham (1960), Fay, (1964), and Johnson (1967). Jordan and Vosburg (1963) described units equivalent in age to the Nippewalla Group of Kansas from Oklahoma and the Texas Panhandle.
Swineford (1955) apparently made the only study of the petrography of the sediments of the Nippewalla Group in Kansas, and her study was confined to exposures in south-central Kansas. Spores from the Flower-pot Formation in Oklahoma were described by Wilson (1962).
A report on the suitability of the salt section cored in western Kansas for radioactive waste disposal was prepared for the Atomic Energy Commission by the staff of the Kansas Geological Survey and consultants (Bayne and Brinkley, 1972).
Permian red beds and evaporites occur throughout the western Midcontinent. They are thought to have been deposited in extensive shallow brackish-saline seas subject to periodic influxes of marine water from the south (Hills, 1942).
Sediments were deposited throughout the region in southwestern Nebraska, western Kansas, western Oklahoma, western Texas, and eastern Colorado and New Mexico, in an area referred to in general terms as the Permian Salt Basin (Bachman and Johnson, 1973). The sediments were shed from the surrounding land masses, the coarse-grained debris being derived from the east and south (Swineford, 1955) and the fine-grained material from the low-lying area to the north (Mudge, 1967). The seas were confined by the Front Range to the west, and the Ozarks, Arbuckles, and Wichitas to the east and south. A low-lying land mass was located to the north and northeast in Nebraska (Fig. 2) (Mudge, 1967). Low-lying positive elements (McKee et al., 1967a, p. 16.) within the major depositional basin of the Midcontinent led to further restrictions of the sea, resulting in the deposition of halite.
In Kansas, evaporites of the Nippewalla Group were deposited in the Hugoton Embayment of the Anadarko Basin (Maher and Collins, 1948), in a saline arm of the sea extending from the south (Hills, 1942) (Fig. 2). Halite was deposited in the Syracuse Basin of the Hugoton Embayment, which was bounded to the west by the Las Animas Arch (Mudge, 1967) and to the east by the Oakley Anticline (Fig. 2) (Merriam, 1963). Halite was also deposited in south-central Kansas in a basin which may have been connected with the Syracuse Basin (Malone, 1962; Campbell, 1963).
Figure 2--Structural elements controlling Leonardian deposition in the Midcontinent (after McKee et al., 1967).
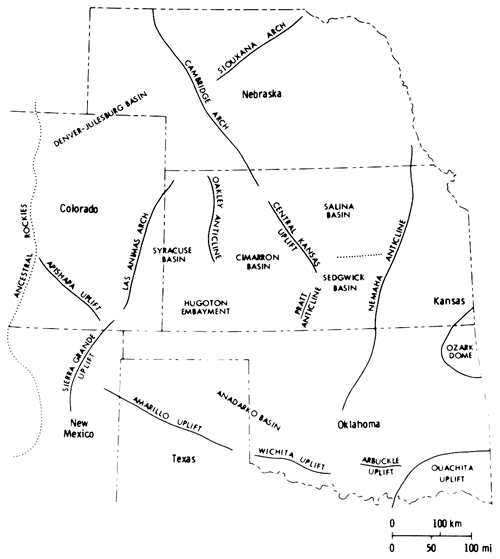
Evaporite deposits equivalent in age to those of the Nippewalla Group in Kansas occur in Nebraska, in the Julesburg Basin (Fig. 2), and in Wyoming, as well as farther south in Oklahoma and Texas. A western connection to the sea was postulated for the basins in Nebraska and Wyoming (Maughan, 1966) and a southern connection for those in Oklahoma and Texas (Hills, 1942). Previous investigations have linked Kansas to the sea via the Hugoton Embayment and Anadarko Basin (Hills, 1942; Malone, 1962; Campbell, 1963; Schumaker, 1966).
Correlation of upper Leonardian-lower Guadalupian sediments of the western Midcontinent is problematical, and at the present time has not been satisfactorily resolved. Continental red bed evaporite facies occur in western Nebraska, Kansas, and Oklahoma, and in each state a different classification is used. In Kansas, the Leonardian-Guadalupian boundary is apparently not a well-marked hiatus; thus, the Cimarronian Stage was introduced to include all deposits from the Wellington Formation to the base of the Whitehorse Formation (O'Connor, 1963).
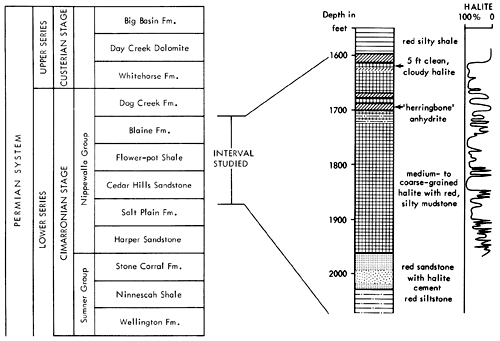
The sediments forming the basis of the present study fall within the Nippewalla Group of Cimarronian age. The formations included in the Nippewalla Group by the Kansas Geological Survey are the Harper Sandstone, Salt Plain Formation, Cedar Hills Sandstone, Flower-pot Shale, Blaine Formation, and the Dog Creek Formation (Fig. 3). These units crop out in south-central Kansas, with a total thickness of about 930 ft. (284 m). The sediments are typically unfossiliferous, and consist of conformable red beds and gypsum beds (O'Connor et al., 1968). Correlation and nomenclature are made difficult by the fact that evaporites, including thick salt beds, generally occur in the subsurface, where they are protected from solution, and the formations with which they are associated are described and classified in outcrop. In the subsurface of western Kansas, unnamed salt and anhydrite beds occur in most of the formations of the Nippewalla Group.
Due to the unfossiliferous nature of the sequence, correlation of the sediments of the Nippewalla Group in Kansas with similar deposits in Nebraska and Oklahoma is based upon tracing unconformities, and anhydrite and dolomite marker beds, such as the Blaine and Stone Corral formations (Rascoe, 1968; Johnson et al., 1975). Many of the thin marker beds do not extend into Kansas, and the time-stratigraphic position of the beds within the Nippewalla Group is difficult to ascertain. As Swineford (1955) remarked, "The problem of correlation of non-fossiliferous red clastics with a type marine section in West Texas seems almost insurmountable . . . ."
The units present in the core from A.E.C. Test Hole 5 are the Harper and Salt Plain formations, Cedar Hills Sandstone, Flower-pot Shale, and Blaine Formation (Fig. 3). Descriptions of these units in outcrop were given by Swineford (1955), Kulstad et al. (1956), Fay (1964), Schumaker (1966), and Zeller (1968). A summary of the main features of the units in outcrop, taken from these reports, is included below for completeness and to permit comparison with the textures and lithologies of the same units in the subsurface. The Stone Corral Formation and Dog Creek Formation were not cored, and are therefore not included in the present discussion.
Figure 3--Stratigraphic position of interval studied with generalized lithologic section of core from AEC 5 and curve showing percent halite in cored sediments.
The dominant sediments of the cored section are red clay, silt and sand, associated with varying amounts of halite and anhydrite (Fig. 4). Minor amounts of dolomite, magnesite and quartz, and traces of the iron oxides geothite and/or hematite also occur. No potassium or magnesium salts, other than magnesite, have been found in this evaporite sequence. The cored sediments form a conformable sequence (Swineford, 1955; Rascoe, 1968) and, in most cases, the formation boundaries are clearly gradational. The boundaries are placed according to changes in lithology, as indicated by a change in response on geophysical logs.
Figure 4--Graphic lithologic log and gamma ray log for AEC Test Hole 5.
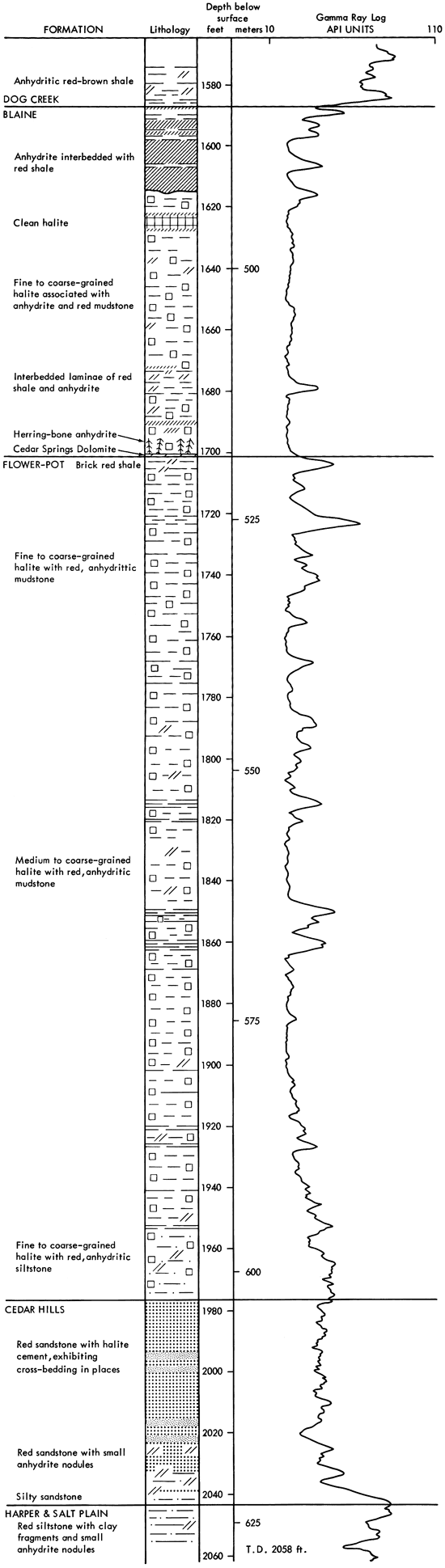
The lowermost sediments of the A.E.C. core (Fig. 4) belong to the Harper and Salt Plain formations, and are composed of mottled, red, silty shale and siltstone. The overlying Cedar Hills Sandstone consists of coarser-grained sediments, ranging from silt to sand size, cemented by halite. The boundary between these formations is gradational, and is placed at a depth of 2044 ft. (623 m). The contact between the Flower-pot and the underlying Cedar Hills is also gradational, occurring through a thickness of sediments of approximately ten feet (1977-1967 ft.) (603-600 m). The clastic sediments grade upward from sandy through silty, to clay size higher in the Flower-pot sequence. The Flower-pot is also marked by the development of halite as discrete crystals, not just as a cement. The Cedar Hills-Flower-pot boundary is placed at 1977 ft. (603 m), as no individual salt crystals occur beneath this level, and it corresponds to a change in the response on the geophysical logs (Fig. 4). It is thus a traceable horizon within the Syracuse Basin. The contact of the Flower-pot with the overlying Blaine Formation is abrupt, and is marked by a red clay bed in the Flower-pot in contact with gray, dolomitic clay of the Blaine, at a depth of 1701.5 ft. (519 m). The Blaine contains a number of anhydritic beds which give prominent deflections on geophysical logs. It grades upwards into red, anhydritic shale of the Dog Creek, and the contact is placed at 1587 ft. (484 m), corresponding to the top of the uppermost anhydrite bed.
Halite is the most abundant evaporite mineral in the sediments studied. In the Flower-pot and Blaine formations, fine to coarse crystals of halite occur intimately associated with varying amounts of red, silty, anhydritic mudstone (Fig. 4). In the Flower-pot, halite makes up approximately eighty percent of the sediment and, in places, approaches ninety-five percent in purity. The only section of halite which is free of red, silty mudstone occurs in the Blaine Formation (1627.5-1623 ft.) (496-494.8 m).
Anhydrite is the second most abundant mineral, but is much less abundant than halite. It occurs dispersed as fine acicular crystals throughout the salt deposits of the Blaine and Flower-pot, mixed with various amounts of clay minerals. Small anhydrite nodules are common in the lower part of the Flower-pot Formation, and in the underlying red beds of the Cedar Hills, Harper and Salt Plain formations, but are absent in the upper part of the Flowerpot and in the Blaine. Thin anhydrite beds, and wavy stringers of anhydrite occur in the Flower-pot, but the main development of anhydrite beds in the cored sequence is found in the Blaine Formation (Fig. 4).
A number of other studies of the Nippewalla Group in the subsurface of western Kansas have been made; however, all relied heavily upon well logs and sample logs, as no core was available. Cuttings taken during drilling of a salt section when no special measures, such as the use of salt-saturated drilling mud, have been taken, are unreliable. Not only is much of the salt dissolved during drilling, but caving-in from above may lead to difficulty in the interpretation of the cuttings. The cored section of the formations of the Nippewalla Group studied here allows better interpretation of the correlation of these units in the subsurface of western Kansas. The boundaries between the formations are readily identifiable on geophysical logs (Fig. 5), which were used in preference to sample logs in the present study.
Figure 5--Gamma ray and resistivity logs from southern part of Syracuse Basin showing positions of stratigraphic boundaries.
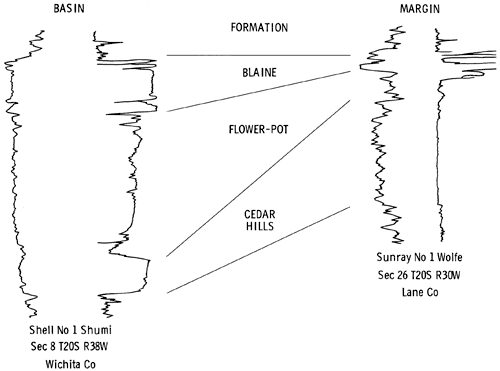
Isopach maps and cross sections of the Nippewalla Group (Plates I-V) based on geophysical logs supplement and differ in some respects from those presented previously (Malone, 1962; Campbell, 1963; Schumaker, 1966; Rascoe, 1968; Rascoe and Baars, 1972). The area considered was restricted to the Syracuse Basin and its margins in Kansas.
The similarity in lithology and response on geophysical logs makes it very difficult to distinguish between the Harper Sandstone and Salt Plain Formation in the subsurface (Schumaker, 1966) (Plate I), and for subsurface correlation purposes, these formations are undifferentiated. The Harper Sandstone was named by Cragin (1896, p. 18-19) from exposures in Harper County, Kansas. In outcrop it is composed of brownish-red, spotted argillaceous siltstones and silty shales, with a few thin silty sandstones. The maximum thickness is 220 ft. (67 m) (Swineford, 1955). The Salt Plain Formation was named by Cragin (1896, p. 23), but according to Swineford (1955) it has no type locality. In outcrop it is composed of reddish-brown flaky siltstones, thin, sandy siltstones, and fine-grained sandstones (Swineford, 1955). The total thickness in outcrop is about 265 ft. (81 m) (Moore et al., 1951).
These formations were encountered between 2044 ft. and 2366 ft. (623-721 m) in A.E.C. Test Hole 5, but only the uppermost 14 ft. were cored. The occurrence of salt below this interval is indicated on the geologist's log. The sediments are composed of red, argillaceous siltstones in the upper part, and halite and anhydrite in the lower part of the sequence, which was not cored. The lowermost 10 ft. (3 m) of sediment penetrated by coring is composed of angular to sub-rounded fragments of laminated red clay, in a matrix of sub-angular to sub-rounded silt grains. The clay fragments do not show a preferred orientation, and are not curled upward at the outer edges (Fig. 6), as in the case of desiccation features. The chaotic, angular texture of the rock suggests that the clay fragments were not produced by bioturbation. It is possible that these sediments may represent a soil horizon (E. F. McBride, oral comm., 1975). The occurrence of small anhydrite nodules (Fig. 7) indicates either that these sediments were deposited in an evaporitic environment, or that the environment became evaporitic shortly after deposition. The uppermost two to three feet (0.6-0.9 m) of the Harper and Salt Plain formations are composed of red, sandy siltstone, with small anhydrite nodules, coarsening upwards into the overlying Cedar Hills Sandstone.
Figure 6--Fragments of red shale in silt matrix. Harper and Salt Plain formations, 2054.7 ft. (x 1.8).
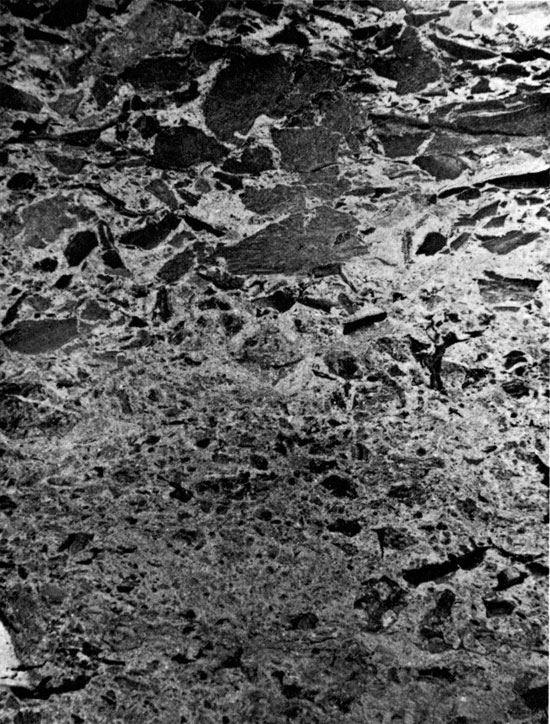
Figure 7--Angular anhydrite nodules in argillaceous siltstone. Cedar Hill Sandstone, 2032 ft. (x 0.9).
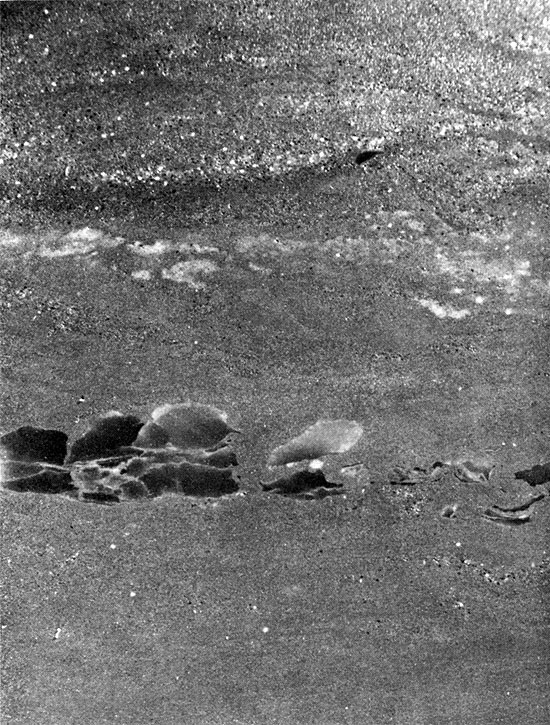
From where it crops out in south-central Kansas, the Cedar Hills Sandstone can be traced in the subsurface throughout western Kansas. The Cedar Hills was named by Cragin (1896, p. 24) from the Cedar Hills in Barber County, Kansas. In outcrop it consists of brownish-red feldspathic sandstone, siltstone, and silty shale. Shaly siltstones are interbedded with the more resistant and more massive coarse siltstones and very fine sandstones. Beds of white, fine-grained sandstone occur at the base and top of the unit, the top sandstone containing many white to pink "snowballs" of granular gypsum (O'Connor et al., 1968). The thickness is about 180 ft. (55 m).
The interval generally considered to be the Cedar Hills in the subsurface is distinctive on geophysical logs (Fig. 5). The upper boundary is marked by a thin shale horizon throughout much of the basin (Plate I), but in the northern part of the basin a thin anhydrite bed occurs at the top of the Cedar Hills section. The lower boundary of the Cedar Hills is picked where there is a slight increase and change in response on the resistivity log (Fig. 5). The boundaries of the Cedar Hills in the subsurface may not coincide exactly with those of the unit in outcrop. The occurrence of salt and anhydrite in the Cedar Hills and Flower-pot in the Syracuse Basin leads to some difficulties in correlating sediments of Cedar Hills age.
Two east-west cross sections, and one north-south cross section (Plate I) through the Syracuse basin show the correlations used in this report. The sections depict the general pattern of sedimentation in the Syracuse Basin. The Cedar Hills thins from east to west and south to north within the area mapped (Plate II). It is thinnest in the central part of the Syracuse Basin, where it is less than 100 ft. (30.5 m) thick. In this area, the overlying salt-bearing horizons reach a maximum thickness.
In the extreme southeastern corner of the area mapped, in Seward County, the Cedar Hills is thinner than it is immediately to the northwest, in Stevens and Haskell counties. A region of thicker Cedar Hills trends northeastward, within the area corresponding to the "Saltless Tract" of Malone (1962), the salt-free area in the subsurface between the two Flower-pot salt bodies (Fig. 1).
Facies of the Cedar Hills interval were interpreted through geophysical and sample logs. Around the southern margin of the basin, the Cedar Hills is generally described as a free-drilling, shaly sandstone, characterized by amber and pink, fine to coarse, polished, rounded grains. Similar sediments occur within the southern part of the basin.
The change of expression of this horizon on geophysical logs (Plate I, C-C') probably results from the halite or anhydrite cement of the sandstone within the basin. To the north and west, the unit becomes more shaly and anhydritic and, in the northwest part of the area mapped, is composed of red, shaly anhydrite, less than 50 ft. (15 m) thick. North of T.19 S., a thin white-gray anhydrite bed, referred to as the "Y anhydrite" (Schumaker, 1966), occurs at the top of the Cedar Hills interval (Plate I, A-A').
Shale beds within the Cedar Hills in outcrop are fairly continuous (Swineford, 1955), and can also be traced in the subsurface. On the eastern edge of the basin, a prominent shale bed occurs approximately midway up the Cedar Hills section, and can be traced westward, part of the way into the basin (Plate I, C-C'). The Cedar Hills thins from east to west above and below the shale bed. Shale beds and salt overlie the Cedar Hills interval in sec. 34, T. 22 S., R. 35 W. (Plate I, B-B'), indicating that the main salt section farther west is correlative with the Flower-pot.
A thin anhydrite bed (Y on Plate I, A-A', B-B') occurs at the top of the Cedar Hills north of T. 19 S., in the area mapped. This anhydrite was mapped by Campbell (1963), and Schumaker (1966), and was noted by Rascoe (1968) to be a valuable marker bed in northwestern Kansas. The anhydrite is generally between 10 and 15 ft. (3-4.6 m) thick, but in a few areas it is more than 30 ft. (9 m) thick. In sample logs, the anhydrite is described as a white or gray, massive or shaly anhydrite. Separation of the anhydrite bed from the rest of the Cedar Hills becomes increasingly difficult to the north, because north of T. 3 S. the Cedar Hills is composed of shale and anhydrite interbedded with shaly anhydrite.
As Schumaker (1966) noted, this persistent anhydrite bed is not restricted to the Syracuse Basin, but is very extensive, blanketing most of northwestern Kansas. This anhydrite can be traced northward into Nebraska, where it corresponds to the Opeche Formation of western Nebraska and the Dakotas. Apparently, anhydrite with some shale was deposited in the northern part of the area during Cedar Hills time, while sandy facies were deposited to the south. Towards the end of Cedar Hills time, anhydrite deposition extended southward, overlapping onto the sandy facies as far as T. 19 S.
The entire Cedar Hills section was cored (2044-1967 ft. or 623-600 m). The red beds of the Cedar Hills are composed of fine to medium-grained sandstone, which is generally massive but occasionally exhibits horizontal stratification (Fig. 8) or high-angle cross-bedding. The sandstone has a pronounced bimodal grain-size distribution (Fig. 9). It is cemented by halite, and anhydrite nodules occur at several horizons.
Figure 8--Approximately horizontal laminae of fine- and medium-grained sand. Cedar Hills Sandstone, 2030.5 ft. (x 1.8).

Figure 9--Laminae of well-rounded, medium-sized quartz sand grains, inclined at approximately 30°. Interstices are partly filled with fine sand grains. Many grains are floating in halite cement. Cedar Hills Sandstone, 2016.6 ft. (Plane Polarized light, x 32).
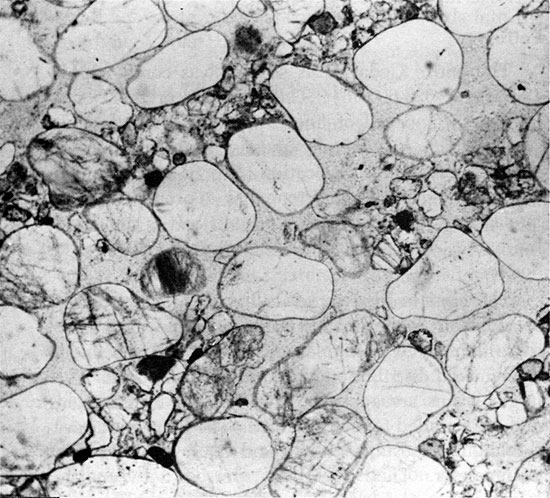
The cross-bedded intervals have laminae inclined as much as 34° from the horizontal, and are rarely more than a few feet thick. Even in hand specimen, the bimodal distribution in grain size is apparent. The larger grains are approximately 0.5 mm in diameter, and are rounded to well rounded. The finer grains are sub-angular to sub-rounded, and many are 0.15 mm in diameter. They occur interspersed between the larger grains, and in laminae alternating with laminae of coarse grains (Fig. 8). Very little clay is present. The larger grains are frequently orange colored, pitted, and are often regarded as a distinctive feature of the Cedar Hills in the subsurface of western Kansas. They should be used with caution for identification purposes, however, as similar sand grains have been reported from the Harper and Salt Plain formations (Swineford, 1955).
The Cedar Hills Sandstone is usually a quartz arenite, but ranges in composition to a lithic subarkose (McBride, 1963). The sandstone grains are predominantly quartz, mostly vein quartz with minor amounts of polycrystalline quartz. Approximately 5-10 percent of the clastic grains comprise feldspar. This occurs in the fine fraction, is usually fresh, and often exhibits microcline twinning. Also in the finer fraction are small amounts of rock fragments that were derived mainly from fine-grained felsic intrusives and opaque minerals. Hematite is common as a coating on most of the grains and as small patches in the matrix. Traces of authigenic clay are present. The sandstone is cemented by halite, and many grains appear to be floating in the cement, although some point contacts between grains can be observed. The shape of adjacent large grains suggests that they may originally have been in concavo-convex contact.
The Cedar Hills Sandstone is probably of eolian origin, having been deposited under desert conditions in an oxidizing, continental setting as a deflation-flat lag deposit. The recognition of eolian sediments is a difficult problem (Pettijohn et al., 1972; Glennie, 1970), particularly as eolian sediments may have been transported by water at some stage. Sandstones with a pronounced bimodal grain-size distribution are thought to represent deflation-lag deposits, in which larger grains are left behind as a lag during wind transport, and finer grains remain trapped between them (McBride, oral comm., 1975; Glennie, 1970). Both horizontal and cross stratification can result from deposition in this environment.
The well-rounded nature of the larger grains suggests that they may have been inherited from a previous cycle (Pettijohn et al., 1972), possibly from Cambro-Ordovician orthoquartzitic sediments underlying the Permian Basin area, which were exposed to erosion in isolated uplifted areas (Swineford, 1955). The more arkosic, sub-angular to sub-rounded silt and fine sand may have been derived from the Ancestral Rockies (Swineford, 1955). The time of reddening of the grains (Walker, 1967) cannot be established, because most of the grains are not in contact. Frosting and pitting of sand grains, while common in the Cedar Hills, is no longer regarded as a diagnostic feature of eolian deposition, as it can result from the replacement of quartz during cementation.
Eolian erosion of loose sand is limited to sediment above the level of the water table (Stokes, 1968; Kinsman, 1969; Glennie, 1970). The largest known depression thought to have been formed by deflation alone is the Qattara Depression in northwest Egypt, which covers an area of 6500 sq. mi. (18,000 sq. km.), and reaches a depth of 400 ft. (122 m) below sea level (Glennie, 1970). Sediment removed from the depression by wind accumulates in a dune field farther south. Evaporation at the surface of the deflation flat frequently leads to the precipitation of gypsum and halite, and salt marshes occur at several levels near the bottom of the Qattara Depression. This environment seems analogous to that of the Syracuse Basin in Cedar Hills time. The basin may initially have been a deflation depression in which deflation-lag deposits collected and were later cemented by halite prior to or at the onset of halite deposition in the Flower-pot. The thicker Cedar Hills section to the southeast of the basin may represent dune sands transported downwind from the depression, analogous to those at Qattara.
Few other reports of halite-cemented sandstones have appeared in the literature. Jacka and Franco (1974) described a deflation-flat halite-cemented red sandstone from Guadalupian sediments around the margin of the Permian Basin in New Mexico. This deposit is a marginal facies, however, while the Cedar Hills apparently occurs throughout much of the Syracuse Basin.
Several important different interpretations of the Cedar Hills have been given in the past. Rascoe (1968) interpreted the Cedar Hills unit in the basin to be a salt lens as, from geophysical logs alone, there is little difference in appearance between the Flower-pot salt and the Cedar Hills. He proposed that an unconformable relationship exists between this lens and the Cedar Hills Sandstone surrounding the basin. The cored section from A.E.C. Test Hole 5 shows that this is not the case, and that the Cedar Hills Sandstone was deposited within the basin, the only difference being that within the basin it is anhydritic or halite-cemented, and outside the basin it is not presently cemented.
Malone (1962) interpreted the main body of salt underlying the Blaine Formation to be of Cedar Hills age, with the Flower-pot Shale occurring as a very thin deposit between the top of the salt section and the overlying Blaine Formation. The position of the anhydrite marker bed at the top of the Cedar Hills in the northern part of the basin (riot studied by Malone), and the occurrence of an almost complete Cedar Hills section, overlain by shale and salt in sec. 26, T. 19 S., R. 33 W. (Pl. I, C-C') indicates that the salt is of Flower-pot age. This interpretation is supported by the occurrence of a persistent thin shale bed between the Cedar Hills and the overlying salt section, which, when traced for some distance, suggests that the Cedar Hills is thinning into the salt basin, rather than thickening and changing into a salt section.
The Flower-pot Shale was named by Cragin (1896, p. 24) from Flower-pot Mound, a butte in Barber County, Kansas. It is composed of about 180 ft. (55 m) of gypsiferous shale and silty shale, with a few thin beds of sandstone and siltstone (Swineford, 1955). Outcrops are generally littered with white, pink, and red satin spar, and clear selenite (O'Connor et al., 1968).
The Flower-pot Shale occurs in the subsurface west and northwest of the area where it crops out in south-central Kansas. The top of the formation has been eroded in the northeastern part of the area mapped. Overall, the Flower-pot thins to the west and north, except in the two areas where evaporites were deposited, one in extreme western Kansas and contiguous Colorado (Pl. III), and the other in Gray, Meade, and Clark counties in southwest Kansas, continuing to the south in Oklahoma (Jordan and Vosburg, 1963). Only the western salt body was considered in the present study.
The Flower-pot Shale is usually a distinctive unit on geophysical logs (Fig. 5). The occurrence of a thin shale bed at the top of the Flower-pot enables it to be distinguished from the Blaine. This shale, which is only a few feet thick above the salt-bearing section, was considered by Malone (1962) to represent the entire Flower-pot section. In this report, the salt section below the Blaine Formation is referred to as Flower-pot salt, following Schumaker (1966).
East-west cross sections through the basin (Pl. I, A-A', C-C') show the development of salt in the Flower-pot. Within the basin (Pl. III), two areas occur where the Flower-pot is thicker than 300 ft. (91 m): a narrow zone trending approximately southeast, just north of, and parallel to, the southern margin of the basin; and a small pocket near the southeastern margin of the basin which includes the area of greatest thickness, 343 ft. (105 m), in T. 21 S., R. 35 W. Elsewhere within the southern part of the basin, the Flower-pot is remarkably uniform in thickness (270-280 ft.) (82-85 m). Outside the basin, the Flower-pot thins slightly from about 60 ft. (18 m) in the south, to about 40 ft. (12 m) to the north of the basin.
Lithologies of the Flower-pot were interpreted from sample logs and geophysical logs. Outside the basin, the Flower-pot consists of reddish-brown, silty shale. Approaching the margins of the basin, and around its edges, the Flower-pot Shale is anhydritic, and there may be thin beds of anhydrite (Fig. 5). Within the northern part of the basin, the Flower-pot consists of reddish-brown anhydritic and gypsiferous shale, with small amounts of halite. The occurrence of halite in the southern part of the basin is noted with much more confidence on sample logs, and this information is supported by the response on geophysical logs (Pl. I, C-C'). Where the Flower-pot is thicker than 200 ft. (61 m), the presence of halite is almost certain. In the present study, the thickness of the complete Flower-pot unit was mapped, regardless of lithology (Pl. III). Since anhydritic and halitic shales give a similar response on gamma ray and resistivity logs, it is difficult to assess the halite content of the section. In the southern part of the basin, the cored section in Wichita County consists of 276 ft. (84 m) of salt with intercrystalline red clay matrix and thin beds of red clay. Several of these clay beds can be traced throughout much of the southern part of the basin (Pl. 1, C-C'). The beds of clay are marked by deflections on the geophysical logs (Fig. 4), but the clay intermixed with the halite (5-15 percent), is not detectable from the geophysical logs alone.
Halite--The Flower-pot is composed of fine to coarse crystals of halite, intimately associated with varying amounts of red, anhydritic, silty mudstone. The halite is not bedded, but several thin beds of mudstone and anhydrite occur within the sequence (Fig. 4 and Pl. I C-C').
Halite crystals commonly range in size from about 0.5 cm to about 4 cm across, but can be as large as 15 cm in maximum dimension. Throughout most of the sequence, the halite is composed predominantly of coarse crystals containing a few large fluid inclusions. Less commonly, abundant, small fluid inclusions are observed, but these are restricted to the fine-grained halite in the upper part of the Flower-pot. The differences in grain size and texture indicate differences in the mode of deposition and diagenesis of the sediments.
Halite in some parts of the Flower-pot, especially lower in the section, appears to have grown within the sediments, displacing soft, silty mud as it grew (Fig. 10). This interpretation is supported by the disruption of anhydrite and mudstone beds by salt growth (Figs. 11 and 12) and by the development of euhedral faces on large halite crystals in contact with patches of mudstone (Figs. 13 and 14). During halite growth, clay-sized impurities were displaced and became concentrated around grain margins. The salt was unable to displace coarser silt grains, however, and these were enclosed within the halite crystals (Fig. 15). This relationship was also observed by Haude (1970), in a study of the development of salt pseudomorphs. The crystallographic orientation of the halite crystals is generally random (Fig. 16) but, in some cases, halite grains oriented with coigns pointing upward are more common (Fig. 13). This may reflect the more favorable orientation of these crystals during competitive growth (Wardlaw and Schwerdtner, 1966). Some crystals, which grew until coalescing with each other in the horizontal direction, continued to grow vertically, forming large, elongate crystals (Fig. 17).
Figure 10--Fine- to coarse-grained halite in silty clay. Flower-pot Formation, 1967.5 ft. (x 0.7).

Figure 11--Fine- to coarse-grained halite in silty clay. Halite growth has caused displacement of thin anhydrite bed (center). Flower-pot Formation, 1961 ft. (x 0.7).

Figure 12--Fine- to coarse-grained anhedral halite crystals with white anhydrite stringers deformed by halite growth. Flower-pot Formation, 1813 ft. (x 0.4).

Figure 13--Fine- to coarse-grained halite displaying cubic crystal faces where it is in contact with patches of mottled, red mudstone. Flower-pot Formation, 1886 ft. (x 1.6).

Figure 14--Fine- to coarse-grained halite, with well-developed cubic crystal faces where in contact with red mudstone. Flower-pot Formation, 1788 ft. (x 0.8).

Figure 15--Halite (gray) containing laminae of silt oriented parallel to halite crystal faces. Flower-pot Formation, 1951.25 ft. (Partly crossed nicols, x 1.5).
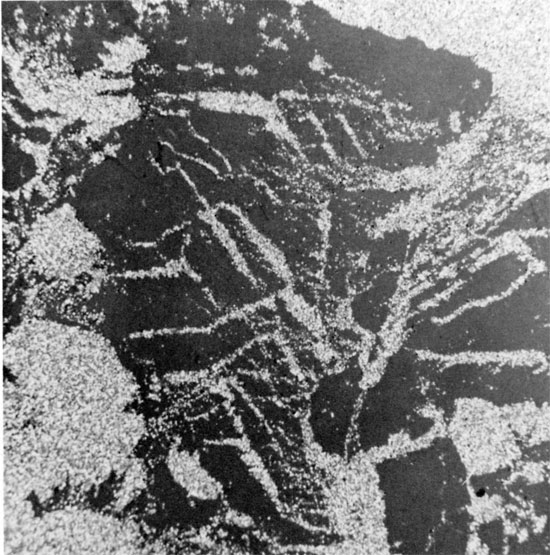
Figure 16--Anhedral, coarse-grained halite, with thin, discontinuous laminae of red mudstone within grains and along grain boundaries. Sub-horizontal clay parting occurs in lower third of specimen. Flower-pot Formation, 1835 ft. (x 0.9).

Figure 17--Fine- to coarse-grained halite with interstitial mudstone. Large, vertically elongated crystals at top of specimen. Halite crystals anhedral, except where in contact with patches of mudstone. Flower-pot Formation, 1830 ft. (x 0.8).

The occurrence of individual anhydrite nodules in silt beds of the lower Flower-pot, and the displacive textures of the halite suggest that the evaporites of this interval grew within the host sediments either under a shallow brine pool or during subaerial exposure, and were not deposited in an extensive evaporite basin.
Halite in the upper part of the Flower-pot usually contains fewer impurities than halite lower in the Flower-pot. Mudstone occurring interstitially with this cleaner salt often exhibits a chaotic mottled texture, and any trace of primary sedimentary features is usually absent. These textures suggest that the clays have been reworked by repeated solution and reprecipitation of the halite. Smith (1971) suggested the term "haloturbation" for this process. Large, anhedral halite grains occurring in some parts of the Flower-pot include some thin, arcuate clay inclusions, which may trace former grain boundaries (Fig. 16). These clay inclusions, as well as the large size and lack of fluid inclusions in the halite grains, suggest that the halite is of secondary, or diagenetic, origin.
The finely crystalline halite with abundant fluid inclusions in the upper part of the Flower-pot indicates that not all of the halite grew displacively; some was precipitated from an open brine body, either at the surface of the brine, or at the sediment-brine interface (Figs. 18 and 19). The finely dispersed clay associated with this halite was probably included in the crystallizing salt. Some chevron structures can be distinguished in the fine-grained halite, but in many cases only rows or clusters of fluid inclusions can be seen (Fig. 20). These may represent the remains of chevron structures, the rest having been removed by recrystallization. The coarser halite crystals appear to have formed by recrystallization of the fine crystals of halite, with which they are associated, with the expulsion of clay and other impurities from the crystals (Fig. 21). The coarser crystals could have formed by bottom growth in a brine-filled basin, but the general lack of primary lamination in the salt suggests that this was not the case. The presence of occasional horizontal mudstone beds and anhydrite and mudstone laminae which occur within the salt sequence (Figs. 18 and 21) suggest that the large halite crystals grew during deposition or early in diagenesis, rather than at a later stage.
Figure 18--Patches of fine-grained halite, associated with medium to coarse halite grains. Some grams display cubic faces where in contact with mudstone. Suggestion of thin clay laminae in lower part of specimen. Flower-pot Formation, 1730 ft. (x 1.1).
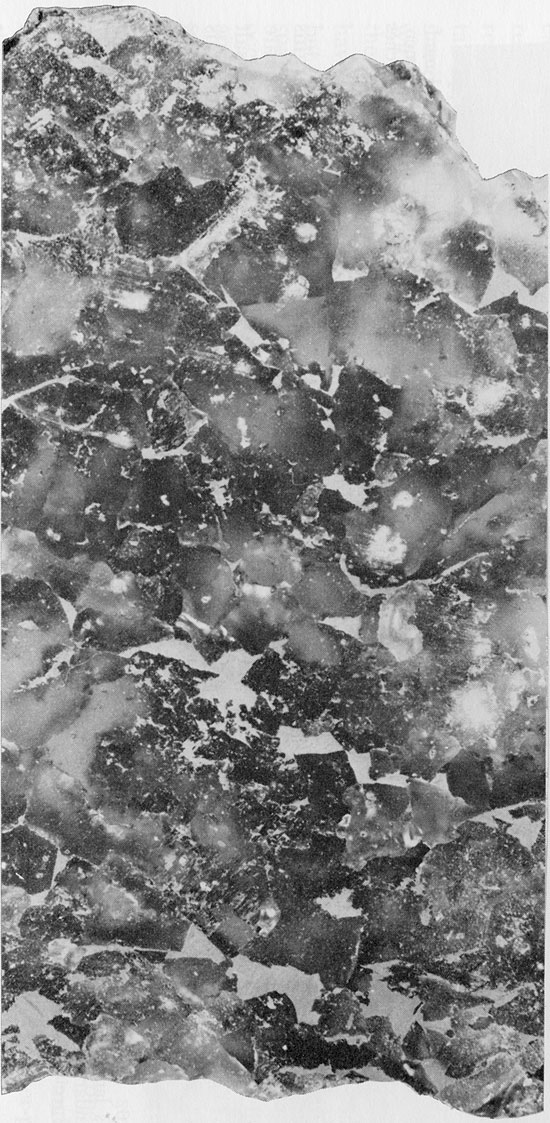
Figure 19--Fine-grained halite with finely disseminated clay and interstitial patches of red clay. Small patch of green clay (top center). Flowerpot Formation, 1745 ft. (x 1.2).
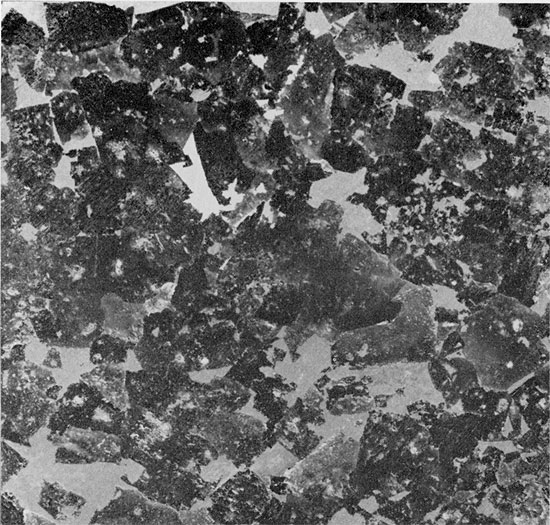
Figure 20--Fine-grained halite with clay occurring as clots and finely disseminated within grains and interstitially between grains. Chevron structures show some replacement by clear halite. Euhedral quartz crystals (white) project from clay patches into halite. Flower-pot Formation, 1745 ft. (Partly crossed nicols, x 32).

Figure 21--Patches of fine-grained halite with dispersed clay, in contact with coarse-grained halite associated with patches of interstitial clay. Horizontal clay lamina at base of specimen. Flower-pot Formation, 1770 ft. (x 1).
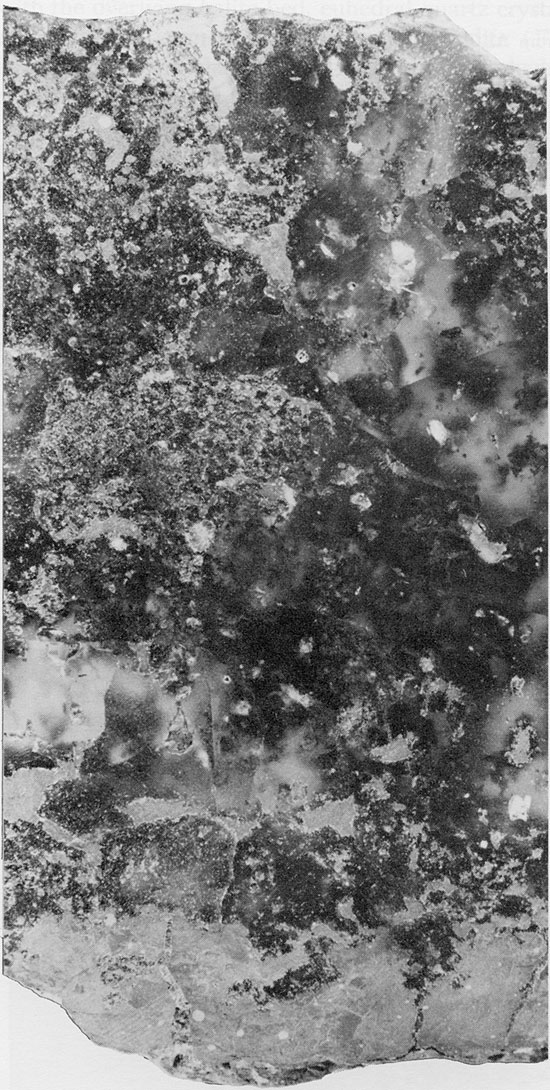
Red Mudstone--The red, anhydritic mudstone associated with the Blaine and Flower-pot salts is generally massive, very fine-grained to silty, and usually structureless, although it may exhibit a mottled texture and, more rarely, fine laminations. The mudstone beds reach a maximum thickness of about 3 ft. (1 m). Only quartz, anhydrite, and clay minerals can be identified in thin section, because of the fine grain size. Small, acicular anhydrite crystals, randomly oriented or with an aligned, felted texture (Maiklem et al., 1969), occur in the mudstone layers (Fig. 22). Larger, rectangular crystals of anhydrite sometimes occur along the margins of the mudstone lenses. Quartz occurs as angular, silt-sized grains, and is more common in the lower part of the Flower-pot salt. Throughout the salt sequence, it is common to find euhedral quartz crystals projecting from the margins of mudstone patches into the surrounding halite crystals (Fig. 20). Authigenic quartz crystals in salt deposits are relatively common, and euhedral crystals of similar occurrence were described from halite with mudstone by Stewart (1951a).
Figure 22--Anhydrite with aligned, felted texture, associated with red mudstone. Euhedral quartz and anhydrite crystals project from the anhydrite and mudstone into halite. Flower-pot Formation, 1925 ft. (Partly crossed nicols, x 32).

The composition of the non-halite fraction in the salt sections was determined by X-ray diffraction (Table 1). The major constituents of the insoluble fraction were found to be anhydrite, magnesite, dolomite, and the clay minerals chlorite-vermiculite and illite. Quartz is present in minor amounts, and the iron oxides hematite and/or goethite occur in trace amounts. Studies of other deposits have shown that mixed-layer clays, illite and chlorite, are the most common clays associated with halite sequences (Braitsch, 1960; Droste, 1963; Kühn, 1968; Adams, 1969).
Table 1--Mineralogy of water-insoluble residues from AEC Test Hole 5 (from James, 1972).
| M = magnesite MgCO3 | C-V = chlorite-vermiculite (?) |
| D = dolomite (Ca,Mg)CO3 | I = illite |
| A = anhydrite CaSO4 | F = hematite Fe2O, and/or goethite Fe2O3·H20 |
| Q = quartz SiO2 |
| Insol. wt. % |
Sample Depth |
Major (25-100%) |
Minor (5-25%) |
Trace (1-5%) |
|---|---|---|---|---|
| 50 | 1617.5 | M | C-V,I | F |
| 30 | 1618.8 | C-V,I | Q | F |
| 19 | 1620.8 | M | C-V,I | F |
| 6 | 1624.8 | A | ||
| 31 | 1626.7 | A | ||
| 10 | 1628.8 | M | C-V,I | F |
| 13 | 1632.8 | A,M | C-V,I | F |
| 8 | 1636.8 | D,A | C-V,I | F |
| 19 | 1640.8 | C-V,A | M | I,F |
| 4 | 1644.8 | C-V,I | F | |
| 1 | 1648.7 | M | C-V,I | F |
| 13 | 1650.8 | A | ||
| 23 | 1654.8 | M | C-V,I,A,F | |
| 10 | 1658.8 | C-V,I | Q | F |
| 13 | 1662.8 | Q,A,C-V,I | F | |
| 2 | 1666.8 | A | C-V,I,Q,F | |
| 50 | 1672.8 | A | C-V,I,Q | |
| 73 | 1676.8 | A | ||
| 88 | 1678.8 | A,M | C-V,I | F |
| 4 | 1680.8 | D,C-V,I,Q | F | |
| 75 | 1710.8 | D | C-V,I | F |
| 68 | 1788.8 | A,Q,C-V,I | F | |
| 81 | 1814.8 | A,Q,C-V,I | F | |
| 75 | 1850.6 | C-V,I,Q | F | |
| 61 | 1938.8 | D | C-V,I | Q,F |
Anhydrite--The near absence of anhydrite beds underlying or within the salt of the Flower-pot which was cored is unusual in comparison with sequences of evaporites thought to be of marine origin. From geophysical log correlations and sample studies, it appears that anhydritic sediments are more common near the margins of the basin and in northwestern Kansas than in the south-central part of the basin where the sequence was cored. Most of the anhydrite in the Flower-pot and Blaine salts occurs as fine-grained material, intimately associated with clay minerals and fine silts of detrital origin, and with lesser amounts of dolomite and magnesite. The anhydrite and dolomite may also have been transported into the basin as clastic sediments, or may have precipitated in situ. In either case, the present distribution of disseminated anhydrite and clays has been affected by the repeated early solution and recrystallization of the halite.
Anhydrite nodules occur at various horizons in the red, silty, and sandy sediments of the Harper and Salt Plain, Cedar Hills, and the lower part of the Flowerpot formations (Fig. 5). The nodules are usually discrete, and rarely coalesce (Figs. 23 and 24), but the anhydrite is never abundant enough to form the "chicken-wire" or mosaic textures which have been described from many other deposits. The nodular anhydrite in the Flower-pot is similar to the distorted nodules or distorted bedded nodules of Maiklem et al. (1969), and is composed of lath-shaped anhydrite crystals (Fig. 25).
Figure 23--Halite layer with sharp, inclined contact with overlying mottled, argillaceous siltstone containing coalesced anhydrite nodules (top). Flower-pot Formation, 1951 ft. (x 0.8).
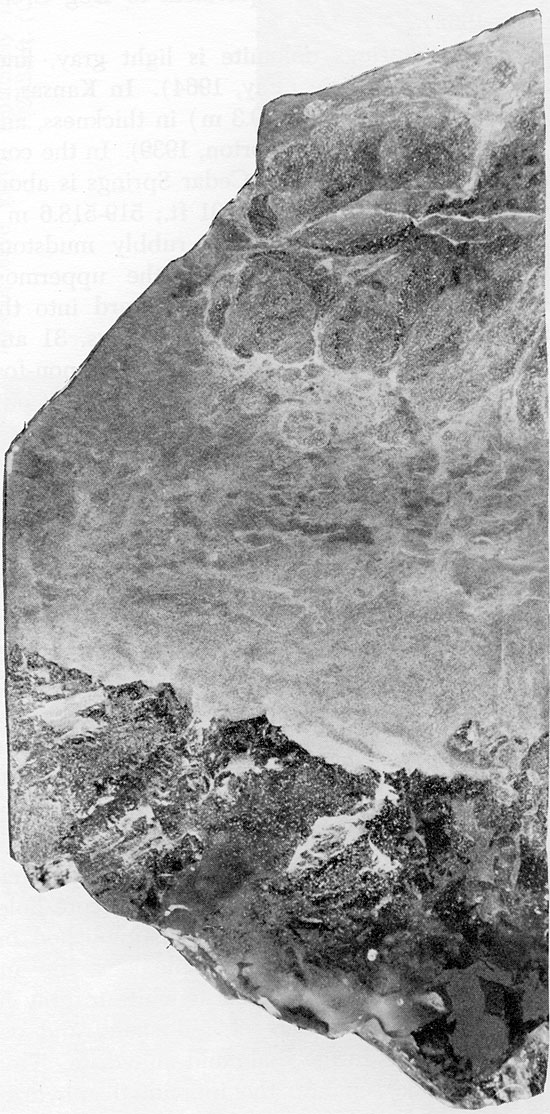
Figure 24--Small, rounded anhydrite nodules in mottled siltstone matrix. Cedar Hills Sandstone, 1952 ft. (x 0.8).
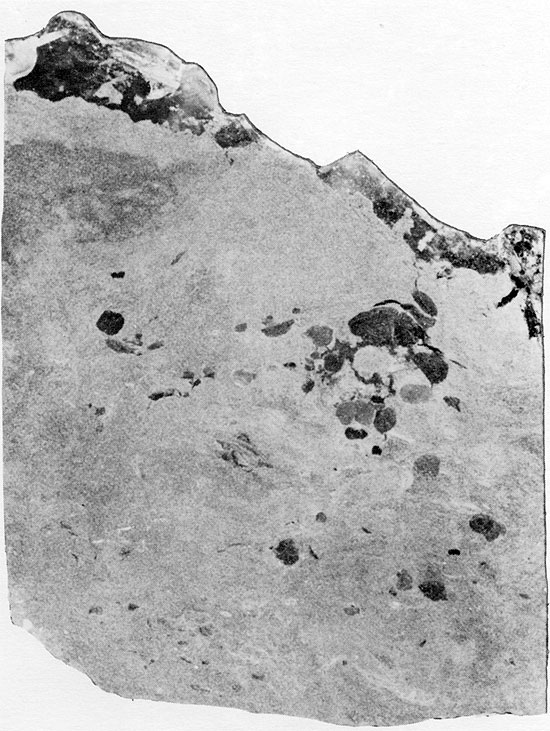
Figure 25--Anhydrite nodules displaying aligned, felted texture in matrix composed of angular silt-sized grains. Flower-pot Formation, 1951 ft. (Crossed nicols, x 32).

These individual anhydrite nodules probably originated by nucleation within the host sediment, perhaps originally as gypsum. Although the nodules are restricted in occurrence to certain layers, they do not form a continuous bed such as would have resulted if the anhydrite or gypsum had been deposited from an open brine. Early diagenetic nodular anhydrite, similar to that in the Flower-pot, has been described in a sabkha environment in the Persian Gulf (Curtis et al., 1963; Shearman, 1966; Kinsman, 1969; Butler, 1970). These modern nodules grow displacively in the host sediment, above the water table, in a subaerial environment. Although not all nodular anhydrite indicates displacive growth in a subaerial environment (Dean et al., 1975), the texture of the anhydrite nodules in the cored sediments, and their association with red bed clastics which are in part of eolian origin, suggests that these sediments were deposited in a continental sabkha environment.
A thin, horizontal bed of anhydrite approximately 5 cm thick occurs at a depth of 1925 ft. (589 m) within the Flower-pot salt. The anhydrite is in contact with red mudstone underneath, and is overlain by halite associated with red mudstone (Fig. 26). The anhydrite is white to gray in color, with a few discontinuous laminae of red clay, and an overall streaky appearance. In thin section, the anhydrite has an aligned-felted texture (Fig. 27), and the streaky appearance of the anhydrite is due to the pervasive replacement of anhydrite by euhedral crystals of quartz (Fig. 28). Immediately above the contact of the anhydrite bed with the overlying halite bed, euhedral quartz crystals are embayed because of replacement by halite (Fig. 29). Within the halite bed itself, large euhedral quartz crystals occur around the margins of patches of silt (Fig. 22). There is also recrystallization of fine-grained anhydrite to large, rectangular crystals, projecting into the halite (Fig. 22).
Figure 26--Fine-grained anhydrite with streaky appearance due to quartz replacement, overlain by red mudstone and medium-grained halite. Flower-pot Formation, 1925 ft. (x 1.6).
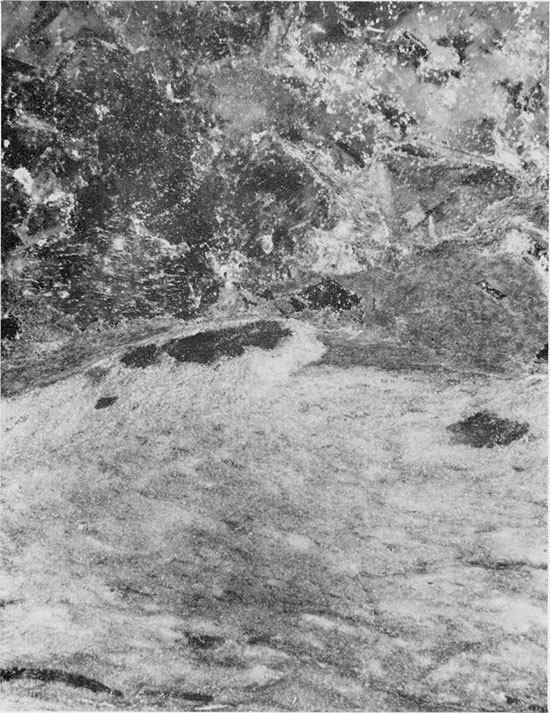
Figure 27--Anhydrite with aligned, felted texture. Small euhedral quartz crystals have grown replacively in the anhydrite. Flower-pot Formation, 1925.25 ft. (Crossed nicols, x 32).

Figure 28--Replacement of aligned, felted anhydrite by quartz with relicts of anhydrite showing preservation of the anhydrite fabric. Flower-pot Formation, 1925.25 ft. (Crossed nicols, x 32).
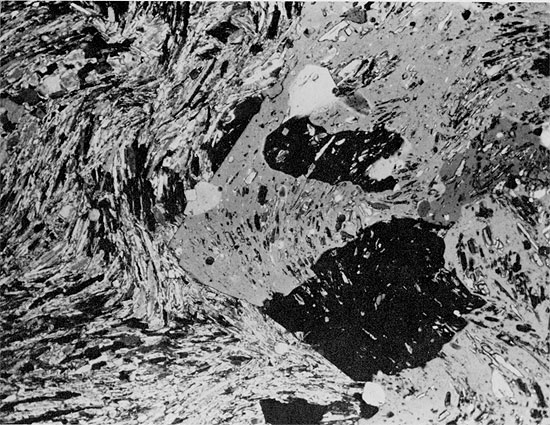
Figure 29--Euhedral quartz crystals embayed and apparently being replaced by halite (small black patches in gray and white grains) in siltstone matrix. Flower-pot Formation, 1925.25 ft. (Partly crossed nicols, x 32).
The mineralogic textures and relationships which occur within this short stratigraphic section indicate a complex diagenetic history. Quartz is relatively common in evaporite deposits, as clastic grains and euhedral authigenic crystals. The most likely source of the replacing silica is the clays in the overlying and underlying beds. Solution of amorphous silica is followed by the precipitation of less soluble crystalline silica (Krauskopf, 1956; Millott, 1970). Amorphous silica removed from clays during diagenesis (Siever, 1962) could have reprecipitated on small silt grains within the anhydrite, with simultaneous removal of the anhydrite in solution. The chemical conditions favoring the replacement of anhydrite by quartz are not known, but it is generally thought that the replacement occurs early in diagenesis (Grimm, 1962; Stoffers and Kühn, 1974).
The Blaine Formation was named by Gould (1902) from Blaine County, Oklahoma. In outcrop in south-central Kansas, the formation consists mainly of gypsum beds separated by dolomite and thin shale beds (O'Connor et al., 1968). A thin dolomite bed which is usually present at the base of the formation was named the Cedar Springs dolomite by Fay (1964) (Fig. 4). The remainder of the Blaine Formation in outcrop is divided into four members on the basis of the most prominent gypsum beds. In ascending order these are the Medicine Lodge Gypsum Member, Nescatunga Gypsum Member, Shinier Gypsum Member, and Haskew Gypsum Member (O'Connor et al., 1968).
The Blaine can be traced in the subsurface to the north and west of the outcrop area. North of T. 4 S., and east of R. 35 W., the Blaine does not occur in the subsurface due to removal by pre-Jurassic erosion (Rascoe and Baars, 1972). The Blaine is an important marker bed in the subsurface of western Kansas. It has a characteristic expression on geophysical logs (Fig. 5), and the white color of the cuttings contrasts with the red sediments above and below (Malone, 1962). The lower boundary is placed at the resistivity-log inflection above the Flower-pot, and the upper boundary at the top of the uppermost anhydrite bed, also indicated by a sharp inflection on the log. The Dog Creek Shale (Fig. 3) is also anhydritic in the subsurface and, in some cases, particularly in southwestern Kansas, the upper part of the section mapped as Blaine may be equivalent to Dog Creek (Malone, 1962).
The Cedar Springs dolomite is light gray, fine grained and often oolitic (Fay, 1964). In Kansas, it ranges from 0.5 to 1 ft. (0.15-0.3 m) in thickness, and is ripple marked in places (Norton, 1939). In the core from A.E.C. Test Hole 5, the Cedar Springs is about 0.5 ft. (0.15 m) thick (1701.5-1701 ft.; 519-518.6 m). It overlies several feet of red, rubbly mudstone probably a solution breccia) of the uppermost Flower-pot (Fig. 30), and grades upward into the herring-bone anhydrite of the Blaine (Figs. 31 and 32). The Cedar Springs is a fine-grained, non-fossiliferous, dolomitized mudstone often with faint outlines of pelletoid grains grading upward into coarse-grained dolomite rhombs. The dolomite becomes progressively more anhydritic towards the top of the bed; and dolomite grains in the overlying anhydrite are pellet-shaped, often containing inclusions in the centers of the grains (Figs. 33 and 34).
Figure 30--Cedar Springs cross-laminated dolomite, with cross-cutting halite vein, grading upward into horizontally bedded anhydrite. Anhydrite and mudstone breccia at base marks contact of Blaine Formation with underlying Flower-pot Formation, 1701.75 ft. (x 0.8).
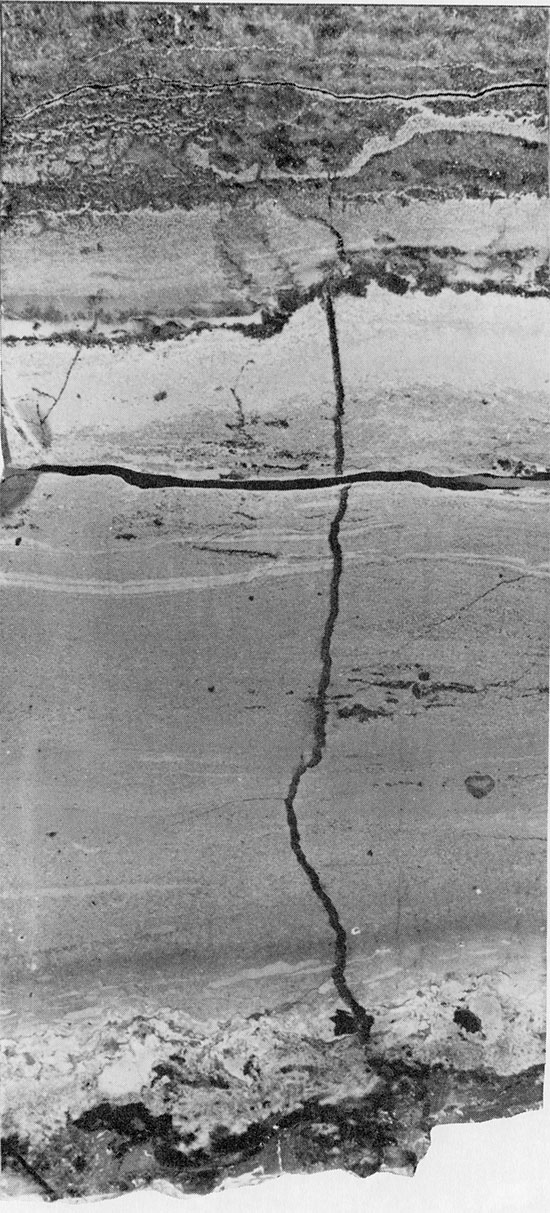
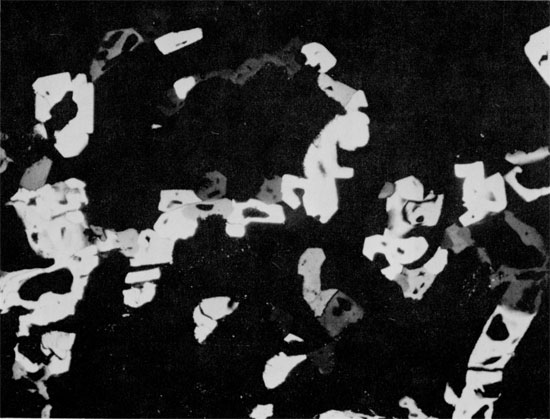
Figure 31--Herring-bone anhydrite with carbonate grains (light) along axes of "branches." Halite fills interstices. Horizontally laminated carbonate occurs in upper center of specimen. Blaine Formation, 1698 ft. (x 0.45).

Figure 32--Core showing vertically oriented herring-bone structures of anhydrite, with interstitial halite. Blaine Formation, 1697 ft. (x 0.9).
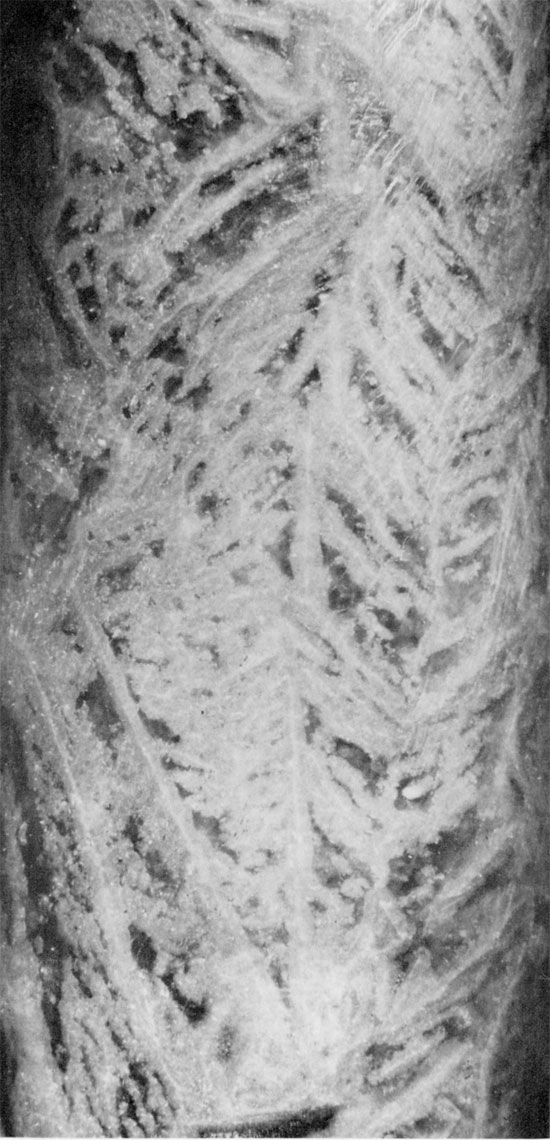
Figure 33--Anhydrite with wheat-sheaf fabric projecting into halite (black). Subbedral to euhedral carbonate grains often contain inclusions. Blaine Formation, 1700.5 ft. (Crossed nicols, x 32).
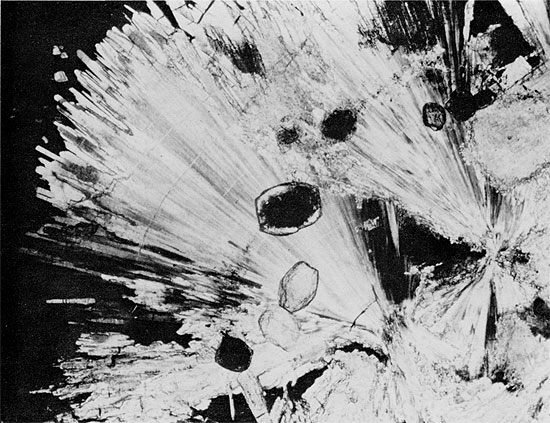
Figure 34--Anhydrite from herring-bone structure showing at least three generations of anhydrite: aphanitic, wheat-sheaf, and euhedral rectangular crystals. Large rectangular grain (center) has grown around carbonate grain. Blaine Formation, 1698.25 ft. (Crossed nicols, x 32).

The Cedar Springs was probably deposited in shallow, rather saline water. This extensive dolomite bed may mark the transgression of a shallow, epeiric sea into the area of present Kansas and Oklahoma at the beginning of Blaine time, following a brief period of mild erosion which brought deposition of the Flower-pot salt to an end.
The four gypsum beds which have been recognized in the Blaine in outcrop are represented by interbedded anhydrite and halite in the A.E.C. core hole. These resistive beds can often be distinguished on geophysical logs (Fig. 5). In sample logs, the anhydrite of the Blaine is described as white to gray in color, with varying amounts of red shale, and occasional halite casts in the shale and anhydrite. Thin beds of red shale separate the individual anhydrite beds, and a thicker shale sequence is developed between the two middle anhydrite beds in much of the area mapped. The Blaine is as thick as 125-140 ft. (38-43 m) within the Syracuse Basin (Pl. IV), where the red shale in the middle Blaine is salt-bearing (Pl. I). The total thickness of halite in the Blaine is about 65 ft. (19.8 m). Most of the halite is associated with varying amounts of red, anhydritic mudstone, and is similar in texture to salt in the upper part of the Flower-pot sequence (Fig. 35). The halite-mudstone sediments have only traces of primary bedding, as thin, sub-horizontal laminae of anhydrite or mudstone. This contrasts with the main anhydrite beds of the Blaine, which have conspicuous horizontal laminations of clay and anhydrite (Fig. 36). The anhydrite beds grade into overlying halite, but contacts of halite and overlying anhydrite beds are abrupt.
Figure 35--Fine- to coarse-grained halite with dispersed mudstone within grains and in patches between grains. Blaine Formation, 1680.75 ft. (x 0.7).
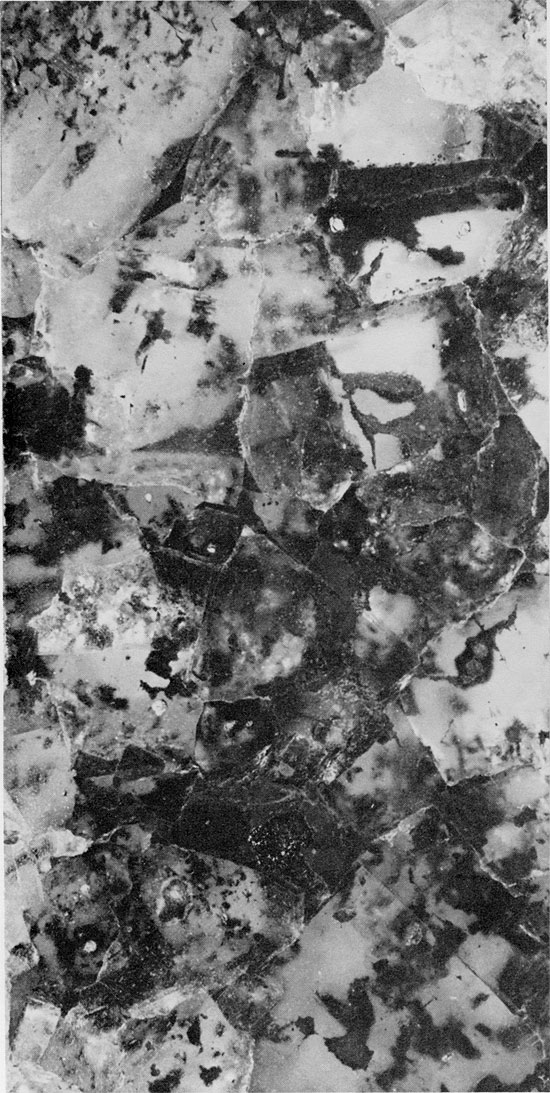
Figure 36--Cross-laminated shale with thin laminae of anhydrite (light). Blaine Formation, 1679 ft. (x 1.1).

Halite--Most of the halite in the Blaine Formation is similar to that in the Flower-pot and probably bad a similar origin. The only occurrence, in the core studied here, of halite free of red, anhydritic mudstone is found in the Blaine between 1627.5 and 1623 ft. (496-494.8 m) (Fig. 4). The halite in this section is very different in texture from the rest of the halite in the Blaine and Flower-pot. The halite is composed of fine, cloudy crystals, between 0.5 and 2 cm in diameter, and coarse, clear crystals, between 3 and 4 cm in diameter. The fine-grained salt contains numerous, small fluid inclusions, arranged in chevron structures (Fig. 37). Thin layers of anhydrite, composed of small acicular crystals, ranging in thickness from a film to 6 cm, occur at regular intervals within the sequence (Fig. 38). Laminations in the thicker anhydrite layers are pronounced (Fig. 39). Immediately above the anhydrite layers, the halite crystals are as large as 6 cm in diameter and contain a few large fluid inclusions. Within these halite-anhydrite cycles, the halite crystals decrease in size upward, and those underlying the next higher anhydrite bed contain chevron structures (Fig. 40). The structures in crystals at the contact with the overlying anhydrite are truncated. Anhydrite also occurs in variable amounts within the salt, often in irregular masses, giving the salt a milky appearance. In only one place within the clean salt sequence is there a small amount of red clay (1623.5 ft.; 495.2 m), occurring in discontinuous layers because of halite growth (Fig. 41). The clean salt section is overlain by irregular, small blocks of red clay and associated anhydrite and halite, interpreted as a solution residue (Fig. 42).
Figure 37--Fine-grained halite with chevron structure outlined by fluid inclusions. Fine-grained anhydrite occurs along grain boundaries. Blaine Formation, 1626.25 ft. (Partly crossed nicols, x 32).
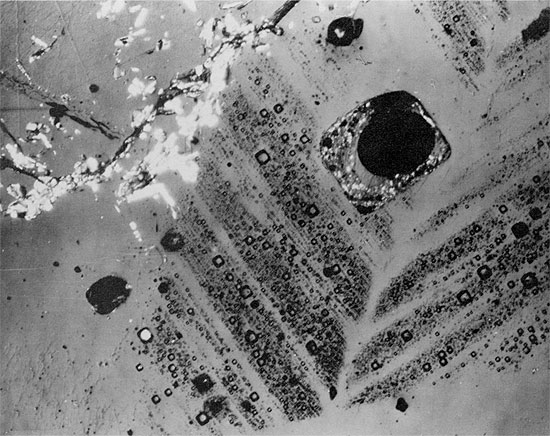
Figure 38--Aphanitic anhydrite grading upwards into large anhydrite crystals, pseudomorphous after gypsum, in halite (black) (from anhydrite layer in clean halite). Blaine Formation, 1627.5 ft. (Crossed nicols, x 32).
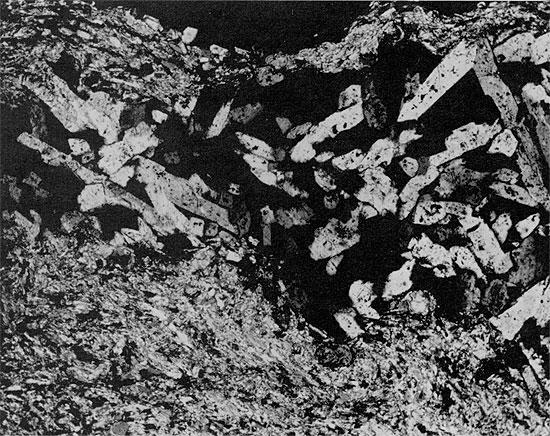
Figure 39--Clean halite with horizontally laminated anhydrite layer. Halite underlying the anhydrite is fine-grained with abundant fluid inclusions arranged in chevron structures. Halite overlying the anhydrite is mainly coarse-grained with a few large fluid inclusions. Blaine Formation, 1627 ft. (x 0.7).
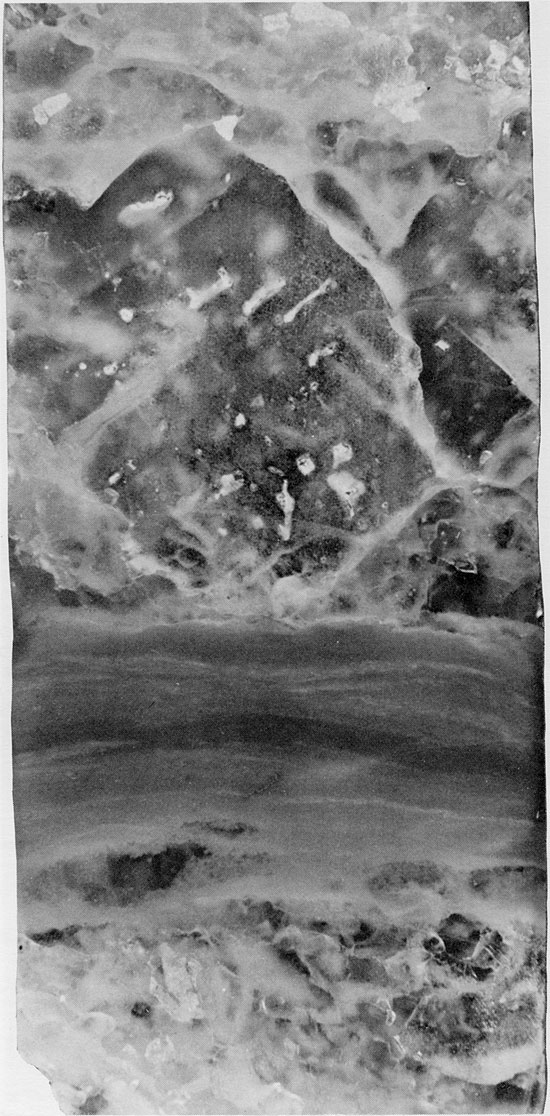
Figure 40--Fine-grained halite with randomly oriented chevron structures (white). Thin anhydrite lamina occurs near top of specimen. Halite grains underlying this display truncated chevron structures. Blaine Formation, 1627 ft. (Partly crossed nicols, x 2).
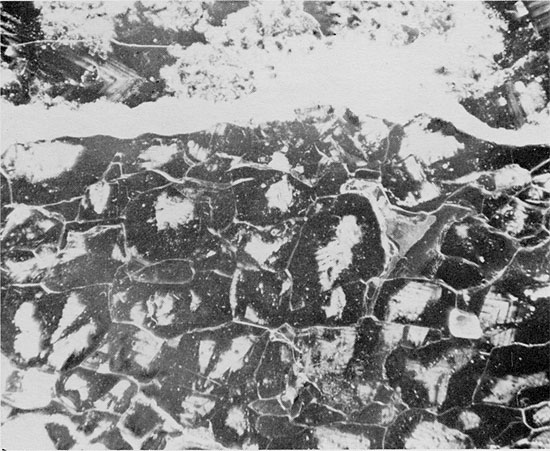
Figure 41--Thin anhydrite lamina underlain by fine-grained halite (gray) with abundant fluid inclusions and fine-grained anhydrite (white), and overlain by coarse-grained halite with discontinuous, sub-horizontal laminae of anhydritic mudstone. Blaine Formation, 1623.5 ft. (Partly crossed nicols, x 1).
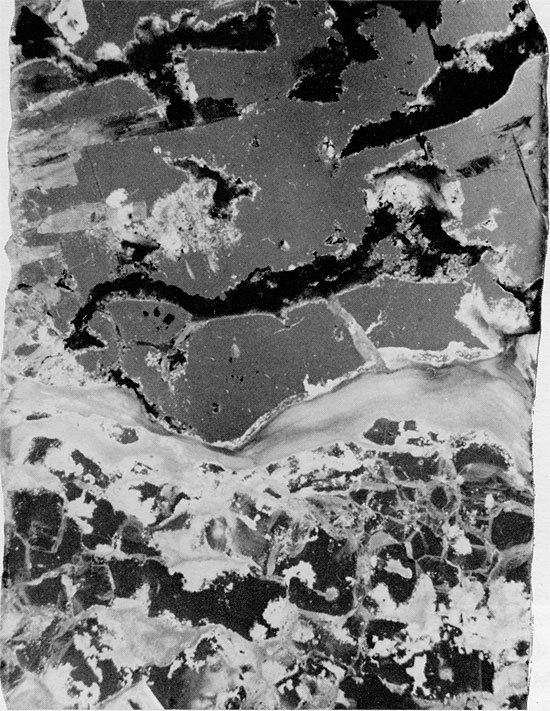
Figure 42--Halite, anhydrite, and mudstone immediately overlying clean halite section (appears to be a solution residue, in part). Blaine Formation, 1622.75 ft. (x 1).

The chevron structures in the more finely crystalline halite are randomly oriented in the lowest halite bed (Fig. 40), but in higher halite beds they are oriented preferentially, with the apex of the chevron pointing upward. Vertically oriented chevrons in salt of the Devonian Prairie Evaporite Formation have been interpreted to result from the growth of halite crystals upward from the bottom of a basin, with the (111) crystallographic direction parallel to bedding (Wardlaw and Schwerdtner, 1966). However, Shearman (1970) described chevrons with a preferred vertical orientation from Holocene halite rock in brine pans on the supratidal flats off the mouth of the Colorado River, in Baja California. Alternating layers of halite and anhydrite in these Holocene deposits in Mexico are the result of repeated reworking, as each flooding of the brine pans by seawater or freshwater causes dissolution of previously deposited halite and initiates a new cycle.
The clean salt section of the Blaine has a number of similarities to the seasonal layers of halite and anhydrite described by Wardlaw and Schwerdtner (1966), and the Holocene brine pan deposits described by Shearman (1970). It is now well documented that "seasonal layers" should not imply yearly, or even regular events. Monitoring of a number of marginal marine lagoons by satellite, and by more direct observation, has shown that flooding of the lagoons occurs irregularly, and depends upon tides, winds, and other factors which, although dependent to some extent on seasonal fluctuations, are not entirely predictable (Phleger, 1969). In the Blaine Formation, then, the alternation of halite and anhydrite may represent some kind of cyclic event, but this is not necessarily the result of annual or even seasonal flooding of the basin.
Wardlaw and Schwerdtner (1966) interpreted the deposition of the halite in the Prairie Evaporite Formation to have taken place on the bottom of a deep basin. If abundant fluid inclusions are the result of rapid crystal growth, Shearman's (1970) observation that these textures originate in shallow bodies of brine, which are subject to more rapid changes in concentration than are deeper bodies, seems more attractive. The clean halite with thin anhydrite laminae in the Blaine was probably deposited in a shallow brine pan.
Anhydrite--An unusual anhydrite-halite-dolomite bed, the subsurface equivalent of the Medicine Lodge Gypsum, overlies the thin dolomite bed at the base of the Blaine Formation (1701-1694 ft. in Fig. 4). Horizontal laminations of halite and anhydrite occur at the base of the bed, and these are overlain by anhydrite exhibiting a pronounced herring-bone texture, similar to that described by Stewart (1951b) from the Zechstein sequence in Britain. The white or blue-gray anhydrite displays a dendritic texture, with thin branches from central, almost vertical, "stems" (Fig. 31) outlined by dolomite grains along the axes of the branches. The interstices between the anhydrite branches are filled with coarse crystals of halite (Fig. 32). The size of the dendritic structures decreases upward within the bed, eventually grading into finely interlaminated anhydrite and shale. Small amounts of red clay occur throughout the bed. In some cases, the remains of original horizontal bedding are visible within the patches of dolomite. Horizontal cross sections through the core (Fig. 43), display a polygonal framework of anhydrite and carbonate filled with coarse crystals of halite. The outlines of the anhydrite framework suggest that it is pseudomorphous after euhedral gypsum crystals.
Figure 43--Horizontal section through core showing herring-bone structures in cross section. Anhydrite-carbonate framework appears to be pseudomorphous after gypsum. Halite fills interstices. Blaine Formation, 1696.5 ft. (x 0.7).
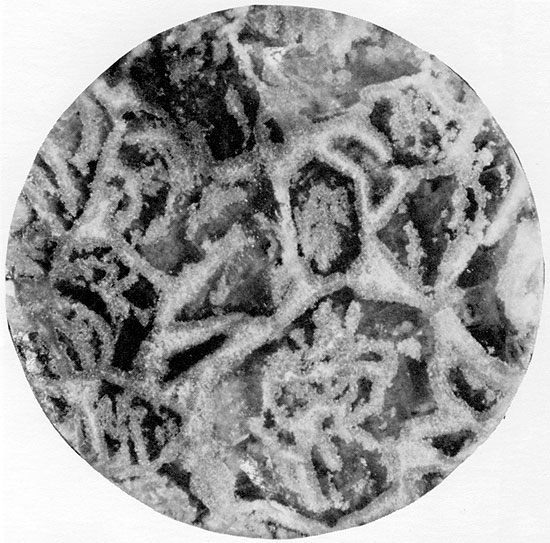
In vertically oriented thin sections, the anhydrite structures are complex, and a diagenetic sequence of at least three stages of anhydrite growth can commonly be recognized (Figs. 33, 34, and 44). Fine-grained anhydrite represents the earliest anhydrite observable, followed by fibro-radiate anhydrite which grew at the expense of the fine-grained anhydrite. Large, rectangular euhedral crystals, the last to form, cross-cut the other crystal forms (Fig. 34). This same sequence of anhydrite crystal forms was also recognized by Kosmahl (1969). The secondary replacement origin of the halite within the herring-bone anhydrite seems apparent from its coarsely crystalline, void-filling nature and from the fact that the anhydrite ribs are often discontinuous because of partial replacement by halite. The halite replacement must have occurred preferentially in certain zones, in order to have resulted in the regular herring-bone pattern now present. The carbonate laminae along the axes of the anhydrite framework may represent original growth planes within the gypsum crystals which were more resistant to replacement, possibly because they were less soluble.
Figure 44--"Branch" of herring-bone anhydrite showing wheat-sheaf anhydrite projecting into halite (black). Aphantic and euhedral rectangular anhydrite crystals occur along axis of "branch." Blaine Formation, 1699 ft. (Crossed nicols, x 32).
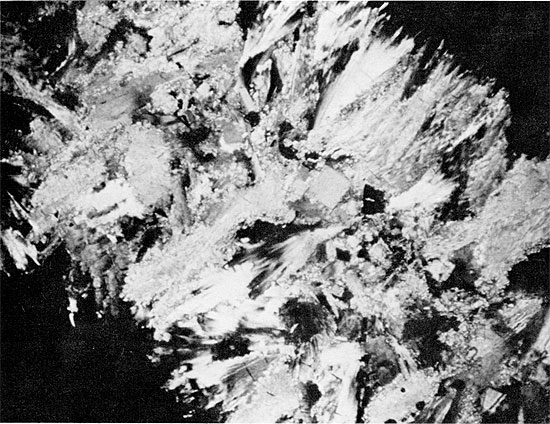
Replacement of gypsum by fine-grained anhydrite presumably occurred early in diagenesis. Subsequent development of fibro-radiate anhydrite may have been contemporaneous with the onset of halite replacement, as the fibro-radiate anhydrite rarely appears to be replaced by halite (Fig. 33). The growth of the large rectangular crystals of anhydrite marks the latest event in the diagenetic sequence.
Similar herring-bone textures have been described from Zechstein deposits in Germany and Britain. In Germany, they occur in the Pegmatite Anhydrite, which is the basal anhydrite in the fourth Zechstein cycle of Germany (Richter-Bernberg, 1955). In Britain, they occur in the Billingham Main Anhydrite, the basal anhydrite of the third English Zechstein cycle (Smith, 1971). The herring-bone textures in the Pegmatite Anhydrite are also associated with continental red bed evaporites. Richter-Bernberg (1955) considered that the unusual dendritic structures may be characteristic of shallow shelf deposition.
The similarity of the herring-bone anhydrite bed of the Blaine Formation to portions of these older deposits, and the diagenetic textures observed in the present study, support previous interpretations that such structures are pseudomorphous after gypsum (Zimmerman, 1909; Borchert and Baier, 1953). The original gypsum crystals may have looked very similar to the Upper Pleistocene and Holocene gypsum crystals from Marion Lake, Yorke Peninsula, Australia (Hardie and Eugster, 1971, Fig. 21B), and the Pleistocene gypsum crystals described from Sicily (Schreiber and Kinsman, 1975). These gypsum crystals exhibit upward-pointing apices, and they contain carbonate grains as fine laminae within the crystals. Carbonate grains also fill pockets between crystals. Large, vertical gypsum crystals were also described by Ogniben (1957), although be ascribed a secondary origin to these.
The herring-bone anhydrite of the Blaine overlies a thin, cross-bedded, pelleted carbonate mud layer, and it is overlain by fine-grained anhydrite and coarse-grained halite associated with red clay (Fig. 4). The small pockets of carbonate grains within the anhydrite bed which show horizontal laminations (Fig. 31), the laminae of carbonate grains along the axes of the anhydrite branches,, and the inclusion of small amounts of red shale suggest that the original gypsum crystals were primary and grew within, or at the surface of the sediment. The stable conditions necessary to produce a 7 ft.-thick (2.1 m) bed of large gypsum crystals suggests that the sediments were protected by a cover of brine during deposition, although this was probably shallow. Deposition probably occurred in an environment similar to that of the shallow lagoonal deposits in Australia (Hardie and Eugster, 1971). The small amount of red clay present was probably blown into the basin during deposition. Replacement of the gypsum by halite may have occurred as brines moved downward during later salt deposition or upward from underlying salts.
The next higher anhydrite bed in the Blaine, 1679.25-1672.5 ft. (512-509.9 m) in Fig. 5, consists of interbedded laminae of red mudstone and fine-grained anhydrite. This anhydrite bed is underlain and overlain by halite and red mudstone. The lower contact is abrupt and irregular, whereas the upper contact is gradational. The anhydrite laminae are much paler in color than the red mudstone laminae, and have irregular upper and lower surfaces (Fig. 36). Mudstone layers frequently are cross-laminated.
The anhydrite laminae are composed of fine lath-shaped crystals, up to 0.5 mm in length, with a felted or aligned-felted texture (Fig. 45) and intercrystalline red clay. Where the anhydrite crystals are aligned, they are parallel or subparallel to bedding. More commonly, however, the crystals are randomly arranged.
Figure 45--Fine-grained lath-shaped anhydrite crystals, generally with random orientation, in mudstone matrix (black). Equi-dimensional anhydrite grains (center) may have resulted from the breakdown of lath-shaped crystals. Blaine Formation, 1678.5 ft. (Crossed nicols, x 32).

Similar textures have been described from anhydrite-carbonate laminites in the Winnipegosis Formation in Saskatchewan (Shearman and Fuller, 1969), Miocene evaporites from the Mediterranean Sea (Friedman, 1972), and Purbeck evaporites in Sussex, U.K. (Holliday and Shephard-Thorn, 1974). In all of these deposits, the lath-shaped anhydrite has been interpreted to have formed displacively, early in diagenesis, in a sabkha environment.
The lath-shaped anhydrite of the Blaine Formation is very similar in texture to the anhydrite in these other deposits, but it is found in association with halite deposits and red beds, instead of supratidal carbonates. A displacive origin for the anhydrite is indicated by the random arrangement of the anhydrite crystals and the irregular, disruptive boundaries of the anhydrite layers. The nature of the anhydrite laminations contrasts with those in the Castile Formation of the Permian Basin area, which have much sharper boundaries, are more uniform in thickness, and form a repetitive, alternating sulfate-carbonate sequence (Anderson and Kirkland, 1966). The irregularity of evaporite laminations and the cross lamination of associated mudstone layers makes it unlikely that the Blaine anhydrite was deposited in deep water.
The anhydrite probably grew within the mudstone host sediment, early in diagenesis while the mud was still soft, with subsequent breakage of the lath-shaped crystals during compaction of the sediments. Deposition could have occurred in shallow water or in subaerial conditions, but the absence of desiccation features, the low angle cross-bedding of the mudstone, and the nature of the associated sediments supports the interpretation of a subaqueous origin.
The two uppermost anhydrite beds of the Blaine occur above the Blaine halite and grade upward into the thinly bedded, red, anhydritic shales of the Dog Creek Formation (Fig. 5). The anhydrite beds are interbedded with red shale, and have a variety of textures (Figs. 46-50). Deposition of these beds, probably as gypsum, most likely occurred in quiet, shallow water. The absence of halite indicates a decrease in salinity in late Blaine-early Dog Creek time.
Figure 46--Anhydrite with crinkly laminae of carbonate grains. Blaine Formation, 1612 ft. (x 1.1).

Figure 47--Fine-grained anhydrite with thin clay laminae displaying distorted massive texture. Horizontal clay parting occurs at top of specimen. Blaine Formation, 1589 ft. (x 0.7).
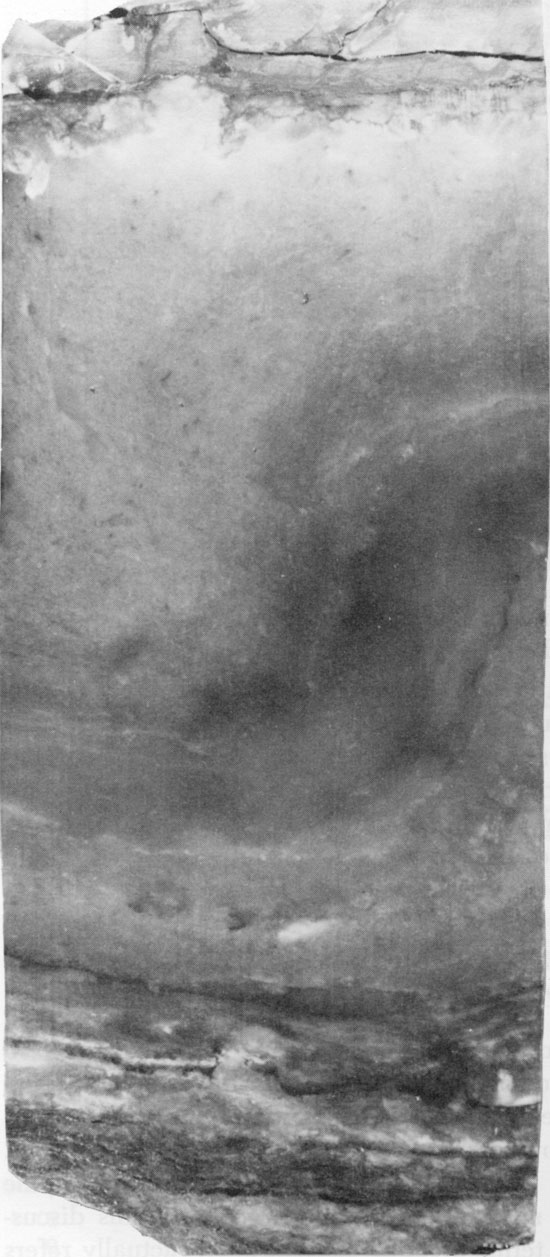
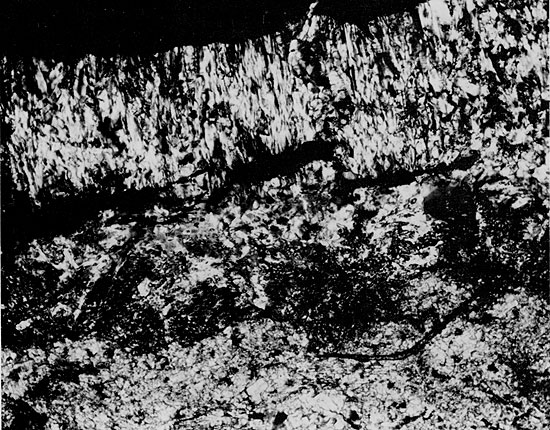
Figure 48--Coarse-grained anhydrite with wheat-sheaf texture to the right and later stage equidimensional crystals to the left. Small carbonate grains occur in the wheat-sheaf anhydrite. Blaine Formation, 1621.25 ft. (Crossed nicols, x 32).

Figure 49--Aphanitic anhydrite pseudomorphous after gypsum. Blaine Formation, 1598.5 ft. (Crossed nicols, x 32).

Figure 50--Fine-grained anhydrite, largely replaced by satin spar gypsum. Blaine Formation, 1589.5 ft. (x 32).
Comparison of Plates II, III, and IV shows that the Syracuse Basin reached maximum development during Flower-pot time. Cross section C-C' (Pl. 1) indicates that the basin expanded in late Flower-pot time, leading to salt deposition farther to the east than in early Flower-pot time. In T. 26 S., R. 19 W., non-evaporite facies of the Cedar Hills and the lower Flower-pot are overlain by evaporitic sediments of the upper Flower-pot. The consistency of separation of the clay beds in the southern part of the demonstrates the uniformity in thickness of units within the Flower-pot. The lower part of the Flowerpot thickens slightly in the central to western part of the area, suggesting that salt deposition began in this area. Comparison of Plates III, IV, and V shows that the basin shrank to the southwest before or during Blaine time. Campbell (1963) postulated that a northern and a southern salt body occur within the area of Flower-pot deposition in the Syracuse Basin, but the evidence from the present investigation supports the interpretation made by Schumaker (1966) that a single salt body exists.
As discussed in a previous section, the Cedar Hills thins where the Flower-pot thickens, and it has been suggested (Schumaker, 1966) that almost all thickening of the Flower-pot is at the expense of the Cedar Hills. If this idea were valid, the outlines of the basin should disappear in an isopach map of the combined thickness of the Cedar Hills and the Flower-pot (pl. V), and the contours should reflect only the overall thinning of the units from east to west and south to north. Plate V, however, clearly shows the presence of the Syracuse Basin, the morphology of the basin being similar to that present during time of deposition Of the Flower-pot (Pl. III), demonstrating that the thinning of the Cedar Hills is not enough to compensate for the thick section of salt above.
The nature of the margins of the basin in Kansas is of particular interest. To the north and northeast, the margins are irregular in plan view and presumably reflect the original morphology of the depositional basin. The southern margin of the basin, in contrast, is extremely abrupt (Pl. III and V), the Flower-pot thinning about 200 ft. (61 m) in less than one mile (1.7 km). The contours along the southern boundary of the basin are also much more linear than those in the northern part of the basin. The thinning of the Flower-pot is apparently not due to tectonic control during deposition, as no structures are reflected in the underlying sediments (Fig. 51). The thinning of the Flower-pot is thought to be the result of salt dissolution in groundwater, leading to compaction of the overlying anhydrite beds (Fig. 51). Evidence to support this hypothesis is found in the occurrence of sink holes along the present basin margin, which corresponds to the Bear Creek Fault (Gutentag et al. 1972; Hershey and Hampton, 1974; Lobmeyer and Sauer, 1974). Solution of salt along this linear zone may be occurring at the present time.
A north-south cross section through the Syracuse Basin (Fig. 51) shows the present-day attitude of the beds. The overall thickening of the beds of the Nippewalla Group southward, indicates that they originally were deposited in a basin which deepened slightly to the south; but the tabular nature of the deposits suggests that deposition occurred on an almost flat, gently dipping surface.
Figure 51--Present attitude and thickness of beds of the Nippewalla Group showing solution collapse structure between localities 20 and 22 (Geographic location of the localities is given in Table 2).
Table 2--Location of wells used to plot Figure 51.
| No. | Location | Company | Lease | ||
|---|---|---|---|---|---|
| Sec. | Twp. | Rge. | |||
| 1 | 13 | 4 | 38 | Safta, Brauer and McDevitt | Weaver, No. 1 |
| 2 | 29 | 5 | 39 | H. E. Bangert | Rudder, No. 1 |
| 3 | 35 | 6 | 38 | Schafer | Hicks, No. 1 |
| 4 | 18 | 7 | 37 | Miami | W. W. Bear 'C' |
| 5 | 28 | 8 | 39 | Musgrove | Trachel No. 1 |
| 6 | 31 | 9 | 38 | Musgrove | Van Donge, No. 1 |
| 7 | 24 | 11 | 37 | Texaco | Kleckner, No. I |
| 8 | 28 | 13 | 36 | Virginia | Hanson, No. 1 |
| 9 | 7 | 14 | 36 | Schafer | Fotopoulos, No. I |
| 10 | 21 | 17 | 38 | Toto Gas | Bauck, No. 1 |
| 11 | 6 | 18 | 39 | Caulkins | Brunswig, No. 1 |
| 12 | 17 | 19 | 39 | Hadson | Schmidt, No. 1 |
| 13 | 8 | 20 | 38 | Shell | Shumi, No. 1 |
| 14 | 4 | 21 | 38 | Pan American | G. B. Reniker, No. 1 |
| 15 | 4 | 22 | 38 | National Coop. and Durbin Bond | Burnett Estate, No. 1 |
| 16 | 14 | 23 | 38 | Kansas & Nebraska Nat. Gas | Miles, No. 3 |
| 17 | 28 | 24 | 38 | Kansas & Nebraska Nat. Gas | Turner, No. I'A' |
| 18 | 9 | 25 | 38 | Stanolind | Beaty Gas Unit, No. 1 |
| 19 | 16 | 26 | 38 | Stanolind | Warner Gas Unit, No. 1 |
| 20 | 16 | 27 | 38 | Atlantic Richfield | W. W. Ackerman, No. 2 |
| 21 | 21 | 27 | 38 | Amoco | Hohner Gas Unit 'C', No. 1 |
| 22 | 25 | 28 | 39 | Atlantic Richfield | E. M. Phillipi |
| 23 | 25 | 29 | 39 | Atlantic Richfield | Williams, No. 1 |
| 24 | 26 | 30 | 39 | Stanolind | Citizens State Bank |
The measurement of bromine concentration in chloride minerals is a valuable tool in the interpretation of the origin and diagenesis of halite and potash deposits. Boeke (1908) first investigated the behavior of bromine during the crystallization of salt solutions, and, since the work of D'Ans and Kühn (1940), the analysis of bromine in chloride salts has become a standard technique in the study of salt deposits. Bromine concentrations can be used for correlation of chlorides within a salt basin, calculation of depth of brine within a basin at the time of precipitation of the salts, and to help distinguish between primary marine deposits and recrystallized salts, as the latter generally have a much lower bromine concentration (Kühn, 1955; Valyashko, 1956; Baar, 1963, Adams, 1969; Kühn and Hsü, 1974; Stoffers and Kühn, 1974).
Most large salt deposits are attributed to the evaporation of a large volume of sea water. The concentrations of the major elements in sea water are well known, and the evidence suggests that these concentrations have remained essentially the same since the Paleozoic (Rubey, 1951; Holland, 1972). During the evaporation of sea water, minerals are deposited as their saturation point is reached. The ideal sequence, determined from the experimental evaporation of sea water, is precipitation of calcium carbonate, followed by calcium sulphate (as gypsum), halite and, finally, potassium and magnesium salts, the, so-called "bitterns."
In nature, the theoretical sequence rarely occurs, as it would require evaporation of a totally isolated body of sea water. Instead, due to reflux of concentrated brines out of the basin, and influx of sea water and of freshwater draining from the land, repetition of parts of the sequence is generally observed within an evaporite deposit.
The concentration of bromine in sea water is 65 ppm. Bromine minerals do not form from the evaporation of sea water, although minerals of elements present at lower concentrations than bromine do occur. Bromine occurs substituting for chlorine in the chloride minerals, halite and sylvite. In this discussion, the term "bromine concentration" actually refers to the concentration of bromide ions substituting for chloride ions in the chloride minerals such as halite and sylvite. Potassium chloride and potassium bromide form a solid solution series at the temperatures at which evaporite deposits form, but sodium chloride and sodium bromide do not. Most of the bromine, therefore, occurs in potassium chloride.
The behavior of bromine in the evaporation of brines under experimental conditions was discussed by Valyashko (1956), Braitsch (1962; 1971) and Holser (1966), and in the evaporation of sea water in salt pans by Herrmann et al. (1973). As halite crystallizes, a small amount of bromine is removed in the solid phase, but most of it becomes concentrated in the remaining brine. As the amount of bromine substituting for chlorine is determined by the concentration of bromine in the brine, the amount of bromine in the precipitating chloride increases with increasing concentration of brine. Thus, evaporation of an isolated body of sea water would result in the precipitation of halite with low bromine concentration initially, and successively precipitated halite would contain progressively more bromine. Theoretically, the first halite to crystallize through the evaporation of sea water should have a bromine content of 75 ppm (Holser, 1966).
Graphic plots of bromine concentration versus stratigraphic level in natural salt deposits, commonly referred to as bromine profiles, are more complex than theoretical profiles (Holser, 1966), showing many irregularities and fluctuations (Holser, 1966; Raup, 1966). These irregularities probably result from reflux during salt deposition by which concentrated brines are lost from the basin, and influxes of sea water or fresh water occur.
Analyses of bromine concentration have been reported for many of the world's major salt deposits. Typical concentrations of bromine in chlorides from various natural environments were given by Holser (1966), and for basal marine halites by Holser (1966), and Herrmann et al. (1973). The interpretation of the bromine profiles is not always straightforward, but when combined with the study of the mineralogy and stratigraphy of the sequence, these profiles give important information on the origin of the salt deposit or, as pointed out by Adams (1969): "create confusion where none existed before."
Most salt sequences have a range of bromine concentrations between 50-200 ppm, and are interpreted to be of marine origin. Only a few salt deposits with bromine concentrations lower than these have been reported. Holser (1966) and Holser et al. (1972) recognized an interval in the Lotsburg Formation, of the Lower Elk Point Subgroup in Canada, in which the bromine concentration of the halite is extremely low, ranging from 0 to 10 ppm. Holser (1970) discussed the low bromine concentration (6-10 ppm) of non-marine salt deposits from the Great Basin. Halite from the 3500 ft. (1067 m) thick Luke Salt Body, Arizona (Eaton et al., 1972) has bromine concentrations of 1-6 ppm.
Low concentration of bromine in salt, or a high ratio, Cl/Br, indicates solution and secondary deposition of the salt (Valyashko, 1972). The lowering of bromine content in halite either by recrystallization in the solid state or by leaching have not been demonstrated (Holser et al., 1972) and are generally not regarded as feasible mechanisms. It is possible to derive salts with low bromine concentrations from primary marine deposits by several different mechanisms, though all involve recrystallization. Conclusions concerning the origin of a low-bromine salt deposit depend upon examining other features of the deposit, such as the mineralogy, stratigraphy and paleogeography. Bromine analyses were used here to complement this information for halite from the Flower-pot and Blaine formations (Fig. 52). These salt deposits do not exhibit some of the features commonly seen in marine evaporites, and are associated with large amounts of red bed clastic sediments.
Bromine profiles typical of salt sequences which were deposited under restricted marine conditions (Holser, 1966; Raup, 1966) are different in two respects from those of the Flower-pot and Blaine salts. First, the shape of the profiles is different, the profiles of marine salts having more numerous inflection points and a much greater range in bromine concentration. The slope of a bromine profile is indicative of the rate of change in salinity of the water in the basin during salt deposition. Intervals whose bromine concentration increases upward are interpreted to indicate increasing salinity in the evaporite basin with time, due either to an increase in the rate of evaporation or to a reduction in the frequency of influx of fresher brines and reflux of more concentrated brines. A decrease in bromine concentration indicates dilution of brines by an influx of less concentrated brine or fresh water.
In contrast to salts of marine origin, the bromine concentration of the salts of the Blaine and particularly of the Flower-pot varies only slightly. Only in the upper part of the core of the Blaine Formation is much variability in bromine concentration found (Fig. 52). The concentration of bromine in the Flower-pot apparently ranges between 0 and only 5 ppm.
Figure 52--Bromine profile for halite in the Flower-pot and Blaine formations.
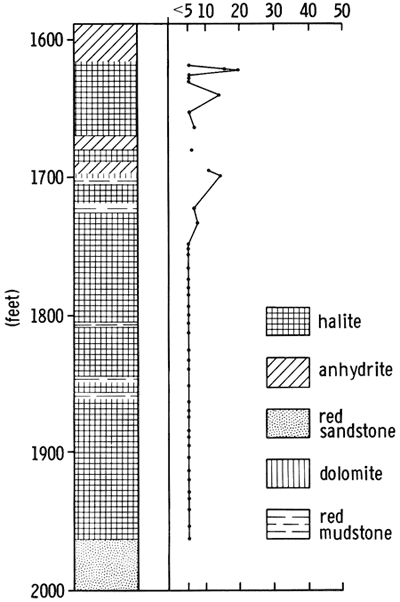
The other major difference between the profiles of the Flower-pot and the Blaine, and those of most other salt sequences is the actual bromine concentration. Values for the Hutchinson salt of Kansas range from 30 to 70 ppm, with the lower concentrations occurring only at the top of the sequence (Holser, 1966). Salt of the Hermosa Formation ranges from 100 to 300 ppm bromine (Raup et al., 1970). In the Flower-pot salt, the actual bromine concentration is below the limits of detection by X-ray fluorescence (5 ppm) for all of the section except the uppermost 40 ft. (12.2 m); but the data suggest that the concentration is somewhat below 5 ppm. In the Blaine, the bromine concentration is more variable, but the maximum concentration noted is 20 ppm (Fig. 52).
Bromine profiles have been used to calculate the depth of water in which salt deposition took place (Kühn, 1955). A sharp change in the bromine profile within a short stratigraphic interval indicates a shallow brine basin, in which evaporation would rapidly decrease the volume of water, with an accompanying rapid increase in bromine concentration, or in which an influx of more dilute brine would tend to lower the salinity and cause a decrease in the bromine content of the crystallizing salt. In contrast, more gradually changing bromine profiles are thought to be the result of salt deposited from deeper, stratified bodies of brine. In this situation, evaporation would cause a less rapid increase in the bromine concentration, and influxes of more dilute brine would tend not to mix with the more dense, stratified brine from which salts are crystallizing.
The bromine content of the Flower-pot salt remains essentially unchanged throughout the vertical sequence, although fluctuations in the range of 0 to 5 ppm may well be present. Very low bromine concentrations such as these indicate that the salts are recycled, and are not primary marine deposits (Holser, 1966; 1970; Valyashko, 1972). Holser et al. (1972), and Holser (1970) calculated that solution and recrystallization of a basal marine halite with a bromine concentration of 75 ppm in fresh water would yield a second-cycle salt with a bromine concentration of 3 ppm, and that a further cycle of solution and precipitation would yield a salt with a bromine concentration of 0.3 ppm. If recycling were to occur in sea water, which contains 65 ppm bromine, the bromine concentration of a second-cycle salt would be 10 ppm, and the third-cycle salt would contain 7.2 ppm. For any number of additional cycles, _however, the bromine concentration would fall no lower than 6.9 ppm. Recycling in sea water, therefore, cannot explain the low bromine concentration of the Flower-pot and Blaine salts. Fresh water must be involved.
Deposition of late Leonardian sediments in western Kansas occurred on a broad, flat, arid alluvial-eolian plain, bordering a shallow epeiric sea (Hills, 1942; Swineford, 1955). In situ recycling of the salt could have occurred in such an environment. In support of this theory, the bromine content of the small amount of halite occurring in the interstitial clays is much higher than that of the larger individual halite crystals occurring in contact with the clays. This suggests that the halite in the clays crystallized from residual brines. There is no evidence that the bromide ion is absorbed onto the lattice of clay minerals, as are such elements as potassium and rubidium; further, this explanation would be unlikely because of the large size of the bromide ion. Recycling of marine salts in situ by groundwater is also a possibility, and was cited by Smith (1971) as the most likely mechanism for the recycling of salts of the fourth English Zechstein cycle.
The low bromine concentration of the Flower-pot and Blaine salts could also result from the solution of an ancient salt body in freshwater, producing a brine with a high Cl/Br ratio, and subsequent precipitation of halite with a low bromine concentration. Salt of the Blaine and Flower-pot could have been derived from such solutions, but in order to produce the bromine profile observed, such brine could not have been evaporated in a deep, stratified body of brine, as this would have resulted in a bromine profile with greater variation in bromine concentration. It is more likely, then, that the low bromine content of the salt is the result of deposition in a shallow basin, which underwent periodic emergence, with influxes of fresh water causing reworking of the salts. This model fits in well with the petrographic observations and with subsurface data presented here. The slightly higher bromine concentrations near the top of the Flower-pot and in parts of the Blaine may indicate less dissolution and re crystallization of the salts in these intervals, possibly as a result of more rapid accumulation of the salt and less exposure to low salinity waters.
The origin of other low-bromine salt deposits poses similar problems in interpretation. The low bromine concentration in the Lotsburg and Cold Lake salts of the Lower Elk Point Subgroup was interpreted to have resulted from the solution and recrystallization of salt from a preexisting salt body (Holser et al., 1972). The origin of the Luke Salt Body in Arizona, in which more than 3000 ft. (915 m) of halite with a very low bromine content occurs, has not been resolved, but it has been suggested that it resulted from the evaporation of brines in a closed, continental lake, with salt possibly derived from salt deposits within the drainage basin. The low bromine concentration of halite in other continental basins may have been derived in a similar way (Holser, 1970). The brine of Great Salt Lake has a high Cl/Br ratio, although the salt here is thought to have been carried in the atmosphere from the Pacific Ocean (Eardley, 1970). The low bromine content of the Salado Formation was attributed to the recycling of salt from ancient deposits, combined with influx of sea water (Adams, 1969).
Few salt deposits with a very low bromine content have so far been described, and the interpretation of their origin is a complex problem. It is generally agreed that such salts were deposited and underwent diagenesis in non-marine conditions, although the original source of the salt was probably the sea.
Although the association of evaporites and red beds is not uncommon, few detailed petrographic descriptions of such deposits have been made. As would be expected, most reports of such sediments are from deposits of Permian and Triassic age, when the deposition of evaporites and red beds in the northern hemisphere was widespread.
Red beds are thought to form in an oxidative environment and to be mainly terrestrial in origin, having been deposited in a hot, arid desert environment. The red color is due to a thin coating of hematite on sand and silt-sized grains and to the incorporation of hematite in the lattices of clays. The total iron content of red beds is usually not high, and is frequently lower than that in many sedimentary rocks without red coloring.
The conditions under which the red pigment forms has been the subject of much discussion, and several theories have been advanced to account for the origin and preservation of the red coating (Krynine, 1949; Van Houten, 1964; Walker, 1967; Glennie, 1970; Chukhrov, 1973); however, the exact conditions leading to the formation of red beds are still unknown.
Halite and terrigenous sediments of Triassic age from Cheshire, United Kingdom, were described by Arthurton (1973). Within the sequence, primary halite crystals have mostly been replaced by secondary halite crystals. Laminations of silt and halite are still preserved, and elongation of the halite crystals is perpendicular to the bedding. Both of these features indicate primary deposition of the salt. These sediments probably were deposited in a shallow lake. The laminated sections are intercalated with and subordinate to "haselgebirge" sediments, in which the clay and halite are intermixed and primary sedimentary features cannot be distinguished. More delicate primary sedimentary features are preserved in the laminated sediments than in the halite with red mudstone of the Flower-pot and Blaine formations. This may reflect a lesser amount of reworking in the Cheshire deposits than of those in Kansas.
Salt deposits more similar to those of the Blaine and Flower-pot were described from the fourth cycle of the English Zechstein sequence (EZ 4--Smith, 1971). In these deposits, halite is intimately associated with between 5 and 15 percent red silty mudstone, the halite often exhibiting euhedral cubic crystal faces where it is in contact with patches of mudstone. The lack of bedding and other primary features in the deposit led Smith to conclude that the halite grew displacively within the host sediment during early diagenesis, possibly in a subaerial environment. He suggested that the texture of the sediment resulted from repeated solution and precipitation of the salt, for which be proposed the term "haloturbation."
The textures of the halite with red mudstone in the Blaine and Flower-pot are similar in many ways to those described by Smith (1971), but there is evidence that some of the Blaine and Flower-pot halite was deposited subaqueously. Determinations of bromine concentrations for the halite of the EZ 4 have not been carried out, but, from the mode of deposition proposed by Smith, it would be expected that the bromine profile would be similar to that for the Blaine and Flower-pot salts, and that the bromine content would also be very low.
Deposits of halite and red mudstone also occur in the Salado Formation in New Mexico, and have been interpreted to have formed in a shallow body of water (Adams, 1969). Beds of halite with red mudstone alternate with beds of clean halite, argillaceous material, and soluble potassium minerals. The close association of potash minerals with red mudstone led Adams (1969) to the conclusion that much of the clastic material was introduced into the basin as airborne dust.
The bromine concentration in the Salado Formation is abnormally low for a potash-bearing sequence, not exceeding 105 ppm, even in the potassium mineral horizons. Adams (1969) concluded that the evaporites of the Salado formed from the evaporation of brines which were derived from the solution of preexisting evaporite deposits, accompanied by periodic influxes of sea water.
Holser et al. (1972) discussed the origin of halite in the Lower Elk Point envaporites of Devonian age in Canada. The halite occurs as clear anhedral grains, 1 cm or larger in size. In the lower 30 ft. (9 m) of the sequence, the salt alternates with 1-4 cm thick beds of brown mudstone, composed mainly of dolomite and quartz. The absence of sulphate in the deposits, and the very low bromine concentration of the halite (0-10 ppm) led Holser et al. (1972) to conclude that these deposits may be non-marine in origin.
Sediments of Guadalupian age around the margins of the Permian Basin in New Mexico and western Texas were interpreted to have been deposited in a continental sabkha-deflation flat setting (Kendall, 1969; Jacka and Franco, 1974). Whereas the sequence in New Mexico and western Texas appears to have been deposited in progressively more arid conditions, the Nippewalla Group in Kansas appears to have been deposited in progressively wetter, though not necessarily less saline, conditions.
The reports of halite crystals growing subaqueously in the bottom muds of the Dead Sea (Neev and Emery, 1967) is of particular interest in this discussion. Large halite crystals are found in laminated, clayey subrecent muds dredged from a canal in the South Basin of the Dead Sea. The halite cubes have incorporated laminae of clayey or silty sediment within the crystals, which are thought to have grown beneath the sediment surface.
The association of halite with red clastic sediments is found at several horizons in Permo-Triassic sediments in Germany. The occurrence of "haselgebirge" sediments in the Upper Rotliegendes of the lower Elbe region near Hamburg was described by Richter-Bernberg (1955). These sediments consist of halite and red pelite, devoid of calcium sulphate, in which no primary stratification can be recognized. Richter-Bernberg thought that these sediments were deposited in a continental depression, the carbonate and sulphate salts having been removed by preconcentration of sea water. The salt may, however, have been derived from descendent solutions.
As much as 600 ft. (183 m) of halite with red mudstone is developed in the fourth Zechstein cycle (Z4), the cycle for which the Pegmatite Anhydrite is the basal sulphate. Similar halite deposits occur in the Z3 and Z4 cycles in Poland (Poborski, 1970). Richter-Bernberg (1955), Kühn (1968), and Poborski (1970) believed that the halite of the Z4 cycle was deposited under conditions transitional from lagoonal to continental, in contrast to the predominantly marine deposits of the earlier cycles. This interpretation is supported not only by the association of red clastics with the Z4 halite, but also by the low bromine concentration of the halite (Kühn and Schwerdtner, 1959; Kahn, 1968).
The association of evaporites and red beds usually indicates that the deposition of the sediments took place in a hot, arid to semi-arid, oxidizing, continental environment. This gives a general picture of the conditions of sedimentation, but several important questions remain unanswered. These include: (1) how deposition of the evaporites took place, (2) whether deposition occurred subaerially or subaqueously, (3) if subaqueously, the depth of brine in the basin, and (4) if deposition of the salts took place in a continental basin, what was the source of the salts.
For the Cedar Hills-Flower-pot-Blaine sequence, primary crystallization from normal marine sea water is clearly ruled out by: (1) the intimate association of the evaporite minerals with red beds, (2) the almost complete lack of carbonate beds, (3) the relatively small amount of sulphate, and (4) the very low bromine concentration in the halite. Although the sediments generally do not display bedding, the sequence appears to be primary, or early diagenetic, and not the result of much later post-depositional recrystallization.
The texture of the halite and anhydrite suggests that much of the evaporite deposition occurred by displacive crystallization within the host sediment. Remains of chevron structures in the fine-grained halite in the upper part of the Flower-pot, and the chevron structures in the clean halite of the Blaine Formation suggest that at least some crystals were precipitated from an open body of brine, but they may have been subject to replacement early in diagenesis. The larger halite crystals containing few fluid inclusions appear to have grown displacively. This growth may have occurred subaerially in soft sediments, or subaqueously under a shallow brine cover. In either case, the growth of large, inclusion-free crystals is favored by slow growth in a stable environment. A brine cover, even if very shallow, should reduce the rate of evaporation and the rate of growth of the interstitial halite. The almost complete absence of laminations and other primary sedimentary structures within the salt sequence indicates that deposition did not occur in a deep brine basin.
The herring-bone anhydrite of the Blaine is similar in texture to gypsum crystals growing in a modern lagoon in Australia (Hardie and Eugster, 1971), where the brine pool permits slow growth of large crystals. The anhydrite of the Blaine may have been deposited in a similar way, with conversion of gypsum to anhydrite, followed by partial replacement by halite during diagenesis.
The red silts, sands, and clays which occur in the lower part of the sequence were deposited predominantly in a subaerial environment, under strongly evaporitic conditions. Wind appears to have played an important role in the transport of the clastic sediments. The higher proportion of evaporites, compared to clastics, in the upper Flower-pot and Blaine suggests that a more abundant supply of brine became available during their deposition or that the rate of evaporation increased significantly; the former seems more likely. Sedimentation may have occurred subaqueously in very shallow water, possibly with periods of emergence.
The bromine profile of the Blaine and Flower-pot salts suggests that they were not deposited from a deep, stratified brine body, for the existence of a deep body of brine should result in fluctuations in the bromine concentration of the halite. The very low bromine concentration of the halite of the entire Flower-pot sequence suggests that it was deposited either subaerially or in a very shallow brine pool, which would be affected immediately by influxes of dilute brine. In either case, repeated dissolution and reprecipitation is required to produce a bromine profile in which the bromine concentration is consistently so low. Continuous recycling of the halite caused by exposure to fresh water, and occasionally to sea water, early in its depositional history could have produced the bromine profile observed. Such a mechanism accords well with the petrographic textures of the minerals and with the geometry bf the deposits, which suggests that the basin may not have been topographically well developed.
The source and mode of deposition of the components of the red mudstone (clay minerals, dolomite, and anhydrite) which occurs throughout the Flowerpot, and in much of the Blaine, is problematical. The dolomite and anhydrite could have been brought into the area as detrital particles from surrounding, marginal areas, or may have been precipitated in situ. The occurrence of magnesite in deposits of this type has not been satisfactorily explained to date, and is not discussed here. The fine-grained clastic sediment may have been brought into the area by streams flowing from the north (McKee et al., 1967a). Influx of slow-moving, low-salinity streams carrying fine-grained sediment could have led to the deposition of the clay with the halite; however, significant amounts of fine-grained material can be transported into an area by wind, and this method of transport may correlate better with an evaporitic setting.
Like the clastic components of the mudstone mentioned above, the origin of the laterally extensive clay beds within the halite sequence is also difficult to explain. These beds can be traced for some distance in the southern part of the basin. If the fine sediment were largely wind-blown, these beds could represent periods of exposure, with blowing dust, during which layers of silty clay were deposited over large parts of the basin; however, no desiccation features have been identified in the rocks of the A.E.C. core. Alternatively, the fine-grained material could have been blown into the basin and deposited subaqueously. A less likely mechanism involves having the sediments brought into the area in suspension in dilute brine. The clay would have settled out before halite saturation was again reached by evaporation. The absence of dissolution-erosion surfaces at the contact between the mudstone and underlying halite suggests that this was not the case; further, this mechanism would require the existence of a large body of water in the basin.
If the salts did not originate from an open body of marine water, the question of the source of the salts must be addressed. Possible sources are the sea, either directly as groundwater or as aerosols, or indirectly from ancient salt deposits. The lack of the minerals normally found in playa sediments suggests that this deposit did not result from deposition in a closed-basin playa, but the possibility of deposition of the salt having occurred in a setting similar to that of the Great Salt Lake of Utah, though more arid, cannot be excluded with present evidence.
The sea remains the most convenient source for the halite. An aim of an ephermeral, inland, epeiric sea probably extended into western Kansas from Oklahoma during late Leonardian time. This seaway may have contained water which, because of its distance from the open sea, was not of normal marine composition. Salt deposition could have occurred interstitially in brine-soaked, soft sediment and in shallow brine pools on the margins of this seaway or in bottom sediment during periods of almost complete withdrawal of the sea. Occasional exposure of the evaporites to river- or rain-derived fresh water, with early dissolution and reprecipitation of the salts in situ, may account for their low bromine content.
As in other branches of geology, students of evaporites are constantly searching for present day deposits which are analogues for evaporites in the ancient geologic record. Many believe that it is too soon after the last ice age for the development of large areas of evaporite deposition. In the early 1960s, Holocene anhydrite and halite were found growing subaerially in coastal and continental sabkhas in the Persian Gulf (Curtis et al., 1963). No modern equivalent of a deep evaporite basin has yet been recognized. Thus, many ancient evaporite deposits are without a modern analogue, one reason why there are still many unanswered questions about evaporite deposition.
Deposition of the Nippewalla Group probably occurred in a hot, arid to semi-arid environment. Similar environments, if they occur at the present time, would probably be found in modern deserts. Possible modern analogues include the Great Kavir of Iran (Stöcklin, 1968), which is an extensive central desert depression, covering an area of 1,800 sq. mi. (4700 sq. km.), in which salt is accumulating at the present time. Another possible analogue is Sabkha Matti, of the Trucial Coast, a broad flat plain marginal to the sea. Most of the surface of the area is at the level of the groundwater table (Kinsman, 1966; Glennie, 1970). In the Qattara Depression of northwest Egypt, the processes of deflation and evaporite deposition are currently active. Deposition of the Harper and Salt Plain formations and of the Cedar Hills Sandstone could have taken place in an environment similar to that in Egypt.
The Great Salt Lake of Utah contains a shallow, concentrated brine, whose maximum depth is about 30 ft. (9 m) and which has a high Cl/Br ratio. The area covered by the present salt lake, and ancient Lake Bonneville, is similar in size to the area of the Syracuse Basin. Eardley (1970) believed that much of the salt was carried in the atmosphere from the Pacific Ocean. Although salt deposition has occurred on the margins of the lake, present climatic conditions are leading to the dissolution of this salt. Salt deposits may occur beneath the bottom of the present lake, or underlying the salt flats, but these sediments have as yet received little study.
The climatic conditions in the Permian in the northern hemisphere probably were very different from those of today, and it is not likely that exact analogues for deposition in late Leonardian time in the Midcontinent exist at the present time.
Sediments of the Harper and Salt Plain formations and the Cedar Hills Sandstone accumulated subaerially through eolian transport, with evaporitic conditions and saline groundwater leading to the precipitation of anhydrite nodules within the sediment. The origin of the anhydrite at the top of the Cedar Hills section in northwestern Kansas, and the progressively increasing amount of anhydrite in the Cedar Hills moving northward in the area mapped is difficult to account for, but may be related to the deposition of evaporites farther north in the Denver-Julesburg Basin.
Deposition of the Flower-pot and Blaine sediments in western Kansas probably occurred in shallow bodies of brine or in emergent conditions, on a broad salt flat on the margins of an ephemeral epeiric sea. The bromine concentration and the textures of the halite deposits suggest that they repeatedly were dissolved by fresh water and reprecipitated in situ. The fine-grained red mudstone associated with the halite may have been washed into the basin by slow-moving streams, but it is more likely that much of it was windblown.
The Cedar Springs Dolomite, at the base of the Blaine Formation, marks a flow of less saline brine into the area and even beyond the limits of the Syracuse Basin. The fact that the anhydrite beds of the Blaine and Flower-pot are not confined to the basin, as well as the tabular geometry of the area of thickest salt, suggest that the basin lacked strong topographic expression during the deposition of the Flower-pot and Blaine sequence.
The most likely source of the evaporite minerals in this sequence is the sea, but it is also possible that salt was derived from the solution of older evaporite deposits to the north, with drainage to the south carrying the halite-bearing solutions into the Syracuse Basin. This possibility can be investigated further only by studying evaporites in Nebraska of similar age to those in Kansas (Fig. 1). No well cores and little well log information on these deposits exists at the present time.
Finally, it is of interest to note that the area of evaporite deposition of the Flower-pot and Blaine is comparable to the area occupied by the Great Salt Lake and ancient Lake Bonneville, suggesting that accumulation of salt in a completely continental basin is a possibility.
I should like to thank W. C. McClain, Oak Ridge National Laboratory, for making the entire core from A.E.C. Test Hole 5, Wichita County, Kansas, available for study; W. R. Van Schmus, University of Kansas, G. W. James and L. M. Magnuson, Kansas Geological Survey, for developing the technique for the bromine analyses; Gene Collins, U. S. Bureau of Mines, Bartlesville, Oklahoma, for performing wet chemical analyses for bromine; W. J. Ebanks, Jr., Kansas Geological Survey, for guidance and editorial advice; D. B. Smith, Institute of Geological Sciences, United Kingdom, Bailey Rascoe, Jr., Phillips Petroleum, and L. F. Dellwig and E. J. Zeller, University of Kansas, for helpful discussions.
Financial support from the Kansas Geological Survey, and ERDA Contract AT(05-1)-1642 is gratefully acknowledged.
Adams, S. S., 1969, Bromine in the Salado Formation, Carlsbad Potash District, New Mexico: New Mexico Bureau of Mines and Mineral Resources, Bull. 93, 122 p.
Anderson, R. Y., and Kirkland, D. W., 1966, Intrabasin varve correlation: Geol. Soc. Am., Bull., v. 77, p. 241-255.
Arthurton, R. S., 1973, Experimentally produced halite compared with Triassic layered halite-rock from Cheshire, England: Sedimentology, v. 20, p. 145-160.
Baar, C. A., 1963, Der Bromgehalt als stratigraphischer Indikator in Steinsalzlagerstätten: Neues Jb. Miner. Mh., v. 7, p. 145-153.
Bachman, G. O., and Johnson, R. B., 1973, Stability of salt in the Permian Salt Basin of Kansas, Oklahoma, Texas and New Mexico: U.S. Geol. Survey, Open-file Rept. 73-14, 66 p. [available online]
Bayne, C. K., and Brinkley, D., eds., 1972, Geology, Hydrology, thickness and quality of salt at three alternative sites for disposal of radioactive waste in Kansas: Univ. Kansas, Center for Research, Inc., 63 p.
Boeke, H. E., 1908, Über das Kristallisationsschema der Chloride, Bromide, Jodide von Natrium, Kalium, und Magnesium, sowie über das Vorkommen des Broms und das Fehlen von Jod in den Kalisalzlagerstätten: Zeitschr. Krist., u. Miner., v. 45, p. 346-391.
Borchert, H., and Baier, E., 1953, Zur Metamorphose ozeaner Gipslagerungen: Neues Jb. Mineral. Abh., v. 86, p. 103-154.
Braitsch, O., 1960, Mineralparagenesis und Petrologic der Stassfurtsalz in Reyershausen: Kali und Steinsalz, v. 3, p. 1-14.
Braitsch, O., 1962, Entstehung und Stoffbestand der Salzlagerstätten: Springer-Verlag, 232 p.
Braitsch, O., 1971, Salt deposits: Their origin and composition: transl'd by Burek, P. J., and Nairn, A. E. M., Springer-Verlag, 297 p.
Butler, G. P., 1970, Holocene gypsum and anhydrite of the Abu Dhabi Sabkha, Trucial Coast: an alternative explanation of origin, in Rau, J. L., and Dellwig, L. F., eds., Third Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 120-152.
Campbell, C. L., 1963, Permian evaporites between the Blaine and Stone Corral Formations: Unpub. M.S. thesis, Univ. Kansas, 73 p.
Chukhrov, F. V., 1973, On mineralogical and geochemical criteria in the genesis of red beds: Chem. Geol., v. 12, p. 67-75.
Cragin, F. W., 1896, The Permian System in Kansas: Colorado College Studies, v. 6, p. 1-48.
Curtis, R., Evans, G., Kinsman, D. J. J., and Shearman, D. J., 1963, Association of dolomite and anhydrite in the Recent sediments of the Persian Gulf: Nature, v. 197, p. 679-680.
D'Ans, J., and Kühn, R., 1940, Über den Bromgehalt von Salzgesteinen der Kalisalzlagerstätten: Kali, v. 34, p. 42-46.
Dean, W. E., Davies, G. R., and Anderson, R. Y., 1975, Sedimentological significance of nodular and laminated anhydrite: Geology, v. 3, p. 367-372.
Droste, J. B., 1963, Clay mineral composition of evaporite sequences, in Bersticker, A. C., ed., Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 47-54.
Eardley, A. J., 19-10, Salt Economy of the Great Salt Lake, Utah, in Rau, J. L., and Dellwig, L. F., eds., Third Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 78-105.
Eaton, G. P., Peterson, D. L., and Schumann, H. H., 1972, Geophysical, geohydrological, and geochemical reconnaissance of the Luke Salt Body, central Arizona: U.S. Geol. Survey, Prof. Paper 753, 28 p. [available online]
Fay, R. O., 1964, The Blaine and related formations of northwestern Oklahoma and southern Kansas: Oklahoma Geol. Survey, Bull. 98, 238 p.
Friedman, G. M., 1972, Petrographic data and comments on the depositional environment of the Miocene sulphates and dolomites at sites 124, 132 and 134, western Mediterranean Sea, in Ryan, W. B. F. et al., Initial Reports of the Deep Sea Drilling Project: U.S. Govt. Printing Office, v. 13, p. 695-708. [available online]
Glennie, K. W., 1970, Desert sedimentary environments: Elsevier, 222 p.
Gould, C. N., 1902, General geology of Oklahoma: Oklahoma Geol. Survey, Second Bienn. Rept., p. 17-14.
Grimm, W. D., 1962, Idiomorphe Quartze als Leitmineralien für salinare Fazies: Erdöl und Kohle, v. 15, p. 880-887.
Gutentag, E. D., Lobmeyer, D. H., and McGovern, H. E., 1972, Ground water in Kearny County, southwestern Kansas: U.S. Geol. Survey, Hydrologic Atlas HA-416. [available online]
Ham, W. E., 1960, Middle Permian evaporites in southwestern Oklahoma: Internat. Geol. Congress, 21 St., Norden 1960, Rept. Pt. 12, p. 138-151.
Hardie, L. A., and Eugster, H. P., 1971, The depositional environment of marine evaporites: a case for shallow, clastic accumulation: Sedimentology, v. 16, p. 187-220.
Haude, R., 1970, Die Entstehung von Steinsalz-Pseudomorphosen: N. Jahrbuch f. Geologic u. Palaontologie, Monatshefte, p. 1-10.
Herrmann, A. G., Knake, D., Schneider, J., and Peters, H., 1973, Geochemistry of sea water and brines from salt pans: Main composition and bromine distribution: Contr. Mineral. and Petrol., v. 40, p. 1-24.
Hershey, L. A., and Hampton, E. R., 1974, Geohydrology of Baca and southern Prowers Counties, southeastern Colorado: U.S. Geol. Survey, Water-Resources Invest. 16-74, 1 sheet.
Hills, J. M., 1942, Rhythm of Permian seas--a paleogeographic study: Am. Assoc. Petrol. Geologists, Bull., v. 26, p. 217-255.
Holland, H. H., 1972, The geologic history of sea water--an attempt to solve the problem: Geochem. et Cosmochim. Acta, v. 36, p. 637-652.
Holliday, D. W., and Shephard Thorn, E. R., 1974, Basal Purbeck evaporites of the Fairlight borehole, Sussex: Inst. Geol. Sciences, Rept. 74/4, 14 p.
Holser, W. T., 1966, Bromide geochemistry of salt rocks, in Rau, J. L., ed., Second Symposiuiii on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 248-275.
Holser, W. T., 1970, Bromide geochemistry of some non-marine salt deposits in the southern Great Basin: Mineral. Soc. Amer., Spec. Paper 3, p. 307-319.
Holser, W. T., Wardlaw, N. C., and Watson, D. W., 1972, Bromide in salt rocks: extraordinarily low content in the Lower Elk Point salt, Canada, in Richter-Bernberg, G., ed., Geology of saline deposits: UNESCO, Paris, p. 69-75.
Jacka, A. D., and Franco, L. A., 1974, Deposition and diagenesis of Permian evaporites and associated carbonates and clastics of shelf areas of the Permian Basin, in Coogan, A. H., ed., Fourth Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 67-89.
James, G. W., 1972, Mineralogy and distribution of water-insoluble residues from AEC Test Hole 5, in Bayne, C. K., and Brinkley, D., eds., Geology, hydrology, thickness and quality of salt at three alternative sites for disposal of radioactive waste in Kansas: Univ. of Kansas, Center for Research, Inc., p. 50-57.
Jordan, L., and Vosburg, D. L., 1963, Permian salt in the Anadarko Basin, Oklahoma and Texas: Okla. Geol. Survey, Bull. 102, 76 p.
Johnson, K. S., 1967, Stratigraphy of the Permian Blaine Formation and associated strata in southwestern Oklahoma: Unpub. Ph.D. thesis, Univ. Illinois.
Johnson, K. S., Wolfe, W. D., and Smith, M. V., 1975, Stratigraphy of the Permian Blaine Formation and associated strata in north-central Texas: Am. Assoc. Petrol. Geologists, Abstracts of Ann. Mtgs., v. 2, p. 39.
Kendall, C. G. St. C., 1969, An environmental re-interpretation of the Permian evaporite/carbonate shelf sediments of the Guadalupe Mountains: Geol. Soc. Am., Bull., v. 80, p. 2503-2526.
Kinsman, D. J. J., 1966, Gypsum and anhydrite of Recent age, Trucial Coast, Persian Gulf, in Rau, J. L., ed., Second Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 302-306.
Kinsman, D. J. J., 1969, Modes of formation, sedimentary associations, and diagnostic features of shallow-water and supratidal evaporites: Am. Assoc. Petrol. Geologists, Bull., v. 53, p. 830-840.
Kosmahl, W., 1969, Zur Stratigraphie, Petrographie, Paläogeographie, Genese und Sedimentation des Gebänderten Anhydrits (Zechstein 2), Grauen Salztons und Hauptanhydrits (Zechstein 3) in Nordwestdeutschland: Geologisches Jahrbuch, Beiheft, v. 71, 129 p.
Krauskopf, K. B., 1956, Dissolution and precipitation of silica at low temperatures: Geochim. et Cosmochim. Acta, v. 10, p. 1-26.
Krynine, P. D., 1949, The origin of red beds: N.Y. Acad. Sci. Trans., ser. 2, v. 11, p. 60-68.
Kulstad, R. O., Fairchild, P., and McGregor, D., 1956, Gypsum in Kansas: Kansas Geol. Survey. Bull. 113, 110 P. [available online]
Kühn, R., 1955, Tiefberechnung des Zechsteinmeeres nach dem Bromgehalt der. Salze: Deutsche Geol. Gesell. Zeitschr., v. 105, p. 646-663.
Kühn, R., 1968, Geochemstry of the german potash deposits, in Mattox, R. B., ed., Saline deposits: Geol. Soc. Am., Spec. Paper 88, p. 427-504.
Kühn, R., and Hsü, K. J., 1974, Bromine content of Mediterranean halite: Geology, v. 2, p. 213-216.
Kühn, R., and Schwerdtner, W. M., 1959, Nachweis deszendenter Vorgänge wahrend der Entstehung der Leine-Serie des deutschen Zechsteinsalzes: Kali u. Steinsalz, v. 2, p. 380-383.
Lobmeyer, D. H., and Sauer, C. G., 1974, Water resources of Hamilton County, south-western Kansas: U.S. Geol. Survey, Hydrologic Atlas HA-516. [available online]
Maher, J. C., and Collins, J. B., 1948, Hugoton embayment of the Anadarko basin in south-western Kansas, southeastern Colorado, and Oklahoma Panhandle: Am. Assoc. Petrol. Geologists, Bull., v. 32, p. 813-819.
Maiklem, W. R., Bebout, D. G., and Glaister, R. P., 1969, Classification of anhydrite--A practical approach: Bull. Can. Petrol. Geol., v. 17, p. 194-233.
Malone, D. J., 1962, Stratigraphic relations and structural significance of the Blaine Formation in the subsurface of southwestern Kansas: Unpub. M.S. thesis, Univ. Wichita, 72 p.
Maughn, E. K., 1966, Environment of deposition of Permian salt in the Williston and Alliance basins, in Rau, J. L., ed., Second Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 35-47.
McBride, E. F., 1963, A classification of sandstones: Jour. Sed. Petrol., v. 33, p. 664-669.
McKee, E. D., Oriel, S. S., and others, 1967a, Paleotectonic investigations of the Permian System of the United States: U.S. Geol. Survey, Prof. Paper 515, 271 p. [available online]
McKee, E. D., Oriel, S. S., and others, 1967b, Paleotectonic maps of the Permian System: U.S. Geol. Survey, Misc. Geol. Inv. Map I-450.
Merriam, D. F., 1958, Preliminary regional structural contour map on top of the Stone Corral Formation (Permian) in Kansas: Kansas Geol. Survey, Oil and Gas Invest., No. 17, Map.
Merriam, D. F., 1963, The geologic history of Kansas: Kansas Geol. Survey, Bull., 162, 317 p. [available online]
Millott, G., 1970, Geology of clays: Springer-Verlag, 429 p.
Moore, R. C., Frye, J. C., Jewett, J. M., Lee, Wallace, and O'Connor, H. G., 1951, The Kansas rock column: Kansas Geol. Survey, Bull. 89, 132 p. [available online]
Mudge, M. R., 1967, Central Midcontinent Region, in McKee, E. D., Oriel, S. S., and others, Paleotectonic investigations of the Permian System of the United States: U.S. Geol. Survey, Prof. Paper 515, ch. F., p. 95-123. [available online]
Neev, D., and Emery, K. O., 1967, The Dead Sea, Depositional processes and environments of evaporites: Geol. Survey of Israel, Jerusalem, 147 p.
Norton, G. H., 1939, Permian redbeds of Kansas: Am. Assoc. Petrol. Geologists, Bull., X7. 23, p. 1751-1819.
O'Connor, H. G., 1963, Changes in Kansas stratigraphic nomenclature: Am. Assoc. Petrol. Geologists, Bull., v. 47, p. 1873-1877.
O'Connor, H. G., Zeller, D. E., Bayne, C. K., Jewett, J. M., and Swineford, A., 1968, Permian System, in Zeller, D. E., ed., The stratigraphic succession in Kansas: Kansas Geol. Survey, Bull. 189, p. 43-53. [available online]
Ogniben, O., 1957, Petrografia della Serie Solfifera Siciliana e considerazioni geologiche relative: Mem. Descrit. Carta Geol. Ital., v. 33, p. 275.
Pettijohn, F. J., Potter, P. E., and Siever, R., 1972, Sand and Sandstone: Springer-Verlag, 618 p.
Phleger, F. B., 1969, A modern evaporite deposit in Mexico: Am. Assoc. Petrol. Geologists Bull., v. 53, p. 824-829.
Poborski, J. W., 1970, The Upper Permian Zechstein in the eastern province of central Europe, in Rau, J. L., and Dellwig, L. F., eds., Third Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 24-29.
Rascoe, Bailey, Jr., 1968, Permian System in the western Mid-Continent: The Mountain Geologist, v. 5, p. 127-138.
Rascoe, Bailey, Jr., and Baars, D. L., 1972, The Permian System, in Geologic Atlas of the Rocky Mountain Region: Rocky Mt. Assoc. of Geologists, Denver, Colo., p. 143-165.
Raup, O. B., 1966, Bromine distribution in some halite rocks of the Paradox Member, Hermosa Formation, in Utah, in Rau, J. L., ed., Second Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio,,p. 236-247.
Raup, O. B., Hite, R. J., and Groves, H. L., Jr., 1970, Bromine distribution and paleosalinities from well cuttings, Paradox basin, Utah and Colorado, in Rau, J. L., and Dellwig, L. F., eds., Third Symposium on Salt: N. Ohio Geol. Soc., Cleveland, Ohio, p. 40-47.
Richter-Bernberg, G., 1955, Über salinare Sedimentation: Zeitschr. Dent. Geol. Ges., v. 105, p. 834-854.
Rubey, W. W., 1951, Geologic history of sea water: Geol. Soc. Am., Bull., v. 62, p. 1111-1148.
Schreiber, B. C., and Kinsman, D. J. J., 1975, New observations on the Pleistocene evaporites of Montallegro, Sicily and a modern analog: Jour. Sed. Petrol., v. 45, p. 469-479.
Schumaker, R. D., 1966, Regional study of Kansas Permian evaporite formations: Unpub. M.S. thesis, Wichita State Univ., 87 p.
Shearman, D. J., 1966, Origin of marine evaporites by diagenesis: Inst. Mining Metallurgy, Trans., Sect. B., v. 75, p. B208-B215.
Shearman, D. J., 1970, Recent halite rock, Baja California, Mexico: Inst. Mining Metallurgy, Trans., Sec. B., v. 75, p. 208215.
Shearman, D. J., and Fuller, J. G., 1969, Anhydrite diagenesis, calcitization, and organic laminites, Winnipegosis Formation, Middle Devonian, Saskatchewan: Bull. Can. Petrol. Geol., v. 17, p. 496-525.
Siever, R., 1962, Silica solubility, 0-200°C, and the diagenesis of siliceous sediments: J. Geol., v. 70, p. 127-150.
Smith, D. B., 1971, Possible displacive halite in the Permian Upper Evaporite Group of northeast Yorkshire: Sedimentology, v. 17, p. 221-232.
Stewart, F. H., 1951a, The petrology of the evaporites of the Eskdale No. 2 boring, east Yorkshire. Part II. The middle evaporite bed: Min. Mag., v. 29, p. 445-475.
Stewart, F. H., 1951b, The petrology of the evaporites of the Eskdale No. 2 boring, east Yorkshire. Part III. The upper evaporite bed: Min. Mag., v. 29, p. 557-572.
Stöcklin, J., 1968, Salt deposits of the Middle East, in Mattox, R. B., ed., Saline Deposits: Geol. Soc. Am., Spec. Paper 88, p. 157-181.
Stoffers, P., and Kühn, R., 1974, Red Sea evaporites: A Petrographic and geochemical study, in Whitmarsh, R. B., Weser, O. E., Ross, D. A., et al.: Initial Reports of the Deep Sea Drilling Project, v. XXIII, p. 821-847. [available online]
Stokes, W. L., 1968, Multiple parallel-truncation bedding planes--a feature of wind-deposited sandstone formations:Jour. Sed. Petrol., v. 38, p. 510-515.
Swineford, A., 1955, Petrography of Upper Permian rocks in south-central Kansas: Kansas Geol. Survey, Bull., 111, 179 p. [available online]
Van Houten, F. B., 1964, Origin of red beds--some unsolved problems, in Nairn, A. E. M., ed., Problems in Paleoclimatology: Wiley, p. 647-659.
Valyashko, M. G., 1956, Geochemistry of bromide in the processes of salt deposition and the use of bromide content as a genetic and prospecting criterion: Geochemistry, v. 6, p. 570-589.
Valyashko, M. G., 1972, Discussion of Holser, W. T., Wardlaw, N. C., and Watson, D. W., 1972, Bromide in salt rocks: extraordinarily low content of the Lower Elk Point salt, Canada, in Richter-Bernberg, G., ed., Geology of Saline Deposits: UNESCO, Paris, p. 74.
Walker, T, R., 1967, Formation of red beds in modern and ancient deposits: Geol. Soc. Am. Bull., v. 78, p. 353368.
Wardlaw, N. C., and Schwerdtner, W. M., 1966, Halite-anhydrite seasonal layers in the Middle Devonian Prairie Evaporite Formation, Saskatchewan, Canada: Geol. Soc. Am., Bull., v. 77, p. 331-342.
Wilson, L. R., 1962, Permian plant microfossils from the Flowerpot Formation, Greer County, Oklahoma: Oklahoma Geol. Survey, Circ. 14, 50 p.
Zeller, D. E., ed., 1968, The stratigraphic succession in Kansas: Kansas Geol. Survey Bull., 189, 81 p. [available online]
Zimmerman, E., 1909, Über den Pegmatit Anhydrit: Kali, v. 31 p. 309-312.
Analyses for bromine in sodium chloride were made by X-ray fluorescence. Samples of halite were taken at 10 ft. (3 m) intervals throughout the salt-bearing sequence in the Flower-pot and Blaine formations. As the salt occurs intimately associated with red clay and anhydrite, careful hand-picking of halite from crushed portions of the core was necessary in order to obtain the purest samples possible. Inclusion-free halite was selected in preference to halite with abundant fluid inclusions. The halite was then ground to a fine powder, and made into pellets using a casing of boric acid crystals. Approximately two grams of sample were required to prepare one pellet.
Standards were prepared in order to construct a calibration curve. Analytical grade sodium chloride contains less than 50 ppm bromine, but the exact concentration is not known precisely. Since the concentration of bromine in the salt sequence is very low, it was necessary to prepare standards of low concentration with sodium chloride that was bromine-free, and for which the exact concentrations were known. This was achieved by reacting fumes of concentrated hydrochloric acid with sodium hydroxide solution. The sodium chloride produced by this reaction did not contain detectable bromine when analyzed by X-ray fluorescence. Known amounts of sodium bromide solution were then added to weighted portions of sodium chloride to make standards ranging in concentration from 0 ppm to 25 ppm bromine. Standards of higher concentration were not prepared, as all samples analyzed fell within this range. As both standards and samples consisted of bromine in a sodium chloride matrix, no matrix corrections were required. Measurements made on the standards were used to construct a calibration curve, using regression analysis.
Analyses were made on a Phillips 1410 X-ray fluorescence spectrometer. The determinations were carried out in air, using a chromium target tube operated at 45 kV/20 mA, a lithium fluoride (200) analyzing crystal, and a scintillation counter. Samples were rotated by a spinner during analysis, to ensure uniform exposure to the X-ray beam. A baseline setting of 1.9 V and a window of 1.6 V were used for pulse-height discrimination. Measurements of the samples and standards were made by 100-second counts at each of the background positions (29.67° 2θ, and 30.27° 2θ), and 200-second counts at the first order Br K a peak top (29.97° 2θ). It was found that the theoretical counting error made all other errors, such as sample preparation, virtually negligible. The concentration of bromine in the samples was determined from the regression equation for the calibration curve. The detection level was calculated to be 5 ppm at the 95 percent confidence level, and concentrations between 5 and 20 ppm to be subject to an error of ±3 ppm. The most probable concentration of bromine in a sample is plotted in Figure 52. The Flowerpot salt was found, for the most part, to have a bromine content below detection, that is, a bromine concentration lower than 5 ppm under the conditions used. The salt sequence within the Blaine Formation has a more variable and slightly higher bromine concentration than the underlying salt.
Scans of the fine fraction associated with the halite samples (including clay minerals, magnesite, dolomite, and anhydrite) were made, as these were known to contain halite. It was found that, in every case, the clay fraction had a greater bromine content than the corresponding halite crystals from the same interval sampled. This relationship was also noted by Adams (1969) in the Salado Formation of New Mexico. Furthermore, it was found that if a portion of the clay sample was washed with distilled water and then scanned, no bromine was detected. Actual values for bromine concentration of halite in the clay fraction are not presented, due to the matrix corrections required to determine them.
Kansas Geological Survey, Nippewalla Group, Permian, Western Kansas
Placed on web July 1, 2009; originally published in Sept. 1978.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/215/index.html