Kansas Geological Survey, Bulletin 134, Part 4, originally published in 1959
Originally published in 1959 as Kansas Geological Survey Bulletin 134, Part 4. This is, in general, the original text as published. The information has not been updated.
A detailed spectrographic method for determining the germanium content of coal is described and the results of analyses of twenty Kansas coals from 117 different locations are reported. No definite conclusions are made as to geographic or stratigraphic variation in germanium content. The concentration of germanium in the coal ash ranges from .0018 percent to .0575 percent, and in the total coal from .0006 per cent to .0116 per cent.
The demand for germanium in many phases of the electronic industry and its relatively short supply have prompted extensive research into possible sources of this element. Small amounts of germanium are found throughout the earth's crust, but it has not been found in sufficient concentration to permit its direct recovery. The chief source of germanium in the past has been certain residues derived from the smelting of zinc ores, from which it is obtained by distillation. The uncertain economics of zinc mining and smelting, however, have led to the investigation of other source materials, especially coal. Several plants now in operation in Germany, Japan, and England recover germanium from the fly-ash and residual ash of coal that is being used in industrial quantities. The possibility that Kansas coals might be a source of germanium prompted the State Geological Survey to begin a study, by spectrochemical analyses, of the germanium content of coals found in the state. A preliminary spectrographic investigation of germanium in Kansas coals was published by Schleicher and Hambleton (1954). The chief purpose of the preliminary investigation was to develop an accurate and rapid spectrographic method for determining germanium content of coals; analyses of six coals from 24 locations were included in the report. The present publication reports analyses of twenty coals from 117 locations; the spectrographic method is again described.
Since the publication of the preliminary report, Fredrick and others (1954) and Machin and Witters (1956) have completed similar studies. Results of these studies show good agreement with the results obtained in the Kansas Geological Survey laboratory. The ash of Kansas coals shows, in general, a higher germanium content than the coals from some other localities. Most of the Kansas coal seams are thin, and, seemingly, germanium is more abundant in thin coal beds than in the thicker coal beds.
The element silicon, being atomically, chemically, and physically very similar to germanium, also can be used in semiconducting devices. Silicon transistors have the advantage that, in general, they can operate at higher ambient temperatures than other semiconductors, but the disadvantage that their capacity is somewhat less than those made of germanium. Silicon probably will never completely replace germanium; however, the manufacture of silicon transistors has effectively reduced the shortage of germanium in the electronics industry as shown by price declines.
In April 1954, the price of germanium was $295 per pound (Eng. Mining Jour., 1954). In July 1955, the price had fallen to $250 per pound (Machin and Witters, 1956) and in June 1957 had been reduced to a low of $197 (Eng. Mining Jour., 1957). The price subsequently rose to $206 per pound in June 1958 (Eng. Mining Jour., 1958).
Hughes Product Group of Hughes Aircraft Corporation states (Chem. Eng. News, 1956) that improved chemical and metallurgical methods of producing and refining will lower the price still further to meet the estimated five-fold increase in market by 1960.
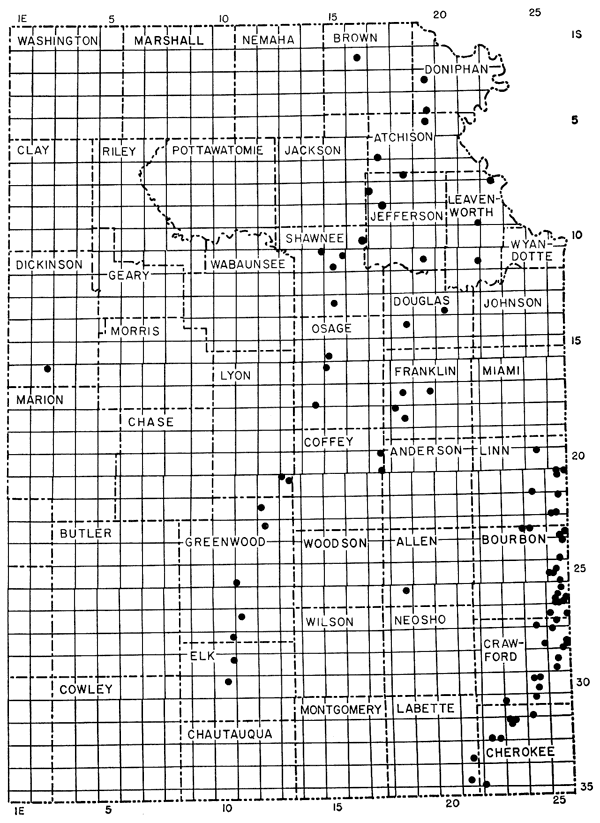
The study includes 93 (117 including Preliminary Study) analyses. The Pennsylvanian coals analyzed include the Weir-Pittsburg, Pilot, Tebo (from western Missouri), Mineral, Fleming, Croweburg, Bevier, and Mulky coals of the Cherokee Group, Desmoinesian Series (Howe, 1956); the Summit and Mulberry (partly from western Missouri) coals of the Marmaton Group, Desmoinesian Series (Schoewe, 1955); the Thayer coal of the Kansas City Group, Missourian Series (Schoewe, 1944); the Blue Mound, Ottawa, Sibley, and Williamsburg coals, two unnamed coals in the Tonganoxie Sandstone, and an unnamed coal in the Lawrence Shale of the Douglas Group, Virgilian Series (Bowsher and Jewett, 1943), and the Nodaway, Elmo, and Lorton coals of the Wabaunsee Group, Virgilian Series (Schoewe, 1946). Analyses also were made of one sample of Permian coal in the Wellington Formation (Schoewe, 1951), two samples each of underclay and rash (impure coal), and eight samples of refuse piles of fines. Data pertaining to the location of the coal samples, name of coal seam, average thickness of the coal, and laboratory number of samples are presented in Table 1. Figure 1 shows the locations sampled.
Figure 1--Map showing localities where coal samples were collected.

Table 1--Coals studied for germanium content, location of samples, and thickness available.
| Lab. no. | County | Location | Coal | Thickness, inches |
|---|---|---|---|---|
| 5693 | Dickinson | 21-16-2E | Unnamed (1) | 5 |
| 5692 | Brown | 15-2-16E | Lorton | 8 |
| 55228 | Atchison | 33-6-17E | Elmo | 14 |
| 55229 | Shawnee | 1-11-14E | Elmo | 20 |
| 54398 | Elk | 9-30-10E | Nodaway | 5 |
| 54399 | Elk | 11-29-10E | Nodaway | 6 |
| 54400 | Greenwood | 12-28-10E | Nodaway | 4 |
| 54401 | Greenwood | 17-27-11E | Nodaway | 4 |
| 54402 | Greenwood | 30-25-11E | Nodaway | 2 |
| 54403 | Greenwood | 9-23-12E | Nodaway | 6 |
| 54404 | Greenwood | 17-22-12E | Nodaway | 2.5 |
| 54397 | Lyon | 9-21-13E | Nodaway | 7 |
| 54396 | Lyon | 6-21-13E | Nodaway | 6 |
| 54395 | Osage | 34-17-14E | Nodaway | 10 |
| 54363 | Osage | 7-16-15E | Nodaway | 16 |
| 54412 | Osage | 29-15-15E | Nodaway | 14 |
| 54413 | Shawnee | 16-13-15E | Nodaway | 9 |
| 54414 | Shawnee | 27-11-15E | Nodaway | 14 |
| 54415 | Shawnee | 12-11-15E | Nodaway | 12 |
| 54416 | Shawnee | 23-10-16E | Nodaway | 15 |
| 54410 | Jefferson | 3-9-17E | Nodaway | 11 |
| 54409 | Jefferson | 18-8-17E | Nodaway | 10 |
| 54408 | Jefferson | 28-7-18E | Nodaway | 9 |
| 54405 | Atchison | 21-5-19E | Nodaway | 8 |
| 54406 | Doniphan | 34-4-19E | Nodaway | 12 |
| 54407 | Doniphan | 15-3-19E | Nodaway | 4 |
| 55227 | Shawnee | 27-11-15E | Nodaway | 13 |
| 55220 | Leavenworth | 36-9-21E | Upper Sibley | 6 |
| 55224 | Leavenworth | 4-8-22E | Upper Sibley | 12 |
| 55225 | Leavenworth | 24-11-21E | Upper Sibley | 26 |
| 55223 | Leavenworth | 24-11-21E | Lower Sibley | 13 |
| 55221 | Jefferson | 22-11-19E | Unnamed (2) | 6 |
| 5547 | Douglas | 28-13-20E | Unnamed (3) | 6 |
| 5548 | Douglas | 28-13-20E | Unnamed (3) | 2 |
| 5546 | Douglas | 28-13-20E | Unnamed (3) | 2.4 |
| 55222 | Coffey | 34-20-17E | Upper Williamsburg | 7 |
| 55194 | Coffey | 3-20-17E | Upper Williamsburg | 7 |
| 55113 | Franklin | 22-18-18E | Upper Williamsburg | 12 |
| 55114 | Franklin | 5-18-18E | Upper Williamsburg | 16 |
| 55226 | Franklin | 15-17-18E | Upper Williamsburg | 15 |
| 55115 | Franklin | 14-17-19E | Upper Williamsburg | 9 |
| 54335 | Douglas | 14-14-18E | Lower Williamsburg | 6 |
| 55117 | Allen | 11-26-18E | Thayer | 7 |
| 53308* | Bourbon | 20-23-24E | Mulberry | 22 |
| 53309* | Bourbon | 24-23-23E | Mulberry | 13 |
| 53310* | Linn | 33-22-25E | Mulberry | 22 |
| 53311* | Linn | 33-21-24E | Mulberry | 23 |
| 53313* | Linn | 3-20-24E | Mulberry | 40 |
| 53314* | Linn | 3-22-25E | Mulberry | 24 |
| 53315* | Linn | 32-22-25E | Mulberry | 24 |
| 55184† | Linn | 1-21-25E | Mulberry | 28 |
| 55185† | Linn | 10-21-25E | Mulberry | 50 |
| 55186† | Bates (Mo.) | 28-41N-33 | Mulberry | 12 |
| 55187† | Bates (Mo.) | 21-40N-33 | Mulberry | 24 |
| 55188† | Bates (Mo.) | 27-40N-33 | Mulberry | 26 |
| 55189† | Bates (Mo.) | 6-39N-33 | Mulberry | 24 |
| 55190† | Bates (Mo.) | 16-40N-33 | Mulberry | 36 |
| 55191† | Bates (Mo.) | 32-41N-33 | Mulberry | 38 |
| 55192† | Bates (Mo.) | 33-40N-33 | Mulberry | 20 |
| 55193† | Linn | 3-21-25E | Mulberry | 45 |
| 5685 | Bourbon | 16-25-25E | Summit | 10 |
| 53305* | Bourbon | 34-25-25E | Mulky | 14 |
| 53306* | Bourbon | 19-25-25E | Mulky | 14 |
| 53307* | Bourbon | 33-26-25E | Mulky | 13 |
| 5691 | Bourbon | 25-23-25E | Mulky | 17 |
| 5690 | Bourbon | 20-25-25E | Mulky | 15 |
| 5689 | Crawford | 25-28-24E | Mulky | 8 |
| 5686 | Bourbon | 11-26-25E | Mulky | 6 |
| 5684 | Crawford | 34-27-24E | Mulky | 8 |
| 5589 | Crawford | 5-28-25E | Mulky | 9 |
| 5595 | Bourbon | 34-24-25E | Mulky | 5 |
| 5598 | Bourbon | 22-26-25E | Mulky | 18 |
| 5687 | Bourbon | 34-23-25E | Mulky | 18 |
| 5682 | Bourbon | 2-24-25E | Mulky | 14 |
| 53312* | Bourbon | 34-26-25E | Bevier | 15 |
| BN-2-B* | Bourbon | 25-26-25E | Bevier | 17 |
| BN-3-B* | Bourbon | 35-26-25E | Bevier | 17 |
| CR-9-B* | Crawford | 28-27-25E | Bevier | 17 |
| CR-6-B* | Crawford | 16-29-25E | Bevier | 10 |
| CR-14-B* | Crawford | 10-30-24E | Bevier | 16 |
| CR-13-B* | Crawford | 7-31-23E | Bevier | 17 |
| CK-6-B* | Cherokee | 31-34-22E | Bevier | 24 |
| 54331 | Crawford | 9-30-24E | Bevier | 24 |
| 5683 | Bourbon | 20-27-25E | Bevier | 16 |
| 5688 | Bourbon | 28-26-25E | Bevier | 13 |
| 5549 | Bourbon | 34-26-25E | Bevier | |
| CR-1-C* | Crawford | 28-29-25E | Croweburg | 12 |
| 53143 | Crawford | 24-28-25E | Croweburg | 12 |
| 5591 | Cherokee | 32-31-24E | Croweburg | 11 |
| 5592 | Labette | 27-33-21E | Croweburg | 8 |
| 5590 | Cherokee | 32-31-24E | Fleming | 4-9 |
| 5593 | Crawford | 24-28-25E | Fleming | 11 |
| 5594 | Cherokee | 4-31-24E | Fleming | 9 |
| 5596 | Labette | U.S. 69 (North of Chetopa) |
Fleming | 8 |
| 54330 | Crawford | 27-30-24E | Fleming | 3 |
| 5597 | Bourbon | 13-27-25E | Fleming | 5.5 |
| 5599 | Cherokee | 8-32-23E | Fleming | 12 |
| 54333 | Crawford | 25-28-25E | Fleming | 9 |
| CK-4-M* | Cherokee | 35-32-22E | Mineral | 20 |
| CR-8-M* | Crawford | 28-29-25E | Mineral | 18 |
| CR-4-M* | Crawford | 35-28-25E | Mineral | 20 |
| 54332 | Crawford | 25-28-25E | Mineral | 16 |
| 53142* | Crawford | 24-28-25E | Pilot | 9 |
| 54334 | Crawford | 24-28-25E | Weir-Pittsburg | (?) |
| 54367† | St. Clair (Mo.) | 9-39N-28 | Tebo | (?) |
| 54368† | St. Clair (Mo.) | 9-39N-28 | Tebo | (?) |
| 5550‡ | Underclay | |||
| 5551‡ | Underclay | |||
| 5552‡ | Rash | |||
| 54355† | Cherokee | 4-32-23E | Composite samples of refuse piles, Fleming, Mineral, and Bevier coal. |
|
| 54356† | Cherokee | 4-32-23E | ||
| 54357† | Cherokee | 4-32-23E | ||
| 54358† | Cherokee | 4-32-23E | ||
| 54359† | Cherokee | 4-32-23E | ||
| 54360† | Cherokee | 5-32-23E | ||
| 54361† | Cherokee | 33-32-22E | ||
| 54362† | Cherokee | 33-32-22E | ||
| ‡Samples supplied by Mr. J. H. Vincent, 701 1/2 West 9th, Pittsburg, Kansas. *Samples analyzed for preliminary report. † Samples obtained from drill holes, Pittsburg-Midway Coal Company. (1)-Unnamed coal in shale below Wellington limestone. (2)-Unnamed coal in Lawrence Shale. (3)-Unnamed coal in Tonganoxie Sandstone. |
||||
The author thanks the Pittsburg-Midway Coal Company for cooperation in providing samples for analysis in this study.
A sample of each coal sufficient in size to produce at least 150 mgm of ash, as calculated from the proximate analysis, was weighed and placed in a platinum dish of about 100 ml capacity. The dish was then covered with a tight pyrex watch glass and 25 to 35 ml of concentrated nitric acid was added through the pourout lip. The dish was heated on an electric hotplate in a fume hood at about 250° F until all the nitric acid either had reacted with the coal or had been distilled out through the pourout lip. The tight watch glass was used to prevent undesirable rapid evolution of the acid, because refluxing seemed to promote more efficient use of the acid's oxidizing properties. When the sample was completely dried by this method it looked like coke. To this hot substance, 15 ml of concentrated nitric acid was added and again permitted to fume off with refluxing as before. After complete dryness was again attained, the dish was placed in a cold muffle furnace and the temperature raised slowly (approximately 75 to 100° C per hour) to 450° C. When the sample was completely ashed, the furnace was immediately shut off. The immediate turning off of the furnace at 450° C is deemed desirable, as prolonged heating, even at the low temperature of 450° C, might result in the loss of volatile oxides, including germanium oxide. According to Tucker and Waring (1954), neither temperature (100 to 200° C) nor time of ignition (1 to 4 hours) affected the concentration of germanium in their coal samples, whereas Goldschmidt and Peters (1933, cited by Ahrens, 1950, p. 215) have reported otherwise. For the purposes of this investigation, the relatively "safe" temperature of 450° C was chosen. The electric muffle furnace used was a Hoskins, equipped with a manually operated panel rheostat and controlled by a Brown recording potentiometer, which automatically turned off the furnace when the desired 450° C temperature was reached. The last 25° C rise in temperature was accompanied by evolution of voluminous fumes both of organic material and inorganic acids, which were dispersed by the use of an efficient exhaust fan. Upon cooling, the ash was weighed and percent "wet" ash calculated. In every case, the percent "wet" ash calculated exceeded the actual ash content of the coal; the addition of the nitrate radical, the oxidation of sulfur to sulfate, and the lack of high-temperature ignition all tend to increase the weight of the ash fraction. The calculated percentage of "wet" ash was used later to calculate the concentration of germanium in the total coal.
Bismuth was chosen as the internal standard element (Rusanov, 1940, as reported by Ahrens, 1950, p. 216). Comparison of the properties of germanium and bismuth indicated the following similarities:
| Ge | Bi | |
|---|---|---|
| Ionization potential | 8.09 V | Ca 8.0 V |
| Excitation potential (For the lines chosen) |
4.94 V | 5.5 V |
The melting points and boiling points of both the elements and their oxides are relatively low, and as nearly as one could judge from theoretical evidence, they seem to be well suited as an element pair. From the weight of ash it was possible to calculate, weigh, and add the amount of bismuth trioxide necessary to produce a concentration of one percent bismuth in the ash. The density of bismuth trioxide would introduce difficulties in the addition and thorough mixing of an amount smaller than one percent. The bismuth trioxide used was germanium-free Johnson, Matthey, and Company, Ltd. "Specpure" grade, distributed by Jarrell-Ash Company. The first samples of ash were ground and mixed with the bismuth trioxide for about two hours each in a 5.5 cm mullite mortar. In the interest of time saving, seven subsequent samples were ground in a Fisher improved mortar grinder with a mullite mortar and pestle for only 30 minutes, inasmuch as after that time the particles had been reduced to a size where compaction in the mortar resulted. Scraping them off the mortar and further grinding resulted in immediate recompaction. It was found that although the mechanical grinder was very efficient in reducing the particle size, its mixing action (at least on samples of only 150 mgm size) was not satisfactory. By experimentation it was found that the hand-grinding time could be reduced to about 20 minutes, maintaining a satisfactorily thorough mixing, but that any attempt to reduce this time further led to a loss of precision in the spectrographic results.
During the grinding of all the hand-ground samples, any compacted sample in the mortar was scraped loose frequently and broken up with a small platinum spatula.
A matrix approximating closely the composition of an average coal ash was prepared by grinding and mixing together 9.14 gm silica, 2.76 gm calcium carbonate, 3.90 gm ferric oxide, and 3.54 gm alumina. As internal standard 9.2787 gm bismuth trioxide was added. Chemical analyses were used to determine the major constituents of the average coal "wet" ash, and the composition of the matrix was so arranged that 7.85 gm of the matrix was equivalent in composition to 10.00 gm of the average "wet" ash. The discrepancy in equivalence was due to the use of oxides and carbonates, which were available in pure form, in the preparation of the matrix; in the "wet" ash, the major metallic constituents were present as sulfates. The effect of sulfate ion on the ignition of ash was compensated for in the prepared matrix by the use of lithium sulfate as buffer; this is standard practice for the Kansas Geological Survey laboratory.
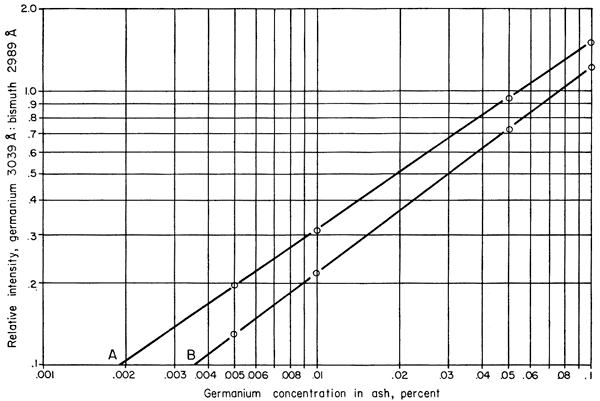
To 3.925 gm of the matrix plus bismuth was added 5 mgm of germanium as germanium dioxide. The resulting mixture contained the equivalent of 0.1 percent germanium. Successive dilutions were made with portions of the original matrix plus bismuth until standards containing 0.1 percent, 0.05 percent, 0.01 percent, 0.005 percent, and 0.001 percent germanium, in essentially identical matrices, were available. These standards and the unknown coal ashes were ignited under identical conditions. Seven spectra were run for each standard, a total of 210 mgm equivalent for each standard, then averaged to give the points from which the working curve for the germanium-bismuth ratio was constructed (Fig. 2).
Figure 2--Graph showing relation of germanium content in coal ash to intensity ratio of spectrum lines used, (A) preliminary study, (B) total study. Displacement is due to changed variables.
The spectrograph used was an Applied Research Laboratories 1.5 meter grating spectrograph powered by a D.C. arc source unit. The electrodes were National Carbon Company standard electrode-grade graphite rods cut to 5 cm in length and formed as an undercut crater electrode similar to the standard Harvey electrode but with thinner wall. The crater is 3.0 mm deep and 5.25 mm inside diameter. The counterelectrode is the standard ARL platform electrode with center-post, selected because the concave platform seems to increase the are sensitivity by reflective increase of the temperature of the sample. The arc was stabilized by a rotating magnet of the type suggested by Meyers and Brunstetter (1947). The rotating sector was set at 10 percent, the grating doors closed to a setting of 4.7 or about 67 percent of the maximum opening, and the electrodes at the beginning of ignition were 4 mm apart, the lower electrode containing the positive charge. No attempt was made to keep the electrode distance constant throughout the ignition. The arc strike was made each time with a rubber-handled sharpened graphite rod from the centerpost of the counterelectrode to the crater edge below; this procedure contributed to the stability of the arc by preventing it from striking from the rim of the platform and thus being able to wander around the outside of the counterelectrode. The film used was Eastman spectrum analysis No. 1.
Several moving film spectra were made to determine persistence of both the germanium and bismuth lines in the samples and their sensitivity at various current ratings and with varying amounts of buffer. Optimum results were obtained using an 8-amp are for an exposure of 60 seconds. The buffer, lithium sulfate, was added experimentally in various amounts. The combination of 10 mgm of ash and 5 mgm of lithium sulfate gave the greatest buffering action, a reasonable cyanogen-band suppression, a higher sensitivity than lesser amounts of buffer, and a more rapid evolution of the element pair than was afforded by a larger proportion of buffer. The ash and buffer were mixed perfunctorily and introduced into the craters of the electrodes after which the mixture was firmly compacted with a flat-faced glass rod of the same diameter as the electrode crater.
After exposure, the film was developed for 3 minutes in D-19, short-stopped for 10 seconds in 3 percent acetic acid, and fixed for 1 minute in Kodak rapid liquid fixer with hardener. After a 1-minute tap-water rinse and a 30-second distilled-water rinse, the film was sponged and dried on an infra-red forced-air film dryer. Density measurements were then read on an ARL densitometer-comparitor.
The lines chosen for density measurements were the germanium line at 3039.0 A and the bismuth line at 2989 A (Harrison, 1946). This particular bismuth line was chosen because of its nearness to the germanium line. The relatively high (1 percent) bismuth concentration caused more sensitive and more commonly used lines in this region to be too intense. The results of the spectral intensity ratios as plotted on the working curve, their averages, the mean deviation, the standard deviation, and the percent mean deviation are shown in Table 2.
To further eliminate the effectt of arc instability, three samples of each ash were ignited consecutively and superimposed as one spectrum, producing a type of "internal average" of the three samples. Three or four spectra of each sample were obtained in this way, and the results averaged; if the first three spectra (nine samples) did not agree, however, a fourth spectrum of three samples was obtained, and the four averaged.
If one of the four spectra differed widely from the other three, statistical methods were employed to determine the validity of discarding the divergent results. If the deviation of one of the results from the mean of the other three was found to be greater than four times the mean deviation of the other three, and greater than three standard deviations from the mean of the other three, the one result was judged to be trivial, on a weighted basis, and was discarded. By commonly accepted statistical principles, 68 percent of all results should be one standard deviation or less from the mean, 28 percent should be one to two standard deviations from the mean, and the remaining 4 percent should fall not farther than three standard deviations from the mean.
Table 2--Results of analyses.
| Lab. no. |
Thickness, inches |
Percent wet ash in coal |
Percent Ge in ash |
Percent Ge in coal |
Ge in coal, ounces/ton |
Number of determinations |
Mean deviation |
Percent mean deviation |
|---|---|---|---|---|---|---|---|---|
| Unnamed coal in Wellington | ||||||||
| 5693 | 5 | 90.85 | .001 | .0011 | .35 | 1 | ||
| Lorton coal | ||||||||
| 5692 | 8 | 48.37 | .0021 | .0010 | .32 | 3 | .03 | 5.0 |
| Elmo coal | ||||||||
| 55228 | 14 | 39.46 | .0099 | .0039 | 1.25 | 3 | .0005 | 5.05 |
| 55229 | 20 | 26.84 | .0056 | .0015 | .48 | 4 | .00035 | 6.2 |
| Nodaway coal | ||||||||
| 54398 | 5 | 17.06 | .0199 | .0034 | 1.09 | 3 | .0015 | 7.54 |
| 54399 | 6 | 12.11 | .049 | .0059 | 1.89 | 7 | .005 | 10.2 |
| 54400 | 4 | 20.32 | .0167 | .0034 | 1.09 | 3 | .0010 | 6.0 |
| 54401 | 4 | 35.12 | .0094 | .0030 | .96 | 3 | .0002 | 2.4 |
| 54402 | 2 | 10.92 | .099 | .0,108 | 3.46 | 3 | .003 | 3.0 |
| 54403 | 6 | 16.54 | .0186 | .0031 | .99 | 4 | .0010 | 5.4 |
| 54404 | 2.5 | 21.35 | .053 | .0113 | 3.62 | 3 | .003 | 5.7 |
| 54397 | 7 | 17.96 | .0343 | .0062 | 1.98 | 3 | .0022 | 6.4 |
| 54396 | 6 | 22.76 | .0153 | .0035 | 1.12 | 3 | .0006 | 3.9 |
| 54395 | 10 | 35.40 | .0184 | .0065 | 2.08 | 4 | .0012 | 6.5 |
| 54363 | 16 | 15.75 | .021 | .0033 | 1.06 | 3 | .0007 | 3.3 |
| 54412 | 14 | 20.20 | .0148 | .0031 | .99 | 7 | .0019 | 12.8 |
| 54413 | 9 | 57.02 | .002 | .0011 | .35 | 1 | ||
| 54414 | 14 | 6.48 | .107 | .0069 | 2.21 | 5 | .008 | 7.5 |
| 54415 | 12 | 10.81 | .0475 | .0051 | 1.63 | 3 | .0022 | 4.2 |
| 54416 | 15 | 64.44 | N.D. | N.D. | ||||
| 54410 | 11 | 10.11 | .061 | .0062 | 1.98 | 3 | .003 | 4.9 |
| 54409 | 10 | 40.48 | .0188 | .0076 | 2.43 | 4 | .0022 | 11.7 |
| 54408 | 9 | 9.19 | .075 | .0069 | 2.21 | 3 | .006 | 8.0 |
| 54405 | 8 | 45.30 | .0089 | .0040 | 1.28 | 3 | .0004 | 4.5 |
| 54406 | 12 | 14.87 | .0216 | .0032 | 1.02 | 3 | .0016 | 7.4 |
| 54407 | 4 | 49.15 | .0125 | .0061 | 1.95 | 3 | .0004 | 3.2 |
| 55227 | 13 | 20.83 | .0142 | .0030 | .96 | 3 | .0004 | 2.82 |
| Upper Sibley coal | ||||||||
| 55220 | 6 | 49.15 | .0033 | .0016 | .51 | 3 | .0001 | 3.3 |
| 55224 | 12 | 36.20 | .0062 | .0022 | .70 | 3 | .0003 | 4.84 |
| 55225 | 26 | 50.15 | .0027 | .00135 | .44 | 3 | .0001 | 3.7 |
| Lower Sibley coal | ||||||||
| 55223 | 13 | 32.65 | .0037 | .0012 | .38 | 3 | .0002 | 5.41 |
| Upper Williamsburg coal | ||||||||
| 55222 | 6 | 40.87 | .0120 | .0049 | 1.57 | 3 | .0004 | 3.3 |
| 55194 | 7 | 21.91 | .0066 | .0014 | .44 | 3 | .0003 | 4.5 |
| 55113 | 12 | 20.20 | .0575 | .0116 | 3.71 | 4 | .0040 | 7.0 |
| 55114 | 16 | 14.69 | .0543 | .0080 | 2.56 | 5 | .0010 | 1.8 |
| 55226 | 15 | 52.17 | .0029 | .0015 | .48 | 3 | .0001 | 3.45 |
| 55115 | 9 | 35.79 | .0103 | .0037 | 1.18 | 3 | .0001 | 1.0 |
| Lower Williamsburg coal | ||||||||
| 54336 | 6 | 20.24 | .047 | .0095 | 3.04 | 3 | .001 | 2.1 |
| Unnamed coal in Lawrence Shale | ||||||||
| 55221 | 6 | 43.44 | .0211 | .0092 | 2.94 | 6 | .0022 | 10.43 |
| Unnamed coals in Tonganoxie Sandstone | ||||||||
| 5547 | 6 | 20.44 | .0272 | .0056 | 1.79 | 3 | .0019 | 6.9 |
| 5548 | 2.4 | 58.10 | .0038 | .0022 | .70 | 3 | .0003 | 7.9 |
| 5546 | 2.4 | 11.25 | .037 | .0042 | 1.34 | 3 | .0033 | 8.9 |
| Thayer coal | ||||||||
| 55117 | 7 | 15.15 | .0203 | .0031 | .99 | 4 | .0012 | 5.9 |
| Mulberry coal | ||||||||
| 53308 | 22 | 15.03 | .0125 | .00188 | .60 | 4 | .0002 | 1.6 |
| 53309 | 13 | 15.54 | .0148 | .00230 | .74 | 3 | .0004 | 2.7 |
| 53310 | 22 | 19.78 | .0065 | .00129 | .41 | 3 | .0003 | 4.6 |
| 53311 | 23 | 7.06 | .0680 | .00480 | 1.54 | 3 | .0013 | 1.9 |
| 53313 | 40 | 20.80 | .0075 | .00156 | .50 | 3 | .0003 | 4.0 |
| 53314 | 24 | 13.23 | .0184 | .00243 | .78 | 4 | .0013 | 7.1 |
| 53315 | 24 | 18.26 | .0105 | .00175 | .56 | 4 | .0016 | 16.7 |
| 55184 | 28 | 20.41 | .0090 | .0018 | .58 | 4 | .0006 | 6.7 |
| 55185 | 50 | 17.41 | .0035 | .0006 | .19 | 4 | .0001 | 2.9 |
| 55186 | 12 | 19.31 | .0116 | .0022 | .70 | 3 | .0006 | 5.2 |
| 55187 | 24 | 19.06 | .0100 | .0019 | .61 | 3 | .0003 | 3.0 |
| 55188 | 26 | 18.06 | .0130 | .0023 | .74 | 3 | .0008 | 6.2 |
| 55189 | 24 | 23.41 | .0089 | .0021 | .67 | 3 | .0005 | 5.6 |
| 55190 | 36 | 18.96 | .0090 | .0017 | .54 | 6 | .0008 | 8.9 |
| 55191 | 38 | 28.28 | .0045 | .0013 | .42 | 3 | .0003 | 6.7 |
| 55192 | 20 | 22.66 | .0044 | .0010 | .32 | 3 | .0002 | 4.5 |
| 55193 | 45 | 16.65 | .0055 | .0009 | .29 | 3 | .0001 | 1.8 |
| Summit coal | ||||||||
| 5685 | 10 | 48.69 | N.D. | |||||
| Mulky coal | ||||||||
| 53305 | 14 | 11.44 | .0178 | .00204 | .65 | 3 | .0004 | 2.2 |
| 53306 | 14 | 28.96 | .0086 | .00249 | .80 | 3 | .0007 | 8.1 |
| 53307 | 13 | 14.30 | .0290 | .00414 | 1.32 | 4 | .0025 | 8.6 |
| 5691 | 17 | 19.14 | .0044 | .0008 | .26 | 3 | .00057 | 13.0 |
| 5690 | 15 | 10.65 | .0180 | .0019 | .61 | 3 | .00013 | 4.2 |
| 5689 | 8 | 19.06 | .0239 | .0045 | 1.44 | 3 | .0009 | 6.7 |
| 5686 | 6 | 36.02 | .0018 | .0006 | .19 | 3 | .0003 | 12.9 |
| 5684 | 9 | 14.98 | .0236 | .0035 | 1.12 | 3 | .0009 | 3.8 |
| 5589 | 9 | 9.30 | .0525 | .0049 | 1.57 | 4 | .0015 | 2.9 |
| 5595 | 5 | 49.90 | .0025 | .0012 | .38 | 1 | ||
| 5598 | 18 | 30.00 | .0047 | .0014 | .45 | 5 | .0003 | 6.3 |
| 5687 | 21 | 20.65 | .0091 | .0019 | .61 | 3 | .00003 | 0.3 |
| 5682 | 15 | 19.63 | .0048 | .0009 | .29 | 4 | .0006 | 12.5 |
| Bevier coal | ||||||||
| 53312 | 15 | 16.66 | .0134 | .00223 | .71 | 3 | .0006 | 4.5 |
| BN-2-B | 17 | 18.32 | .0101 | .00185 | .59 | 3 | .0011 | 10.9 |
| BN-3-B | 17 | 20.47 | .0044 | .00098 | .31 | 4 | .0006 | 12.5 |
| CR-9-B | 17 | 11.52 | .0274 | .00242 | .77 | 4 | .0032 | 15.2 |
| CR-6-B | 10 | 15.54 | .0153 | .00238 | .76 | 3 | .0003 | 2.0 |
| CR-14-B | 16 | 18.81 | .0078 | .00147 | .47 | 3 | .0004 | 5.1 |
| CR-13-B | 17 | 17.40 | .0135 | .00235 | .75 | 3 | .0006 | 4.4 |
| 54331 | 17 | 10.06 | .0198 | .0020 | .64 | 3 | .0006 | 3.03 |
| 5683 | 16 | 19.14 | .0236 | .0045 | 1.44 | 2 | .0004 | 1.7 |
| 5688 | 13 | 31.18 | .0092 | .0030 | .96 | 3 | .0005 | 5.4 |
| CK-6-B | 24 | 20.31 | .0086 | .00175 | .56 | 3 | .0006 | 7.0 |
| Croweburg coal | ||||||||
| CR-1-C | 12 | 19.60 | .0107 | .00174 | .56 | 4 | .0014 | 15.7 |
| 53143 | 12 | 7.50 | .0209 | .00157 | .50 | 3 | .0009 | 4.3 |
| 5591 | 11 | 7.23 | .0120 | .0009 | .29 | 5 | .0022 | 18.3 |
| 5592 | 8 | 11.41 | .0223 | .0025 | .80 | 4 | .0017 | 7.6 |
| Fleming coal | ||||||||
| 54333 | 9 | 18.53 | .0054 | .0010 | .32 | 3 | .0001 | 1.85 |
| 5590 | 57 | 17.13 | .0083 | .0014 | .45 | 3 | .0005 | 6.0 |
| 5593 | 11 | 9.55 | .0051 | .0005 | .16 | 5 | .0006 | 11.8 |
| 5594 | 9 | 10.21 | .012 | .0012 | .38 | 3 | .0003 | 2.5 |
| 5596 | 8 | 2.95 | .100 | .0030 | .96 | 3 | .0023 | 2.3 |
| 5599 | 12 | 23.45 | .0071 | .0017 | .54 | 3 | .0004 | 5.6 |
| 5597 | 5.5 | 12.34 | .0218 | .0027 | .86 | 3 | .0008 | 3.7 |
| Mineral coal | ||||||||
| CK-4-M | 20 | 13.24 | .0052 | .00069 | .22 | 4 | .0010 | 19.2 |
| CR-8-M | 18 | 15.16 | .0052 | .00079 | .25 | 4 | .00045 | 8.7 |
| CR-4-M | 20 | 19.52 | .0036 | .0007 | .22 | 4 | .0005 | 13.9 |
| 54332 | 16 | 9.75 | .0064 | .0006 | .19 | 3 | .0003 | 4.7 |
| Pilot coal | ||||||||
| 53142 | 9 | 14.98 | .0118 | .00177 | .57 | 3 | .0010 | 8.5 |
| Weir-Pittsburg coal | ||||||||
| 54334 | 13.26 | .0054 | .0007 | .22 | 4 | .0004 | 7.4 | |
| Tebo coal | ||||||||
| 54367 | 29.12 | .0083 | .0024 | .77 | 3 | .0001 | 1.2 | |
| 54368 | 35.18 | .0061 | .0021 | .67 | 4 | .0006 | 9.8 | |
| Underclays | ||||||||
| 5550 | 65.07 | N.D. | ||||||
| 5551 | 96.79 | N.D. | ||||||
| Rash | ||||||||
| 5552 | 26.85 | .0058 | .0016 | .51 | 3 | .0007 | 12.1 | |
| 5549 | 21.15 | .0098 | .0020 | .64 | 3 | .0004 | 4.3 | |
| Refuse piles--Composite of Fleming, Mineral, and Bevier coals | ||||||||
| 54355 | 56.28 | N.D. | ||||||
| 54356 | 42.89 | .001 | .0004 | .12 | 1 | |||
| 54357 | 48.86 | N.D. | ||||||
| 54358 | 70.10 | N.D. | ||||||
| 54359 | 60.21 | N.D. | ||||||
| 54360 | 82.26 | N.D. | ||||||
| 54361 | 20.00 | .0059 | .0012 | .38 | 5 | .0002 | 3.4 | |
| 54362 | 33.33 | .0041 | .0013 | .42 | 4 | .0003 | 7.3 | |
| (N.D.=not detected.) | ||||||||
The range of concentration of germanium found in the samples analyzed was .0018 to .099 percent in the ash, and .0005 to .0116 percent in the air-dry coal (Table 2). Calculated in the more practical unit of ounces per ton of coal, the range of values is 0.16 in Sample No. 5593, Fleming coal of the Cherokee Group, to 3.71 in Sample No. 55113, Williamsburg coal from the Lawrence Shale. On the basis of the current retail price of germanium ($12.90 per ounce), the coals range in value from $2.06 to $47.86 per ton. Evaluation of the coals with respect to, their ash content indicates that the ash of Sample No. 54402, containing 31.69 ounces per ton of ash, would have a value of $408.70 per ton, if no germanium were lost in the ignition. Coal seems to compare favorably with other sources of the metal, both foreign and domestic. Although germanium is a very common constituent of the earth's crust, no deposits of ore-grade germanium minerals have yet been found.
Some investigators have adopted a pessimistic attitude toward the recovery of germanium from coal (Thompson and Musgrave, 1952) because of low concentrations of germanium found in the thicker eastern coals. The analyses shown in this study, however, represent the entire coal at the location sampled, and although the total eastern coals are said rarely to contain as much as 0.003 percent germanium, 55 samples of the coal analyzed in this study showed 0.002 percent or more germanium, and 22 samples contained more than .004 percent germanium.
Ahrens, L. H. (1950) Spectrochemical analysis: Addison-Wesley Press, Inc., Cambridge, Mass., p. 1-330.
Bowsher, A. L., and Jewett, J. M. (1943) Coal resources of the Douglas Group in east-central Kansas: Kansas Geol. Survey, Bull. 46, p. 1-94. [available online]
Chemical and Engineering News (1956) Semi-conductor boom just starting: v. 34, no. 50, p. 6042.
Engineering and Mining Journal (1954) Markets--Trends and prices, Miscellaneous metals, ores, and minerals: v. 155, no. 4, p. 106.
Engineering and Mining Journal (1957) Markets--Miscellaneous metals, ores, and minerals: v. 158, no. 6, p. 71.
Engineering and Mining Journal (1957) (1958) Markets--Miscellaneous metals, ores, and minerals: v. 159, no. 6, p. 24.
Frederick, W. J., White, J. A., and Biber, H. E. (1954) Determination of germanium in coal, coal ash, and flue dust: Anal. Chem., v. 26, no. 8, p. 1328-1330.
Harrison, G. R. (1946) M.I.T. wavelength tables: John Wiley and Sons, Inc., New York, p. 1-429.
Howe, W. B. (1956) Stratigraphy of pre-Marmaton Desmoinesian (Cherokee) rocks in southeastern Kansas: Kansas Geol. Survey, Bull. 123, p. 1-132. [available online]
Machin, J. S., and Witters, Juanita (1956) Germanium in fly ash and its spectro-chemical determination: Illinois Geol. Survey, Circ. 216, p. 1-13.
Meyers, A. T., and Brunstetter, B. C. (1947) Magnetic rotation of the direct current arc in spectrographic analysis: Anal. Chem., v. 19, no. 1, p. 71.
Schleicher, J. A., and Hambleton, W. W. (1954) Preliminary spectrographic investigation of germanium in Kansas coal: Kansas Geol. Survey, Bull. 109, pt. 8, p. 113-124. [available online]
Schoewe, W. H. (1944) Coal resources of the Kansas City Group, Thayer bed, in eastern Kansas: Kansas Geol. Survey, Bull. 52, pt. 3, p. 81-136. [available online]
Schoewe, W. H. (1946) Coal resources of the Wabaunsee Group in eastern Kansas: Kansas Geol. Survey, Bull. 63, p. 1-144. [available online]
Schoewe, W. H. (1951) Coal resources of the Permian System in Kansas: Kansas Geol. Survey, Bull. 90, pt. 3, p. 53-68. [available online]
Schoewe, W. H. (1955) Coal resources of the Marmaton Group in eastern Kansas: Kansas Geol. Survey, Bull. 114, pt. 2, p. 49-112. [available online]
Thompson, A. P., and Musgrave, J. R. (1952) Germanium, produced as a by-product, has become of primary importance: Jour. Metals, v. 4, no. 11, p. 1132-1137.
Tucker, W. P., and Waring, C. L. (1954) Effect of ashing temperatures on the volatility of germanium in low-rank coal samples: Anal. Chem., v. 26, no. 7, p. 1198-1199.
Kansas Geological Survey, Geology
Placed on web April 21, 2009; originally published in May 1959.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/134_4/index.html