Kansas Geological Survey, Bulletin 119, part 6, originally published in 1956
Originally published in 1956 as Kansas Geological Survey Bulletin 119, part 6. This is, in general, the original text as published. The information has not been updated.
The underclay of the Nodaway coal of the Wabaunsee group (Pennsylvanian), in Kansas has been studied with the object of determining its origin. Samples were collected from the underclay and some of the shales next below at 39 localities at approximately 5-mile intervals across Kansas from the Oklahoma border to the Nebraska border. In northeastern Kansas in the position of the Forest City basin the underclay is ash gray and plastic, but in southern Kansas on the Bourbon arch it is yellowish gray, not as plastic, and generally thinner.
Data obtained from mechanical, chemical, and x-ray analyses, and microscopic study of the very fine sand show that the underclay has a different mineralogical assemblage on the Bourbon arch than in the Forest City basin. Furthermore, in most places, the shale is markedly different from the overlying clay.
In the Forest City basin the Nodaway underclay contains illite, kaolinite, and mixed-layer illite-montmorillonite whereas the underlying shale lacks mixed-layer illite-montmorillonite, but has chlorite or chlorite-like clay. The poorly developed underclay on the Bourbon arch more closely resembles the underlying shale. Mechanical analyses show that the underclay in the Forest City basin is finer grained than the shale, and the reverse is true on the Bourbon arch.
Potassium is most concentrated in the topmost portion of the underclay, at the seven localities from which samples were chemically analyzed. Furthermore, where the coal is thick the amount of potassium is greater than under thin coal.
A comparison was made between Nodaway underclay and modern gley (bleached layer under peat), which develops beneath allochthonous peat or autochthonous peat. It is postulated that underclay is "fossil" gley. The characteristics of gley and underclay are due to action of organic compounds that have moved downward from the peat by diffusion. Biotite has been altered to chlorite in the poorly developed underclay, but the "gleying" process has altered chlorite to mixed-layer illite-montmorillonite in the well-developed underclay.
The repetition of varied sorts of sedimentary deposits in remarkably constant stratigraphic sequence is a distinctive feature of the late Paleozoic formations in the Midcontinent region of North America. As now is well known, this characterizes cyclic sedimentation. Much remains to be learned about the lithologic and paleontologic details of these deposits and their significance. For example, only recently clay minerals of the cyclic successions (termed cyclothems) have come to be studied systematically with the object of defining the environments of deposition on the basis of mineral assemblages (Murray, 1954).
The purpose of this study is to add to the knowledge of cyclothems in an area where they are well developed. In one of the early publications describing cyclic sedimentation in Pennsylvanian strata of North America, Weller (1930, p. 122) states:
The presence of an underclay in every cyclical formation is perhaps the most convincing evidence that the cycle of conditions was regional rather than local. It is highly improbable that the long periods of stability necessary for the development of weathering profiles with precisely identical relations to certain stages of sedimentation could have occurred at unsynchronized intervals in numerous local areas. Theoretically, therefore, the underclays should be the most important, most significant, and most persistent members of the cyclical formations. This is substantiated by field observation as the underclays persist more or less uniformly far beyond their accompanying coals and over areas in which other members of the formations change markedly in character and thickness.
Weller's conclusions have been tested by making a sedimentological study of the underclay beneath the Nodaway coal in Kansas, as the subject of this report.
Clay is used for ceramic products, and research that contributes to a better understanding of the petrology of clay deposits aids the ceramic industry. The present study of the variations of an underclay from Oklahoma across Kansas to Nebraska may aid in locating deposits satisfactory for commercial exploitation.
The light-gray layer below most coal beds has been variously referred to as seat earth, ganister, and underclay by previous writers, without proper regard to grain size and texture. In Britain the term seat earth is used widely when reference is made to any gray layer next below coal. Where the seat earth is sandy it is called ganister, and where it is plastic when wet (or at least very fine grained) it is termed an underclay. The terms ganister and seat earth are rarely used in North America, but they should have a place. A gray sandy layer next below coal is not an underclay but a ganister ("undersand").
The following definition of underclay is offered: An underclay is seat earth that is plastic when wet.
After preliminary field investigations in eastern Kansas, the underclay beneath the Nodaway coal was chosen for study for several reasons. It can be positively identified because it lies at a predictable distance below the persistent, readily identifiable Church limestone. In places the Nodaway coal is not accompanied by a well-developed underclay, and elsewhere the underclay is overlain by only a featheredged coal. These relationships are particularly desirable for study because they are judged likely to yield the greatest difference in mineralogical assemblages.
The underclay below the Nodaway coal was sampled at approximately 5-mile intervals across Kansas from Oklahoma to Nebraska. At each locality the outcrop was cleaned carefully so that fresh channel samples of the clay and shale could be obtained, leaving no unsampled intervals. Portions to be sampled were chosen on the basis of lithologic changes. Few samples represent more than 0.5 foot of rock and many were only half that. About 3 pounds of underclay or shale were considered representative for each bed. Laboratory studies included mechanical, chemical, and x-ray analyses, and microscopic study of detrital minerals. Details of methods are discussed in the separate section.
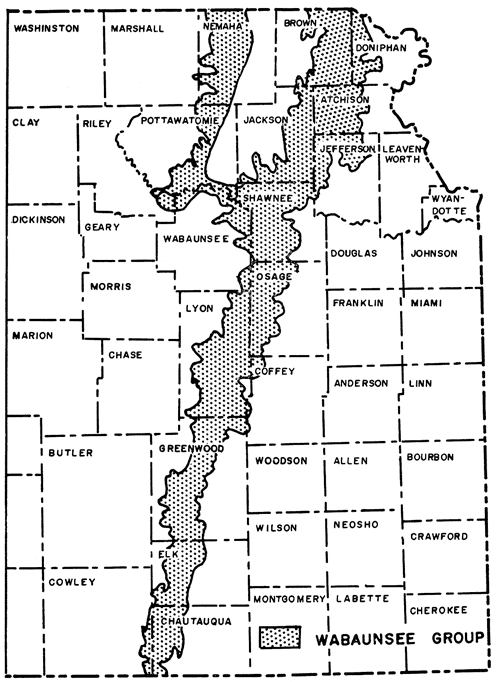
The Nodaway underclay, situated next below the Nodaway coal, is a persistent unit of the Howard formation, which is the lowermost limestone of the Sacfox subgroup of the Wabaunsee group, separated from the Shawnee group below by the Severy shale. Wabaunsee rocks comprise the uppermost beds of the Virgilian Series in Kansas, underlying beds that are classed as Lower Permian. These rocks crop out in a northeast-southwest belt in eastern Kansas (Fig. 1). Outcrops of the Howard formation extend across Kansas from Doniphan, Brown, and Nemaha counties in the northeast to Chautauqua County on the Kansas-Oklahoma border. Figure 2 shows the lower members of the Howard limestone formation.
Figure 1--Map showing outcrop of Wabaunsee group in Kansas (after Moore, 1949).

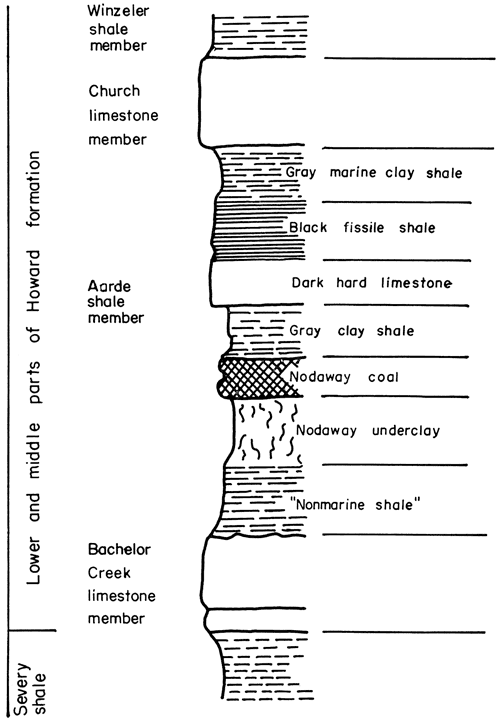
Figure 2--Graphic section of lower and middle parts of Howard formation, Wabaunsee group.
Special thanks are extended to Miss Ada Swineford for advice and assistance in the techniques of x-ray and mechanical analyses, to Mr. Russell Runnels for the chemical analyses, and to Mr. William Miller for x-ray analyses.
The Severy shale (Pl. 1, 3), lowermost unit of the Wabaunsee group, conformably overlies the Topeka formation of the Shawnee group. In southern Kansas, the Severy is bounded at the top by the Bachelor Creek limestone member of the Howard formation, but north of the Osage County the Bachelor Creek limestone is absent. Accordingly, in northern Kansas the Aarde shale (in the type area comprising strata between Bachelor Creek and Church limestones) directly overlies the Severy. Because demarcation of a shale-on-shale boundary is impracticable, the term Severy-Aarde shale has been introduced to designate Severy combined with the lower shale member of the Howard (Moore, 1949).
Plate 3--Lower and middle parts of Howard formation and upper part of Severy shale in sec. 3, T. 34 S., R. 9 E., 1 1/2 miles west of Wauneta, Chautauqua County; (a) Aarde shale including prominent massive limestone, (b) Bachelor Creek limestone, nodular, nonpersistent, red and green, (c) Church limestone, (d) Severy shale, sand unit below notebook.

The Severy shale is thinly bedded, sandy, yellowish brown, and blue gray. Because it lacks hard calcareous bands, topographic discontinuities do not interrupt the smooth slope below the Howard formation, except in southern Kansas where a hard sandstone band commonly forms a bench. No fossils were observed, but Moore (1949) reports "small brachiopods and some other invertebrates are abundant locally just below the Howard formation." Average thickness of the Severy shale across Kansas is about 75 feet.
Haworth (1898, p. 67) reported the geological investigations of G. I. Adams, who recognized that the Howard formation ("Howard Limestones") is a mappable unit. Although Adams realized that the Howard extends over a wide area, he confined his study to the region between the Oklahoma border and Eureka, Kansas.
The Howard formation is a persistent unit of the Wabaunsee rocks and ranges in thickness from about 8 to 30 feet. Except in the glaciated area of northeastern Kansas, the beds form a distinct escarpment, because they are overlain by White Cloud shale and underlain by generally soft Severy shale.
The Howard limestone is best developed near Howard, Elk County, Kansas, where it is composed of three limestone members and two shale members (Moore, 1935). These are called, in upward order, Bachelor Creek limestone, Aarde shale (includes Nodaway coal and underclay), Church limestone, Winzeler shale, and Utopia limestone.
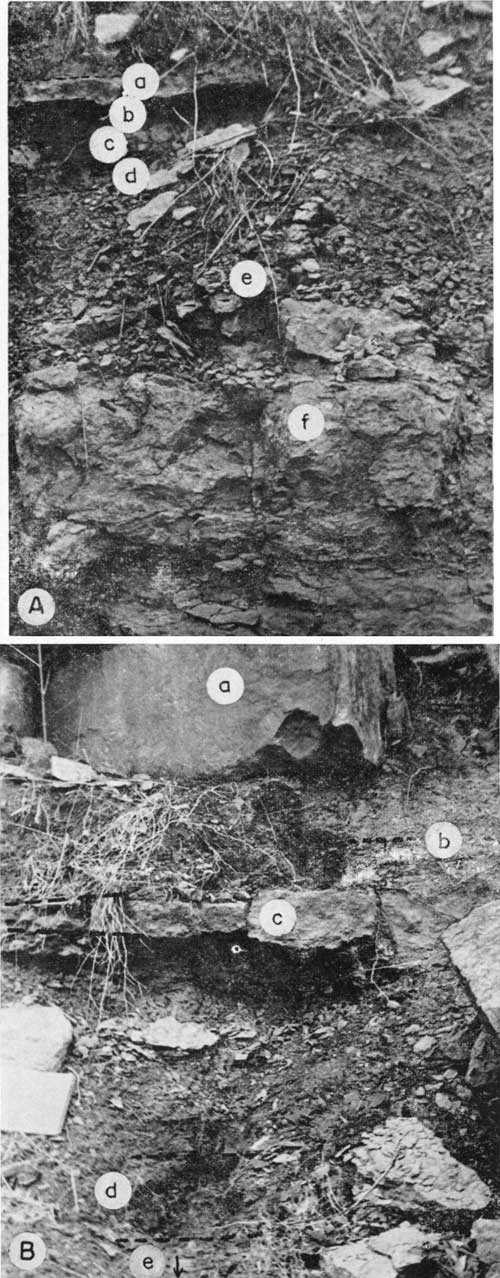
Bachelor Creek limestone member--The lowermost member of the Howard limestone, called Bachelor Creek limestone (Fig. 2), persists from northern Osage County, Kansas, about 30 miles south of Topeka, southward into Oklahoma. In northern Elk and Greenwood counties, this member is a massive slightly arenaceous limestone, mottled bluish gray and yellowish brown, and it conformably overlies thick beds of calcareous sandstone belonging to the Severy shale. Generally, in southern Elk and Chautauqua counties, the massive Bachelor Creek (Pl. 3) gives way to a thin, irregular, reddish to green nodular bed. Where the slightly arenaceous limestone is overlain by underclay or thin shale, the upper part is almost everywhere pitted and soft (Pl. 4A) and weathers to a steep slope. The Bachelor Creek member ranges from a few inches to 7 feet thick. Among its invertebrate remains are crinoid stems, bryozoans, and a few brachiopods. In Elk County, Verville (1951) found that this limestone contains Myalina, Dictyoclostus, small gastropods, and echinoid spines.
Plate 4--A. Bachelor Creek limestone and lower part of Aarde shale in sec. 17, T. 27 S., R. 11 E., 1 mile south of Climax, Greenwood County: (a) hard nonpersistent limestone unit of Aarde shale, (b) marine shale, (c) Nodaway coal, (d) underclay, (e) nodular, rubbly upper part of Bachelor Creek limestone, (f) lower part of Bachelor Creek limestone. B. Lower and middle parts of Howard formation in sec. 17, T. 22 S., R. 12 E., on bank of Verdigris River 1 1/2 miles east of Madison, Greenwood County: (a) Church limestone, (b) black fissile shale, (c) nonpersistent limestone of Aarde shale, (d) zone of Nodaway coal with underclay below, (e) sandy Bachelor Creek limestone.
Aarde shale member (including Nodaway underclay)--The Aarde shale (Pl. 4B) is separated into seven distinct units on the basis of lithology (Moore, 1949). They are, in upward order, (1) gray clay shale, (2) Nodaway underclay, (3) Nodaway coal, (4) gray clay shale, (5) dark hard limestone, (6) black fissile shale, and (7) gray marine clay shale. As noted previously, strata in the northern part of Kansas equivalent to the Aarde shale have no evident boundary with the Severy shale. The Nodaway coal, its underclay, and black fissile shale overlying the coal persist from Oklahoma to Nebraska, however. Thickness of the Aarde shale ranges from 1 foot in the south to slightly more than 12 feet in Osage County.
(1) Nonmarine shale--A nonmarine gray clay shale ranging from slightly less than 0.1 inch to 2 feet in thickness overlies the Bachelor Creek limestone from northern Greenwood County southward into Oklahoma. To the north, where the Bachelor Creek limestone is not present, the nonmarine shale overlies the Severy shale without apparent unconformity. At many places this shale is not present between the underclay and the Bachelor Creek. In few places is the upper contact sharp, for this stratum grades upward to structureless underclay within a thickness of 2 to 5 inches, except at one locality (sec. 12, T. 28 S., R. 10 E.), where it is overlain directly by the Nodaway coal.
Ferric oxides or hydrates impart a yellowish-brown color to the shale. Rafted, black carbonaceous remains of stems, stalks, and leaves, almost invariably arranged parallel to the bedding, are sufficiently abundant in some places to give a gray color. No invertebrate remains were observed.
(2) Nodaway underclay--The Nodaway underclay (Pl. 4 A, B) is situated between the Nodaway coal and the lowermost unit of the Aarde shale. It occurs everywhere beneath the Nodaway coal except at sec. 12, T. 28 S., R. 10 E., where it is not developed. Typically it is ash gray, but variations include gray, blue gray, olive green, and yellowish green. The yellows are most common where the Aarde shale member is thin. No consistent relationship exists between the thickness of coal and underclay, which ranges from a thin film to 1.7 feet in thickness.
Plant fragments, including twigs, stalks, and leaves, are preserved as flattened carbon "slicks". In the northern part of Kansas, fine black carbonaceous matter is finely disseminated throughout the upper few inches of underclay, the greatest concentration occurring next below the coal.
In all places the Nodaway underclay is plastic, but more so where is is ash gray. When dry, it is hard and shows a characteristic "nutty structure". [Note: Soil-structure term meaning that angular fragments crudely have the same shape (Joffe, 1936, p. 50, pl. 2).] It is important to note that iron is not removed from the underclay, but has been translocated short distances within the unit (see section "Chemical Analyses"). At almost every observed locality, ferric oxide is concentrated so that it stands out as rusty streaks or mottled yellow spots. It may be concentrated in very thin seams just below the coal, or near the middle, or at the bottom of the underclay. Commonly it is concentrated in thin tortuous sheets between the nuts.
Calcium carbonate, selenite blades, and pyrite concretions are rare, being found at only a few localities. Horizontal streaks of vitreous coal are common within the uppermost portions of the underclay.
Stigmarias (fossil roots) were observed only at sec. 5, T. 16 S., R. 15 E., Osage County. Rootlets protrude horizontally from the main vertical root into the underclay.
Slickensides are not common in the Nodaway underclay, but do occur at sec. 4, T. 32 S., R. 10 E., sec. 29, T. 15 S., R. 15 E., and sec. 12, T. 11 S., R. 15 E. They resemble tiny striated faults with shiny slippage surfaces, all of which are slightly curved.
At most places the upper contact of the Nodaway underclay is sharp, but at a few places it is gradational, especially where the coal is represented only by carbonized impressions of leaves, twigs, and stalks.
(3) Nodaway coal--Where exposed at natural outcrops, old strip pits, or road cuts, the Nodaway coal (Pl. 4 A, B) is dull and slightly rusty, and breaks easily into small angular fragments. Where not exposed to weathering, it is "bright, shiny, black, brittle and moderately hard" (Schoewe, 1946, p. 27). It was not observed to contain prominent clay bands, except at sec. 23, T. 10 S., R. 16 E., Shawnee County. Schoewe (1946, p. 26) reports that "close examination reveals that it is composed of many thin layers from one-sixteenth to one-half inch thick which give the coal a laminated appearance. The uppermost one-quarter inch or less contains extremely matted highly carbonized material showing the impression and structure of the woody material from which the coal was derived." Chemically, the coal is variable from top to bottom, but seemingly there is no definite order to the changes (Schoewe, 1946, p. 28).
The Nodaway coal, or coaly equivalent, persists without interruption across Kansas from Oklahoma to Nebraska. Its thickness ranges from a featheredge in Chautauqua and Elk counties to 1.2 feet in Shawnee County.
(4) Gray clay shale--Between the Nodaway coal and the dark hard limestone lies a gray clay shale (Pl. 4A.) that tends to show wide variation in thickness, calcareousness, and contained fossils, but these variations were not studied in detail. The gray clay shale is noncalcareous except locally. In some places it contains brachiopods and crinoid stems, but elsewhere abundant plant remains are found without any invertebrates. The thickness ranges from 0.2 to 8.5 feet. Where dark limestone is absent above, the shale is regarded as continuing upward to the black fissile shale unit of the Aarde shale.
(5) Dark hard limestone--This unit (Pl. 3, 4 A, B) of the Aarde shale member extends from central Osage County across southern Kansas into Oklahoma. The bed is dense, vertically jointed, and dark gray on the fractured surface, but weathers brown. Verville (1951) reports the presence of Chonetes, Hustedia, and Lophophyllum in the dark hard limestone. It thins northward from 1:5 feet in Chautauqua County to an average of 0.4 foot in Osage County, where it has a tendency to pinch and swell.
(6) Black fissile shale--Black fissile shale (Pl. 4B) extends from Oklahoma across Kansas into Nebraska as a distinctive, persistent unit of the Aarde shale. In most places the unit directly overlies the dark hard limestone, where present, but locally a gray, laminated shale separates the two. Verville (1951) identified Streptognathodus, Cavusgnathus, Crurithyris, and rare Hustedia in the black shale of the Aarde in Elk County. This unit ranges from 0.1 to 1.7 feet in thickness and is separated from the Church limestone by a gray clay shale.
(7) Gray marine clay shale.-The uppermost division of the Aarde shale is a well-laminated gray clay shale unit, which constitutes the most fossiliferous part of the member. Its fauna includes many marine fossils, such as Bairdia, Amphissites, Triticites, Rhombopora, Chonetes, Crurithyris, and Punctospirifer (Verville, 1951). Thickness of the marine clay shale ranges from 0.3 to 1.5 feet.
Church Limestone Member--The Church limestone (Fig. 2, Pl. 3, 4B) was easily traced from Oklahoma across Kansas to Nebraska. It is vertically jointed, hard, dense, and breaks with subconchoidal fracture. Fresh surfaces are blue gray, but the weathered surfaces are brownish blue to rich brown. Verville (1951) reports that the Church contains assorted brachiopods, bryozoans, fusulinids, and ostracodes. It ranges in thickness from 1.2 to 6 feet.
The Winzeler shale and Utopia limestone members in the upper part of the Howard formation were not considered in this study.
Moore (1935) states that the Howard formation is transitional between the well-developed megacyclothems of the Shawnee group below and the simple cyclothems of the Wabaunsee group above. He suggests that "Cyclothem A" of the typical megacyclothem is represented by the Bachelor Creek member and the shale next below the Nodaway underclay; "Cyclothem B" includes all units up to the black fissile shale; "Cyclothem C" contains the black fissile shale and all the units above in the Howard formation.
The gentle arch in Greenwood and Elk counties shown on Plate 1 coincides with the area occupied by the Bourbon arch (Jewett, 1951, p. 121). Northward in Osage and Shawnee counties, in the southern part of the Forest City basin (Lee, 1943), the Nodaway coal is separated from the bed of black fissile shale above by strata that are 9 feet thicker here than on the Bourbon arch. This supports an opinion expressed by Wallace Lee (personal communication) that tectonic movements affecting the Bourbon arch and Forest City basin were still active during early Wabaunsee time.
During the middle of the 19th century two hypotheses were proposed to account for the origin of underclay, each consequent on hypothese regarding the origin of coal. The autochthonous (or in situ) hypothesis of coal formation, which was first offered, holds that underclay is "fossil" soil. Many relationships support this analysis and it is held by some geologists to be the most plausible explanation of underclay (Moore, 1940). The adoption of this view is due in part to the excellent presentation of the theory by W. E. Logan in 1840 to the Geological Society of London (Logan, 1842). The hypothesis gained additional prestige when sanctioned by Sir Charles Lyell.
Later it was postulated that underclay is not a "fossil" soil, but part of a normal sedimentary cycle, wherein coal, in the form of vegetal debris, was rafted to its present location. Unfortunately for the allochthonous theory, its supporters seemed to muster only negative arguments.
The histories of the two hypotheses follow, with emphasis on their bearing on underclay.
Joffe (1936, p. 37) states that paleopedologists regard soils not found at the earth's surface as "buried soils". Joffe revised Marbut's definition so that it was no longer necessary to consider a soil as the "outer layer of the earth's crust", although he retained the term "biological characteristics". When the "biological characteristics" of a soil are no longer a factor after burial, however, the soil can be regarded as fossilized. Because of the limited use of the term "fossil" soils, the term will be used in quotation marks.
Perhaps the first statement that underclay is a "fossil" soil was made by Dechen in 1832 (Potonie, 1910, p. 116), but it was left to Logan to establish the hypothesis firmly. After his study of more than 100 coal seams in Wales, Logan (1842) concluded that Stigmaria ficoides, found in the underclay, is related to coal, and that the underclay represents soil on which the coal-forming plants grew. It should be emphasized that he only implied equivalence of underclay to a soil, and that he did not directly state it in this way in the reference cited. According to Harrington (1883, p. 63), "Logan had the sagacity to observe and turn to account a fact which has settled forever the question of the origin of coal in favour of the theory of growth in situ." Concerning thick coal beds at Mauch Chunk, Pennsylvania, Logan wrote, "But independent of the astonishment arising from the thickness of the seam, I felt great delight at finding under it my underclay in an its glory, crossed by fibres coated with carbonaceous matter, and presenting precisely the character of the underclay of the South Wales seams." After a visit to Pictou, Nova Scotia, he says, "I now know what the ground contains for about a mile deep in that neighbourhood, and in every case where I have seen a seam of coal it is accompanied by one of underclay filled with Stigmaria ficoides. My fact, therefore, I now consider beyond controversy" (Harrington, 1883).
Lyell (1845, p. 118-160) cited the Great Dismal Swamp in North Carolina as support for the autochthonous hypothesis and for his statement that underclays in Europe and North America, containing Stigmaria, are indications of an equal number of soils.
After such enthusiastic remarks, many other geologists regarded the underclay as soil on which the coal-forming plants grew. The first volume of the Geological Survey of Illinois states that underclay "was the original subsoil on which the vegetation grew" (Worthen, 1866, p. 59). Hutchings (1894), in England, held the same belief while studying the detrital minerals of fireclays. Potonie (1910) supported the hypothesis by comparing the coal-underclay profile to modern moors. Also, he described in minute detail a Stigmaria showing radiating appendages, which he said were fossil rootlets. White and Thiessen (1913) state that roots are present in 26 underclays out of 33 that they studied.
One of the first petrographic investigations of underclay was that by Chapman (1914), who concluded that the Yorkshire underclays are "fossil" soils, as demonstrated by their mineral composition, microstructural characteristics, and chemical relationship with the shale next below.
A variation of the autochthonous hypothesis was introduced by Stout (1923) at the end of a lengthy, detailed publication on coal formation clays of Ohio. He makes no definite statement that underclay is a soil, but the implication is strong. Briefly, according to Stout, underclay is a record of oxidizing conditions, and coal is a record of reducing conditions. While underclay and coal were being deposited, "plant debris" was uniformly distributed, but conditions for preservation varied from place to place and at different times. Oxygen-bearing water circulated freely and destroyed the plant debris until the floor of the swamp started to rise, but as emergence continued the water became more stagnant because of the lack of free circulation, and the resulting reducing conditions facilitated the preservation of plant parts.
Moore (1940, p. 165) believes that underclay was deposited as part of a normal sedimentary succession, but the growing plants helped to destroy stratification and extracted certain soluble salts.
Duparque (1948, 1949) regards underclay as a "fossil" soil, and its upper surface as an erosional one on which plant debris from other localities settled. He admits that this explanation, applied to Westphalian underclays of northern France, does not apply to the rocks studied by Grand 'Eury, for these contain stumps in addition to drifted material, both of which contribute to formation of the coal.
Gresley (1887) was an early opponent of the "fossil" soil theory of underclay. For example, he called for an explanation of the following: (1) Stigmarian clays without coal; (2) the sharp contact between underclay and coal, whereas it should be gradational if the underclay is "fossil" soil; (3) why Stigmaria never pass into the coal bed.
More recently, however, the numerous competent workers who do not adhere to the "fossil" soil hypothesis propose substitutes. Weller (1930) presented arguments to support his opinion that underclay is analogous to a gumbotil. Because underclays are not universal below coal, nor coal universal above underclay, Weller believed that the two are not genetically related. He states that "in almost every case a poorly drained profile of weathering is at least suggested, and that in many places it is well developed." After studying gumbotil profiles on Illinois drift, Weller concluded that underclays were formed similarly under oxidizing conditions, on land surfaces relatively free from erosion or deposition.
A petrographic and mineralogical study by Allen (1932), in Illinois, can be regarded as one of the first important works that do not rely on the "fossil" soil theory to explain underclay. Allen compared beds of underclay of the Pennsylvanian Period with modern soil profiles of poorly drained areas with gumbotil. He concluded that potash beidellite was "purified" and deposited in water with other detrital minerals. Minor leaching transferred calcium carbonate to a lower horizon. Allen also found that underclay is more similar to young, unleached Wisconsinan tills than to older, leached tills of Kansan and Illinoisan age. Later, more information was provided to indicate that underclay was not due to soil-forming processes (Grim and Allen, 1938).
Stainier (1934) found that most Stigmarias have no rootlets. He attributed their absence to abrasion during transportation in water. Later he (Stainier, 1935, 1937) drew additional conclusions from detailed descriptions of 30 underclays, all of which suggest that underclay is not a "fossil" soil. Concerning the relationships he wrote: (1) there should be greater abundance of carbonaceous matter in underclay because distillation of plant parts can be effected there to a greater extent than in shale; (2) dark shale below coal should have greater resemblance to decolorized material at the bottom of the peat in bogs; (3) presence of more rootlets in the bottom than in the top parts of underclay is the reverse of that observed in soils. In a later paper, Stainier (1940) cites fossils such as Lingula and Productus closely associated with coal, and holds that this makes it necessary to regard underclay and coal as part of a marine sequence.
Moore (1940, p. 165) advances one other possibility based on a statement by Mietzsch, who attributes 22 to 26 percent of the ash of living Lycopodiaceae to clayey matter. He suggests that perhaps underclay represents the remains of decayed plants of this type.
Schultz recently (1954) made a valuable contribution of methods and techniques in his study of 200 underclay samples collected in North America in the Midcontinent area, Illinois basin, and Appalachian basin. Schultz believes that underclay results from sedimentation of clay that had its origin in soil profiles at some distance from the site of final deposition. After several pauses in transit from the soil profile to the coal basin, the clay was flocculated by organic compounds at the site of deposition.
The Nodaway underclay and the shale directly below were mechanically analyzed in order (1) to determine variation across Kansas from the Oklahoma boundary to northern Doniphan County, and (2) to find the size relationships between the underclay and shale next below.
Samples from the top of the Nodaway underclay at each of the 39 localities in Kansas where this stratum was studied (Appendix 1) and of the nonmarine shale directly below at 12 localities (Table 1) were analyzed.
Table 1--Localities from which samples of nonmarine shale were obtained for mechanical analyses.
| County | Location of sample | Depth below coal, inches |
|---|---|---|
| Chautauqua | Sec. 3, T. 34 S., R. 9 E. | 14-18 |
| Greenwood | Sec. 12, T. 28 S., R. 10 E. | 6-11 |
| Greenwood | Sec. 36, T. 26 S., R. 10 E. | 3-8 |
| Greenwood | Sec. 33, T. 24 S., R. 11 E. | 5-11 |
| Greenwood | Sec. 1, T. 24 S., R. 11 E. | 4-7 |
| Lyon | Sec. 6, T. 21 S., R. 13 E. | 10-15 |
| Osage | Sec. 32, T. 18 S., R. 14 E. | 7-15 |
| Osage | Sec. 34, T. 17 S., R. 14 E. | 7-23 |
| Osage | Sec. 25, T. 16 S., R. 14 E. | 14-24 |
| Shawnee | Sec. 27, T. 11 S., R. 15 E. | 6-14 |
| Jefferson | Sec. 22, T. 9 S., R. 17 E. | 14-28 |
| Doniphan | Sec. 15, T. 3 S., R. 19 E. | 3-12 |
To obtain a representative portion of the field sample to be analyzed, a 2- or 3-gm portion was selected from each of the lumps of underclay. In this way a 20- to 30-gm random sample was prepared.
The samples were dispersed with sodium oxalate and analyzed by the pipette method as described by Krumbein and Pettijohn (1938).
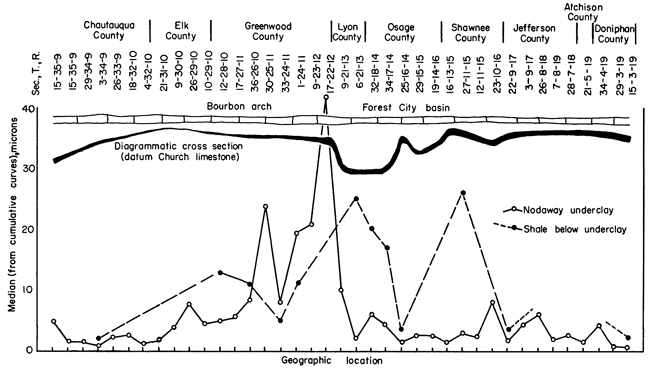
These mechanical analyses (presented in tabular form as Appendix 2) were assembled on 5-in. by 5-in. semi-log paper into cumulative curves, from which was read the median size in microns. These median values in microns are plotted against geographic location in Figure 3. One curve on the figure shows the variations in fineness of the underclay from south to north across Kansas. The striking fact is the relative coarseness of the underclay between sec. 10, T. 29 S., R. 10 E., and sec. 6, T. 21 S., R. 13 E. (Greenwood County), which area overlies the northern flank of the Bourbon arch (Pl. 1).
The medians of the size analyses of the shale next below the underclay in 12 locations also are plotted on Figure 3. This line shows that the coarsest shale is found in the Forest City basin, the finest on the Bourbon arch.
Grain-size relationships within the underclay-shale profile [note: term introduced here to shorten reference to the underclay and the upper part of the shale directly below.] are also illustrated in Figure 3, where it can be seen that in sec. 33, T. 24 S., R. 11 E., and sec. 1, T. 24 S., R. 11 E. (Greenwood County), the underclay is coarser than the shale directly below.
Figure 3--Relationship of median particle size, as determined by mechanical analyses, to position on structure.
Grains having a size range of 0.06 mm to 0.125 mm from selected underclays and shales were studied qualitatively in order to determine differences in gross mineralogical assemblages. These differences, which reflect variations in source rock or weathering history, contribute to an explanation of differences in the assemblages of clay minerals. Eighteen localities distributed across Kansas from the Oklahoma boundary to the northern part of Atchison County were chosen as representative of the Nodaway underclay. The localities are listed in Table 2.
Table 2--Localities from which samples were obtained for microscopic study. *Indicates shale next below Nodaway underclay was also studied microscopically.
| County | Location |
|---|---|
| Chautauqua | Sec. 15, T. 35 S., R. 9. E.* |
| Chautauqua | Sec. 20, T. 34 S., R. 9 E. |
| Chautauqua | Sec. 27, T. 33 S., R. 9 E.* |
| Chautauqua | Sec. 4, T. 32 S., R. 10 E. |
| Elk | Sec. 21, T. 31 S., R. 10 E. |
| Elk | Sec. 9, T. 30 S., R. 10 E. |
| Elk | Sec. 10, T. 29 S., R. 10 E. |
| Greenwood | Sec. 12, T. 28 S., R. 10 E. |
| Greenwood | Sec. 17, T. 27 S., R. 11 E.* |
| Greenwood | Sec. 36, T. 26 S., R. 10 E. |
| Greenwood | Sec. 33, T. 24 S., R. 11 E.* |
| Greenwood | Sec. 9, T. 23 S., R. 12 E.* |
| Lyon | Sec. 9, T. 21 S., R. 13 E.* |
| Osage | Sec. 32, T. 18 S., R. 14 E.* |
| Osage | Sec. 34, T. 17 S., R. 14 E. |
| Shawnee | Sec. 27, T. 11 S., R. 15 E.* |
| Shawnee | Sec. 22, T. 9 S., R. 17 E.* |
| Atchison | Sec. 21, T. 5 S., R. 19 E.* |
The same samples of underclay and shale were used for both microscopic and x-ray study. Samples weighing 20 to 30 grams were used.
The sample was dispersed in distilled water containing NH4OH, and clay finer than. 2 microns was separated for x-ray study by settling and decantation.
The remainder was boiled for 1 hour with dilute HCl to remove iron oxide from the sand grains. Unfortunately, this process also removed other constituents such as carbonates, sulfides, chlorite, and apatite.
After removal of grains finer than sand size (< 60 microns) by decantation, the sand was dried and sieved into three sizes: (1) larger than 0.25 mm, (2) 0.125 mm to 0.25, and (3) less than 0.125 mm. The 60- to 125-micron fraction was chosen for study of detrital material because it was the largest part of most samples.
A representative portion of each sample was immersed in oil of refractive index 1.540 and examined with a petrographic microscope.
Each important mineralogical constituent in the 0.0625- to 0.125-mm size range of the underclay and underlying shale is described.
Quartz--In general two types of quartz grains, which may grade into each other, are present. The more common type occurs as colorless, vitreous, subangular grains, which commonly have conchoidal fracture, and are devoid of crystalline inclusions except for minor unidentified, hairlike inclusions (rutile?) in some grains. The second type of quartz is subround to round, lacks fracture, and is colorless, but contains abundant inclusions of minerals (some identified tentatively as tourmaline and zircon). Quartz grains of this size are more abundant in the shale than in the underclay above.
Feldspars--Plagioclase was noted more commonly than orthoclase. Only a few scattered grains of angular, fairly clear, slightly kaolinized orthoclase were identified on the basis of Carlsbad twinning or refractive index. In general, feldspars occur more commonly and in a less altered state in the shale than in the underclay. The plagioclase studied is probably sodic. All grains are angular and show polysynthetic twinning, and most have prism faces and ragged ends. Almost all grains are cloudy, owing to kaolinization, or because of inclusions of zircon, tourmaline, or both. The underclay contains light-brown angular grains, which exhibit relict twinning under crossed nicols. These undoubtedly are altered plagioclase.
Muscovite--Most samples studied contain readily detectable amounts of muscovite, from traces to 20 percent. Flat, subcircular flakes of muscovite are colorless to greenish, have undulatory extinction, and are relatively clear, except for rare inclusions of zircon and tourmaline. An unsuccessful attempt was made to relate amount of muscovite in either the underclay or the shale to position relative to the Bourbon arch. The only generalization that can be made is that in Chautauqua County muscovite is not important in the underclay and shale. Farther north, in Greenwood County, it comprises as much as 20 percent of the size fraction studied.
Biotite--The flakes are flat, subcircular, brown to greenish brown, and pleochroic. Biotite is restricted to the shale next below the underclay and occurs at five localities, all but one of which are north of the Bourbon arch. Table 3 lists the localities where biotite is found in nonmarine shale. In sec. 32, T. 18 S., R. 14 E., the greenish-brown variety (Milner, 1952) is common.
Table 3--Localities where samples of nonmarine shale contain biotite. All samples contain less than 5 per cent in very fine sand.
| County | Location |
|---|---|
| Greenwood | Sec. 12, T. 28 S., R. 10 E. |
| Osage | Sec. 32, T. 18 S., R. 14 E. |
| Osage | Sec. 34, T. 17 S., R. 14 E. |
| Shawnee | Sec. 27, T. 11 S., R. 15 E. |
| Atchison | Sec. 21, T. 5 S., R. 19 E. |
Chlorite--("Chlorite" refers to the chlorite group (Milner, 1952).) The flakes are flat, subcircular to ragged, green to very pale green, pleochroic, and clear. Some show "ultra blue" extinction. Chlorite persists in various amounts in the shale across Kansas from Oklahoma to Nebraska, but in the underclay it is not everywhere present. Treatment with hot dilute hydrochloric acid probably removed most of the chlorite, making it impossible to get a true percentage relationship within the underclay-shale profile.
Zircon and tourmaline--These constituents were found in all samples studied. Zircon occurs as subrounded grains and as prismatic tetragonal crystals. Grains are colorless to light purple; one grain from sec. 4, T. 32 S., R. 10 E., is yellow. They are vitreous and adamantine, have conchoidal fracture, and some colored grains are pleochroic.
Most of the tourmaline grains are olive green, pleochroic, and rounded, some having conchoidal fracture. Some grains are trigonal, striated crystals, most of which have ragged ends.
Rutile--Two types of rutile are found. Small striated "foxy-red" euhedral crystals are sparsely scattered in the underclay-shale profile, mostly as knee-shaped twins, and are judged to be authigenic. Needles of rutile of this type were noted in the silt size, also. Reddish-brown and amber rounded grains, tending to have conchoidal fracture, are more numerous than the crystalline variety. Both kinds are commonly pleochroic. Rutile of this size range extends from the Oklahoma border northward across Chautauqua, Elk, and Greenwood counties, but farther north only one grain was found, that in sec. 27, T. 11 S., R. 15 E. Chemical analyses show that rutile is by no means restricted to the south, but the size range studied was restricted. A survey of literature reveals that rutile occurs in most underclays (McCaughey, 1923; Grim and Allen, 1938).
Pellets--Rounded aggregates of assorted minerals, referred to as "pellets" by Allen (1932), are a prominent constituent of the underclays in the three size ranges prepared. Typically the particles comprising the pellets project at random from the surface of the pellets, presenting a microscopically ragged aspect. Quartz and very fine muscovite (sericite) and clay minerals are the main constituents. Two types of pellets were observed. One is light brown with black or brown granules locked within the pellet. The second is clear, devoid of dark granules, and in general lacks carbonaceous matter. Variants between these two types are common.
The brown color of the pellets is due to neither iron oxide nor carbonaceous matter, because the color persisted even though all samples were boiled in dilute hydrochloric acid and some were boiled for 4 hours in 6 percent hydrogen peroxide. The sample as a whole does bleach after treatment with hydrogen peroxide, but microscopic inspection indicates this is due to oxidation of detrital carbonaceous matter and not to a basic change in color of the pellets.
As much as 95 percent of the 0.125- to 0.06-mm size grade of Nodaway underclay may be composed of these pellets. Shale next below the underclay commonly contains the same types of pellets, but not in such large amounts. This relationship is consistent at every locality investigated between the Oklahoma border and Nebraska.
The Geochemistry Division of the State Geological Survey chemically analyzed selected samples of Nodaway underclay and shale from the seven localities. Values in percent of silica, alumina, iron oxide, titanium oxide, calcium oxide, magnesium oxide, sodium oxide, potassium oxide, sulfide sulfur, sulfate sulfur, phosphorus pentoxide, and loss on ignition are given in Table 4. and SiO2/R2O3 ratios have been calculated and are included in Table 4.
Table 4--Chemical analyses.
| Location (Sec., T., R.) and County |
Sample depth below coal |
Percentage of constituents | SiO2/Al2O3 | SiO2/R2O3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | P2O5 | SO3 | K2O | Na2O | S | Loss on ignition |
Total | ||||
| 15-35-9 E. Chautauqua |
udc 0-7" | 64.42 | 15.98 | 4.22 | 1.11 | 3.20 | 1.00 | 0.01 | nil | 2.50 | 0.48 | trace | 6.86 | 99.78 | 4.03 | 3.02 |
| trans 7"-15" | 65.52 | 15.09 | 4.09 | 1.18 | 3.31 | 0.94 | trace | nil | 2.32 | 0.57 | trace | 6.69 | 99.71 | 4.35 | 3.22 | |
| 12-28-10 E. Greenwood |
sh 0-6" | 61.60 | 18.10 | 5.02 | 1.10 | 0.61 | 2.01 | 0.16 | 0.29 | 3.69 | 1.45 | 0.02 | 5.89 | 99.92 | 3.40 | 2.44 |
| sh 6"-11" | 45.91 | 12.44 | 5.16 | 0.82 | 14.91 | 1.96 | 0.11 | trace | 1.87 | 0.90 | 0.19 | 15.05 | 99.13 | 3.69 | 2.48 | |
| 33-24-11 E. Greenwood |
udc 0-5" | 63.70 | 16.68 | 6.19 | 1.30 | 1.63 | 1.37 | 0.27 | nil | 3.47 | 1.25 | 0.02 | 5.13 | 100.99 | 3.82 | 2.64 |
| sh 5"-11" | 54.29 | 15.32 | 6.04 | 0.99 | 7.74 | 1.62 | 0.15 | nil | 2.65 | 0.96 | nil | 9.80 | 99.56 | 3.54 | 2.42 | |
| 34-17-14 E. Osage |
udc 0-4" | 59.77 | 21.95 | 4.85 | 0.97 | 0.36 | 1.01 | trace | nil | 3.71 | 0.73 | 0.01 | 6.38 | 99.73 | 2.71 | 2.14 |
| udc 4"-7" | 69.63 | 16.39 | 2.48 | 1.40 | 0.28 | 0.83 | trace | nil | 2.69 | 1.34 | 0.01 | 4.80 | 99.84 | 4.25 | 3.42 | |
| sh 7"-15" | 68.57 | 15.46 | 4.31 | 1.03 | 0.23 | 1.39 | 0.05 | 0.17 | 1.98 | 1.46 | 0.31 | 4.72 | 99.36 | 4.43 | 3.30 | |
| sh 15"-23" | 70.36 | 14.02 | 4.55 | 1.07 | 0.28 | 1.32 | 0.08 | 0.14 | 3.27 | 0.34 | 0.42 | 4.37 | 99.88 | 5.00 | 3.58 | |
| 27-11-15 E. Shawnee |
udc 0-5" | 56.39 | 20.06 | 6.64 | 1.61 | 0.40 | 0.95 | 0.03 | 0.32 | 3.70 | 0.78 | 0.03 | 6.55 | 99.75 | 2.80 | 1.99 |
| trans 5"-10" | 61.84 | 18.31 | 5.39 | 0.86 | 0.37 | 1.03 | 0.02 | 0.32 | 3.39 | 1.11 | 0.05 | 5.35 | 99.79 | 3.37 | 2.52 | |
| sh 10"-14" | 69.55 | 13.67 | 3.93 | 1.39 | 0.29 | 0.79 | 0.04 | 1.15 | 2.44 | 1.55 | 0.30 | 5.22 | 100.02 | 5.10 | 3.48 | |
| 22-9-17 E. Jefferson |
udc 0-7" | 58.66 | 22.50 | 4.86 | 0.78 | 0.34 | 1.29 | trace | nil | 4.28 | 0.60 | 0.02 | 6.01 | 99.32 | 2.60 | 2.08 |
| udc 7"-14" | 55.59 | 23.08 | 6.98 | 0.67 | 0.42 | 1.34 | 0.11 | 0.18 | 3.87 | 0.64 | 0.01 | 6.89 | 99.77 | 2.41 | 1.81 | |
| sh 14"-20" | 57.68 | 22.45 | 6.24 | 0.67 | 0.41 | 1.18 | 0.09 | 0.21 | 4.04 | 0.60 | nil | 6.19 | 99.76 | 2.56 | 1.96 | |
| 21-5-19 E. Atchison |
udc 0-6" | 59.01 | 22.50 | 4.43 | 0.93 | 0.60 | 1.20 | 0.03 | 0.11 | 4.11 | 0.62 | 0.01 | 6.14 | 99.68 | 2.62 | 2.12 |
| sh 12"-18" | 62.17 | 19.72 | 4.43 | 1.66 | 0.53 | 1.00 | 0.08 | 0.22 | 3.73 | 0.92 | nil | 5.73 | 100.19 | 3.15 | 2.40 | |
Potassium--Percentage of potassium in the topmost portions of underclay has been plotted with reference to geographic location in Figure 4. This chart shows that potassium content in the underclay increases towards the north, but the underlying shale does not show the same sort of gradation in potassium content to the northward. Data presented in Table 4 show that in all seven localities potassium is more plentiful in the topmost portion of the underclay.
Figure 4--Interrelationship of chemical constituents in Nodaway underclay.
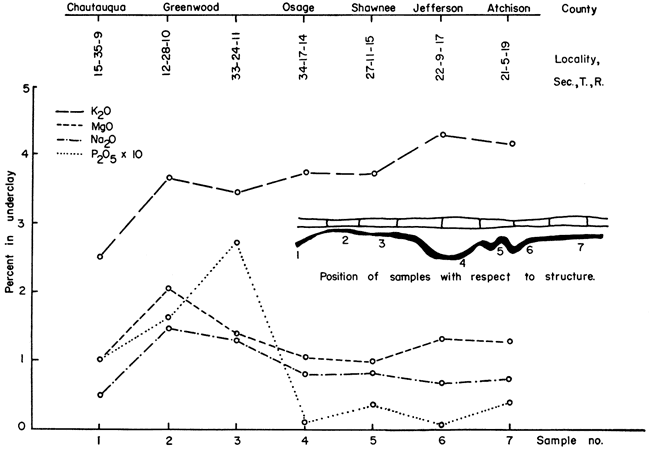
Calcium, magnesium, sodium, phosphorus--Unlike potassium, the magnesium, sodium, and phosphorus do not increase in amount toward the north, nor do they have an orderly arrangement within the underclay-shale profile. Figure 4 shows, however, that magnesium, sodium, and phosphorus are present in greater quantity in Greenwood County. Abnormally large amounts of calcium occur in the lower shales (about 5 inches above the Bachelor Creek limestone) in sec. 12, T. 28 S., R. 10 E., and sec. 33, T. 24 S., R. 11 E., Greenwood County
Iron, titanium, sulfur--These constituents show no orderly arrangement with regard to geographic distribution or position in the underclay-shale profile.
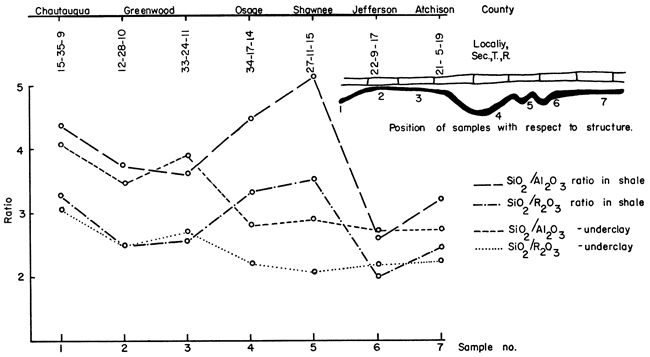
Silica/alumina and silica/sesquioxide ratios--It is to be understood that ratios listed in Table 4 are for the complete sample and not, as reported in many publications, ratios for the clay-size fraction only. Figure 5 shows the silica/alumina and silica/sesquioxide ratios for the underclay and shale plotted against geographic position. The ratios for the underclay are greatest in Chautauqua and Greenwood counties and smaller farther north. The significance of these data will be discussed later in this report.
Figure 5--Relationship of silica/alumina and silica/sesquioxide ratios to geographic location and to position in the underclay-shale profile.
Of 34 samples selected from localities between the Oklahoma boundary and Doniphan County as representative of the underclay and underlying shale, 18 were collected from the topmost portion of the underclay, one from the shale below the coal where no underclay is developed, and the rest from shale and the lower portion of the underclay. See Table 5 for list of samples and their distances below the coal.
Table 5--Localities from which samples were obtained for x-ray analyses.
| County | Location | Depth below coal, inches |
Lithology |
|---|---|---|---|
| Chautauqua | Sec. 15, T. 35 S., R. 9 E. | 7-14 | udc |
| Chautauqua | Sec. 15, T. 35 S., R. 9 E. | 14-20 | sandy sh* |
| Chautauqua | Sec. 29, T. 34 S., R. 9 E. | 0-5 | udc |
| Chautauqua | Sec. 26, T. 33 S., R. 9 E. | 0-3 | udc* |
| Chautauqua | Sec. 26, T. 33 S., R. 9 E. | 3-7 | udc |
| Chautauqua | Sec. 26, T. 33 S., R. 9 E. | 7-12 | sh* |
| Chautauqua | Sec. 4, T. 32 S., R. 10 E. | 0-5 | udc* |
| Elk | Sec. 21, T. 31 S., R. 10 E. | 0-5 | udc* |
| Elk | Sec. 9, T. 30 S., R. 10 E. | 0-5 | udc |
| Elk | Sec. 10, T. 29 S., R. 10 E. | 0-5 | udc |
| Greenwood | Sec. 12, T. 28 S., R. 10 E. | 0-6 | sh |
| Greenwood | Sec. 12, T. 28 S., R. 10 E. | 6-11 | sh |
| Greenwood | Sec. 17, T. 27 S., R. 11 E. | 0-5 | udc* |
| Greenwood | Sec. 36, T. 26 S., R. 10 E. | 0-3 | udc* |
| Greenwood | Sec. 33, T. 24 S., R. 11 E. | 0-5 | udc* |
| Greenwood | Sec. 33, T. 24 S., R. 11 E. | 5-11 | sh |
| Greenwood | Sec. 9, T. 23 S., R. 12 E. | 0-5 | udc* |
| Lyon | Sec. 9, T. 21 S., R. 13 E. | 0-6 | udc* |
| Lyon | Sec. 9, T. 21 S., R. 13 E. | 12-18 | sandy sh |
| Osage | Sec. 32, T. 18 S., R. 14 E. | 0-7 | udc |
| Osage | Sec. 32, T. 18 S., R. 14 E. | 7-20 | sh |
| Osage | Sec. 34, T. 17 S., R. 14 E. | 0-4 | udc* |
| Osage | Sec. 34, T. 17 S., R. 14 E. | 4-8 | udc |
| Osage | Sec. 34, T. 17 S., R. 14 E. | 8-16 | sh |
| Osage | Sec. 34, T. 17 S., R. 14 E. | 16-24 | sh |
| Shawnee | Sec. 27, T. 11 S., R. 15 E. | 0-5 | udc |
| Shawnee | Sec. 27, T. 11 S., R. 15 E. | 5-9 | transition* |
| Shawnee | Sec. 27, T. 11 S., R. 15 E. | 9-14 | sh |
| Jefferson | Sec. 22, T. 9 S., R. 17 E. | 0-7 | udc* |
| Jefferson | Sec. 22, T. 9 S., R. 17 E. | 7-14 | udc |
| Jefferson | Sec. 22, T. 9 S., R. 17 E. | 14-20 | sh |
| Atchison | Sec. 21, T. 5 S., R. 19 E. | 0-6 | udc |
| Atchison | Sec. 21, T. 5 S., R. 19 E. | 10-18 | sh* |
| Doniphan | Sec. 15, T. 3 S., R. 19 E. | 0-4 | udc* |
| *samples glycerated udc abbreviation for Nodaway underclay |
|||
Oriented aggregates were obtained by a method described by Bradley, Grim, and Clark (1937). Oriented samples from which additional data were desired were gently sprayed with glycerol until damp before obtaining a diffraction pattern (MacEwan, 1951, p. 116).
Diffraction patterns were made with a GE XRD-3 Geiger-Mueller counter spectrogoniometer. The patterns were run at 0.2° 2Θ per minute, using a 1° beam slit, 2° detector slit, and nickel-filtered copper radiation at 50 kv and 15 ma.
The Nodaway underclay was found to have different clay assemblages from south to north across the state. Clay minerals recognized are kaolinite, illite, chlorite, mixed-layer illite-montmorillonite, and a trace of montmorillonite. An unidentified mixed-layer clay with 13Å spacing, which expands to 14Å when treated with glycerol, was also found (Fig. 6, 7, 8, 9, 10).
Figure 6--X-ray patterns of minus 2 micron clay, oriented on glass slides, from underclay and shale in sec. 26, T. 33 S., R. 9 E., Chautauqua County. A, underclay 0-3 in. below coal, contains mixed-layer illite-montmorillonite and lacks chlorite. B, shale 7-12 in. below coal, contains chlorite-type clay and has small amount of mixed-layer illite-montmorillonite.
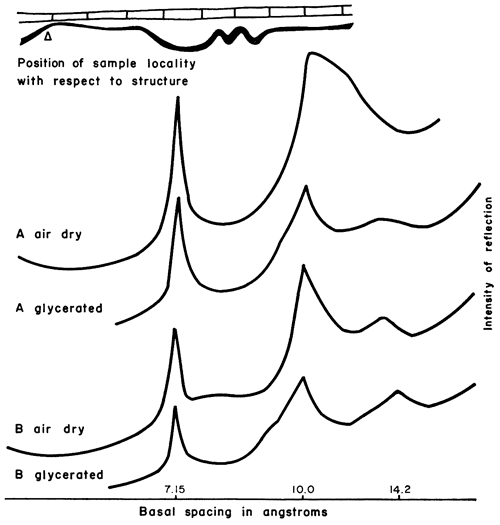
Figure 7--X-ray patterns of minus 2 micron clay, oriented on glass slides, from shale in sec. 12, T. 28 S., R. 10 E., Greenwood County. A, shale 0-6 in. below coal, mainly illite and chloritic clay, 7.15Å judged to be mainly reflection of chlorite (002) plane. B, shale 6-11 in. below coal, mainly illite and chloritic clay having higher degree of crystallinity than sample A. Kaolinite and mixed-layer illite-montmorillonite virtually absent.
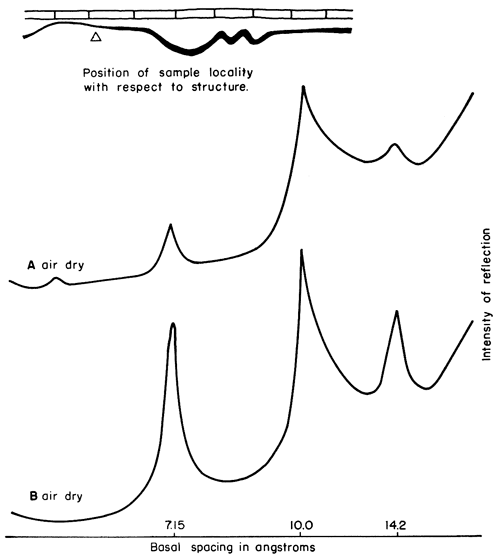
Figure 8--X-ray patterns of minus 2 micron clay, oriented on glass slides, from underclay and shale in sec. 33, T. 24 S., R. 11 E., Greenwood County. A, poorly developed underclay 0-5 in. below coal, mainly illite and chloritic-type or vermiculitic-type mixed-layer mineral, which partly expands on and chloritic clay having higher degree of crystallinity than sample A, Mixed-layer illite-montmorillonite virtually absent; kaolinite basal reflection weak.
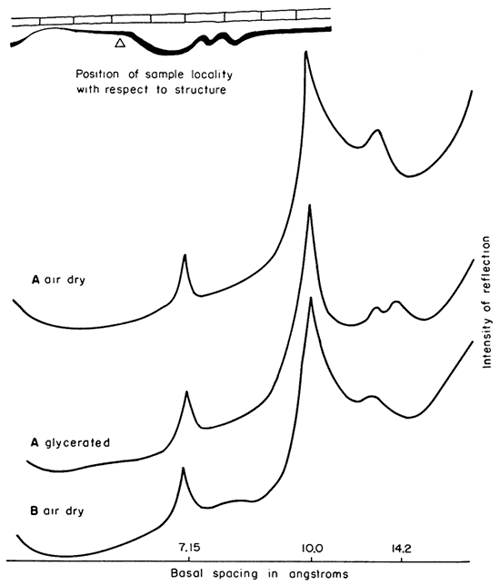
Figure 9--X-ray patterns of minus 2 micron clay, oriented on glass slides, from underclay and shale in sec. 34, T. 17 S., R. 14 E., Osage County. A, underclay 0-4 in. below coal, mainly kaolinite and illite; small amount of mixed-layer illite-montmorillonite. B, shale 16-24 in. below coal, mainly illite, kaolinite (prominent reflection at 7.15Å spacing judged to be due to combined kaolinite 001 and chlorite 002 planes), and chlorite.
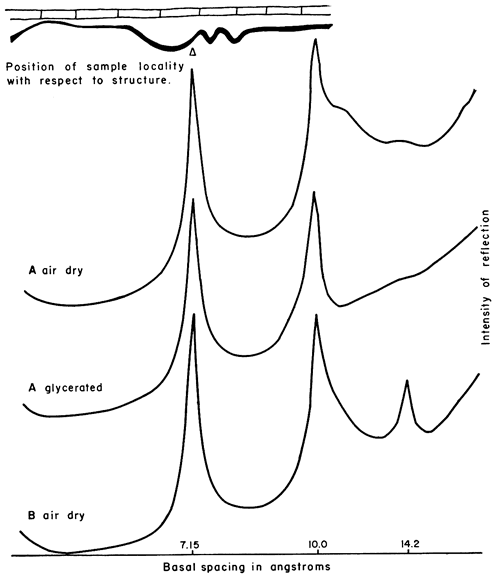
Figure 10--X-ray patterns of minus 2 micron clay, oriented on glass slides, from underclay in sec. 15, T. 3 S., R. 19 E., Doniphan County. Underclay, 0-4 in. below coal, mainly kaolinite and illite, some mixed-layer illite-montmorillonite; small amount of montmorillonite with 17.7Å spacing revealed by glycerol treatment.

Kaolinite--Kaolinite (Fig. 6-10), a nonexpanding mineral with 7.15Å basal spacing, occurs in various amounts from south to north in the underclay. Plate 2 shows that kaolinite is present in moderate amounts in Chautauqua and Elk counties, in parts of Greenwood County is virtually absent, but is a large constituent from Osage County northward. As Plate 2 shows, the amount of kaolinite in the underclay is to some extent a reflection of that found in the laminated shale below.
Illite--Illite (Fig. 6-10) has 10Å basal spacing, and in non-glycerated slides the peak is skewed because of degraded illite or mixed-layer illite-montmorillonite that produces a basal reflection having a d-value slightly greater than that of pure illite. The amount does not vary from top to bottom in the profiles studied, but in southern Greenwood County two underclays seem to have above-average amounts of illite. Glycerated slides indicate that most of the illite is well crystallized and not degraded. Reference to Plate 2 shows the estimated relative proportions of illite from south to north across Kansas.
Chlorite--A nonswelling clay constituent with basal spacing about 14.2Å, regarded as chlorite (Fig. 6-10), but possibly in part vermiculite, occurs in the Nodaway underclay of northern Elk County and southern Greenwood County. It is evident from Plate 2 that chlorite is generally present in the shale directly below the underclay. (Note that the five shales containing biotite also have a 14Å mineral. Table 3; Pl. 2.)
Chlorite-like mixed-layer clay--X-ray patterns of oriented underclay from sec. 36, T. 26 S., R. 10 E., and sec. 33, T. 24 S., R. 11 E., have low, blunt peaks at 6.7°2Θ (basal spacing 13.2Å); the basal spacing expands when the mineral is treated with glycerol. The peak on the pattern of the sample from sec. 36, T. 26 S., R. 10 E., shifts to 14Å and sharpens. Treatment of the other sample with glycerol yields two peaks; one occupies the 6.7° (13.2Å) position and a new one forms at 14Å (Fig. 8).
The lower part of the shale in sec. 33, T. 24 S., R. 11 E., produces a reflection at 6.9° (basal spacing 12.8Å), but it has not been investigated further in this study.
Montmorillonite--A mineral that expands to a basal spacing of 17.7Å on treatment with glycerol is regarded as montmorillonite (Fig. 10). It occurs in sec. 15, T. 3 S., R. 19 E., Doniphan County.
Mixed-layer illite-montmorillonite--The skewness of the illite peak at 10Å is judged to be due to mixed-layer illite-montmorillonite (Fig. 6-10). Treatment of the oriented slides with glycerol expands the mixed layers to a spacing of 13.4Å, thus sharpening the illite peak. This mineral, which may consist of illite and montmorillonite layers in an approximate 1:1 ratio, is present in almost all of the 34 samples studied, and at most localities it is most plentiful in the upper portion of the underclay. Along the underclay outcrop from Oklahoma to Nebraska the mixed-layer mineral is an important constituent, except in northern Elk County and southern Greenwood and Osage counties (Pl. 2). As Plate 2 shows, mixed-layer illite-montmorillonite is present in lesser amounts in the shale than in the underclay, except at the center of sec. 15, T. 35 S., R. 9 E., Chautauqua County.
Characteristics of the Nodaway underclay that have been determined from field observations, mechanical, chemical, and x-ray analyses, and microscopic study of very fine sand are related to each other and to characteristics observed elsewhere by other workers. In this part of the report, relationships are discussed and some explanations suggested.
A low ridge of Mississippian rocks, forming part of the Bourbon arch, trending northwest from Bourbon County, was identified first by Wallace Lee, on the basis of thinning of Pennsylvanian formations over the high area as chief criterion (Jewett, 1951, p. 121). According to Lee (1943, p. 94), the Forest City basin, to the north of the Bourbon arch, was sinking during Shawnee time as is indicated by thinning "northward from about 350 feet in the deeper parts of the basin in Douglas County to less than 250 feet west of the Nemaha anticline." Because data concerning the Wabaunsee group, which contains the Nodaway underclay, are lacking, the effect of conditions in the Forest City basin on the deposition of these rocks is not known. Lee (personal communication) believes that the Bourbon arch moved up relative to the Forest City basin during part of the Wabaunsee time, however.
The evidence in Plate 1 reveals that the Forest City basin sank sufficiently to accommodate a thickening of the shale between Nodaway coal and Church limestone. The thinning of the Aarde shale member in Elk and Greenwood counties indicates that the Bourbon arch was rising during Howard time. These two structures undoubtedly had an influence on the character and formation of the Nodaway underclay.
During nonmarine intervals of Howard time, eastern Kansas was a plain with few, if any, prominent hills. This view is supported by the absence of marked changes in lithology of the Nodaway underclay, and the fact that lateral differences in thickness of each rock unit are slight.
Prior to the deposition of the continental deposits (Nodaway underclay and coal), the gentle undulations of the old sea floor were preserved more or less in their original form. It can be safely inferred from data on Plate 1 that in Elk and Greenwood counties the land surface was underlain by a veneer of shale, approximately 0.5 to 1.0 foot thick, on top of Bachelor Creek limestone. In Chautauqua, Lyon, and Osage counties thickness of the shale covering ranged from 1.0 to 5.0 feet. Farther north, the underlying limestone was not present.
In the discussion that follows the assumption is made that rainfall was abundant and the climate such that the land could support widespread forests. The main disagreement among paleobotanists concerns the temperature, but in all probability the climate was warm temperate or subtropical (Moore, 1940).
The gently undulating surface described above would soon be covered with lush vegetation on the low divides between shallow freshwater basins. Probably small amounts of clay and silt were moved into the basins at first, but as the forests became more dense these contributions were almost stopped. When it is considered that Florida has more than 2,000 shallow lakes (Forsaith, 1916), it is a reasonable hypothesis that eastern Kansas could have had tens of thousands of similar lakes collectively forming a freshwater swamp hundreds of miles inland from and along the shoreline of the Howard sea.
If conditions similar to those in Florida today existed in Kansas during Howard time, almost every small freshwater lake received several feet of vegetal debris, which raised the bottom of the lake sufficiently close to the surface for plants to take root (Forsaith, 1916). In this way every lake would be filled with partly allochthonous and partly autochthonous peat. Analogizing further, the filled lakes of Howard time can be compared to high moors in East Prussia described by Gothan (Stutzer and Noe, 1940, p. 135-136). Gothan noted that peat-filled lakes build up convex bottoms; continuation of the process leaves the old shores (in this case the low divides) topographically lower than the middle of the old lake. Resultant flooding of the divides kills the forest there and builds up peat.
The foregoing is a plausible explanation of how a widespread layer of peat may have been formed in the Pennsylvanian of Kansas.
In Greenwood County and northern Elk County the Nodaway underclay and the shale next below it possess four important characteristics that are not evident in counties to the north or south. These are: (1) a silty underclay grading downward to a less silty shale; (2) relatively small kaolinite content; (3) chlorite and chlorite-like clays in the underclay; and (4) small mixed-layer illite-montmorillonite content.
Grain size--In areas other than Greenwood County along the outcrop of the Nodaway underclay, the median grain size within the underclay-shale profile increases downward (Fig. 3). It is reasonable to infer that vegetation in Howard time was less able at first to prevent transportation of coarser detrital minerals, but as it became more matted, only the finest particles could move, thus accounting for the fine upper portions of underclay.
Curves on Figure 3 show that Nodaway underclay is much more silty in Greenwood County than it is to the north and to the south. If it is assumed that Nodaway underclay is formed in situ, the problem of determining the cause of this silty zone is reduced to consideration principally of the origin of the parent shale. Three factors may have had a bearing: (1) rise of the Bourbon arch may have decreased the depth of water so that finer fractions of sediment were not deposited in the shale there; (2) Greenwood County may have been closer to the source of sediments; (3) the mantle of vegetation may not have been as dense in this area during formation of the underclay, thus allowing removal of the clay fraction by moving water.
The median sizes of the underclay and the underlying shale are 8.0 and 5.0 microns respectively in sec. 33, T. 24 S., R. 11 E., Greenwood County. It is postulated that this anomaly is due to the dilution of the shale with very fine calcium carbonate, as shown by the 7.74 percent calcium oxide in the analysis. The same premise can be adduced to explain the underclay in sec. 1, T. 24 S., R. 11 E., where calcareous shale is mechanically finer than the overlying underclay. Calcium carbonate in the shale does not necessarily result in upward coarsening in the underclay-shale profile, as is shown by the samples from sec. 12, T. 28 S., R. 10 E. In samples from this locality, the shale median is large (13 microns) because of admixed sand in flat-lying, discoidal calcareous sandy patches as much as 3 inches long, which are plentifully scattered throughout the lower 5 inches of the shale between the Nodaway coal and sandy Bachelor Creek limestone. The hard sandy patches are almost indistinguishable from samples of Bachelor Creek limestone at this locality.
Variations in content of kaolinite--Kaolinite is estimated to comprise less than 10 percent of the coarse underclay in northern Elk County and Greenwood County, whereas to the north in Osage County, in the southern part of the Forest City basin, it is judged to constitute more than 40 percent of the underclay. In the underclay-shale profile, kaolinite is included as a constant proportion of the total clay downward into the shale (Pl. 2).
Either a difference in source or a difference in environment of the formation of clay minerals is adequate to explain the distribution of kaolinite across Kansas. Variations in composition and in relative quantity of the 0.06- to 0.125-mm fraction of sand-size grains are negligible, both in the Nodaway underclay and the underlying shale on the Bourbon arch and Forest City basin, so it is believed that kaolinite distribution is not explained by differences in the source of this mineral. Regarding the environment of deposition or formation, Millot (1949, p. 288) has shown that kaolinite formation is retarded by the presence of calcium ion in lake water. Applying Millot's observations to this underclay-shale profile, it can be postulated that kaolinite formation or preservation was retarded in Greenwood County because of locally high calcium-ion concentration, and facilitated in Osage County where the calcium-ion concentration was low. (See chemical analyses in Table 4.) High calcium content does not necessarily indicate low kaolinite content, as is evident from a study of x-ray and chemical analyses of the sample from the center of sec. 15, T. 35 S., R. 9 E. Here the original clay assemblage is unchanged because, presumably, calcium ions from sandy Bachelor Creek limestone have only recently been raised (in solution) by capillary action during the formation of modern soil, which is only 0.5 foot above the underclay.
Variation in content of chlorites--As Plate 2 shows, chlorite is present in all samples of the shale below the Nodaway underclay except those from Jefferson and Atchison counties and one from southern Chautauqua County. [Note: Chlorite, as used in this heading, refers to true members of the chlorite group and chlorite-like mixed-layer clay.] It occurs in Nodaway underclay at one locality in northern Elk County and three of five in Greenwood County.
There is little doubt that in well-drained soils chlorite is produced by alteration of biotite through a series of intermediate clay minerals (Walker, 1949; Stephen, 1952). In addition, it has been shown that on the bottom of Chesapeake Bay in the James estuary chlorite is forming by diagenesis of illite (Powers, 1954). Powers found that the chlorite is least stable when formed in a brackish-water environment, but with increase of salinity (in bayward direction), the stability increases. Murray (1954), in studying shale of Pennsylvanian cyclothems in Illinois and Indiana, found the amounts of kaolinite and chlorite to be approximately equal (in nonmarine and marine shale) but in most samples overshadowed by even larger quantities of illite.
Little published information has been found describing what happens to chlorite in clay deposits. Allen (1928) described alteration of biotite to chlorite and chlorite to anauxite (a silica-rich kaolinite type of clay). He considers "the anauxite of the Ione formation to have formed by surface weathering under tropical or subtropical climate. The calcium, magnesium, iron, and alkalies were removed from the biotite as bicarbonates and carbonates" (p. 152).
The indirect effect of bacteria as an agent of decomposition is too often overlooked. Takahashi (1939, p. 511) points out that where organic acids and bacteria are both present the reactions involving Recent bottom sediments are three times more rapid than where Recent sediments and organic acids are not associated with bacteria. Thiel (1928, p. 71) reports conflicting data concerning the origin of greenalite (a chlorite related to glauconite): Gruner recorded fossil bacteria associated with this mineral, but Hawley formed structures like Gruner's "fossil bacteria" inorganically in the laboratory.
I believe that in the shale next below the Nodaway underclay, some of the observed chlorite has been derived from biotite, and in the five Nodaway localities where biotite also has been found, the alteration is not complete. Moreover, chlorite has not been found in well-developed Nodaway underclay, seemingly because the agents that produce the lightest-gray, most plastic, and thickest underclay have altered it to mixed-layer illite-montmorillonite (see under next heading). In contrast, where silty underclay is poorly developed, as on the Bourbon arch, thin, yellow chlorite has been preserved.
Variations in content of mixed-layer illite-montmorillonite--On the Bourbon arch (Greenwood County) where poorly developed Nodaway underclay is silty, chloritic, and virtually nonkaolinitic, the mixed-layer illite-montmorillonite is sparse, but where the underclay is well developed, nonchloritic, and kaolinitic, this mixed-layer mineral is plentiful. Almost everywhere across Kansas in the Howard deposits studied, the underclay-shale profile contains more of the mixed-layer mineral in the topmost portions.
Grim and Allen (1938) refer to a montmorillonite-like clay mineral in some underclays of Illinois, which in some places seems to be more plentiful at the top. Brown and MacEwan (1950) describe similar mixed-layer illite-montmorillonite from soils. Schultz (1954) found this constituent most concentrated in the upper portions of underclay and deficient in the shale next below.
It is plausible to conclude that where processes forming Nodaway underclay have been most active (Osage County in the Forest City basin, for example) the original detrital chlorite and biotite have been altered to mixed-layer illite-montmorillonite. In places where biotite is the ultimate source of mixed-layer illite-montmorillonite an intermediate chlorite stage is involved. The total chlorite content, then, according to this hypothesis, is partly authigenic and partly primary detrital. On the Bourbon arch, where biotite has been altered to chlorite, the alteration processes have not been sufficiently intense to form well-developed underclay or to release magnesium from chlorite for the mixed-layer mineral.
Variation in content of illite--As stated previously, illite in the Nodaway underclay is more plentiful in Greenwood County in the position of the Bourbon arch, thus correlating with higher calcium carbonate content in the underclay. Grim and Allen (1938) report that illite is the essential clay constituent in calcareous underclay, but is less important in noncalcareous underclay. Schultz (1954) found illite and mixed-layer illite-chlorite in underclay.
A review of the genesis of illites indicates that either the addition of potassium ion to kaolin or the leaching and oxidation of magnesium-calcium-ferrous-iron rocks will yield illite (Frederickson, 1952, p. 6). Until recently it was believed that kaolinite contributed to the sea is not altered (Dietz, 1941, p. 48), and that montmorillonite is the main constituent in forming illite. Grim, Dietz, and Bradley (1949) have proved beyond reasonable doubt, however, that kaolinite accepts magnesium and potassium ions from sea water into its lattice so as to form montmorillonite and illite. Millot (1952) found illite to be the principal constituent in rocks of salt-lagoon origin, prevalent in rocks of sea origin, and commonly present in rocks of "basic lake" origin.
That illite was stable during genesis of the Nodaway underclay is indicated by the lack of marked difference between illite content of the underclay and that of associated shale. On the other hand attention is focused where illite increases in proportion over the northern flank of the Bourbon arch. Reference to Plate 2 (12-28-10; 33-24-11) shows that a large amount of illite is accompanied by an almost total absence of kaolinite. Perhaps the kaolinite (if ever present in the underclay while being deposited) came in contact with ions, particularly K+, with which it combined to form illite. This hypothesis of ions combining with kaolinite to form illite is presented to explain some of the illite on the floor of the Pacific Ocean off the coast of California (Grim, Dietz, and Bradley, 1949).
The topmost portion of Nodaway underclay is rich in magnesium, sodium, and phosphorus in Greenwood County, but farther north it is much poorer in these elements. Potassium, by contrast, comprises 2.5 percent of the underclay in Chautauqua County and 4.11 percent in Atchison County (Fig. 4). Silica/alumina and silica/sesquioxide ratios reflect mechanical composition, which in turn is controlled chiefly by quartz content.
Variation of magnesium, sodium, and phosphorus content--Relatively large percentages of magnesium, sodium, and phosphorus in samples from localities on the Bourbon arch correlate with poorly developed underclay, chlorite minerals, and low amounts of kaolinite and mixed-layer illite-montmorillonite. There is no correlation with mechanically coarse underlay. Magnesium and phosphorus are essential for plant growth whereas only microscopic amounts of sodium are desirable, except for halophytes, which use large quantities of sodium.
Using this information, one is tempted to suggest that the Nodaway underclay is poorly developed in Greenwood County because vegetation was not lush during Howard time in this region and the elements magnesium, sodium, and phosphorus were not required in large amounts. It can be argued that in the Forest City basin these elements were extracted while flourishing forests created the environment for underclay development and provided the vegetal debris for coal. If this explanation is accepted, the idea can be expanded further by attributing the Nodaway coal in Greenwood County mainly to allochthonous plant fragments, whereas to the north autochthonous coal overlies thick underclay. In Chautauqua County, however, the same reasoning does not apply, because here moderately well developed underclay is overlain by only a thin film of coal.
Chemical analyses of Bachelor Creek limestone were not made, but until proved impossible the hypothesis that Nodaway underclay and the shale next below obtained additional quantities of certain elements by diffusion from the sandy limestone cannot be overlooked.
Variation of potassium content--Increase in content of potassium and illite in the topmost portion of Nodaway underclay is paralleled by a thickening of Nodaway coal northward across Kansas from Oklahoma to Nebraska. Chemical analyses of underclay-shale profiles show that potassium is most concentrated in the topmost part of the underclay.
It was noticed long ago that fireclays below thin coal were more satisfactory refractories than those overlain by thick coal (Hopkins, 1901; Stout, 1923, p. 552). Potassium is a major impurity, and although Hopkins and Stout did not provide chemical analyses, it may be safely inferred that potassium was concentrated in the clays underlying the thickest coals. Grim and Allen (1938) report analyses of the minus 0.002 mm fraction of eight Pennsylvanian underclays. Six show the same upward increase in potassium content as is shown in Nodaway underclay; two are decidedly the reverse.
"The bulk of potassium in plants occurs as K+ ions" (Rankama and Sahama, 1949, p. 442), and analyses of ash from trees reported by Moore (1940, p. 52) show potassium oxide to range from 0.30 to 1.99 percent. Plants obtain all their potassium from the soil on which they grow. The argument is that much of the vegetal matter floated into the swamp from land, say 50 miles away, carrying potassium with it. Undoubtedly some of the plants grew in situ, and in these places the underclay should be depleted. Indeed, some of the analyses in Table 4 suggest this.
The general relationship of increased coal thickness accompanied by greater potassium content in the upper portion of the underclay is herein explained as resulting from the removal of potassium ions from the vegetal matter, and lodgment in the clay as a base-exchange cation, or as a part of the illite structure. Where there is more vegetal matter, more potassium is available. All potassium moving downward by diffusion has been adsorbed near the upper surface of Nodaway underclay, leaving lower portions unenriched.
Other minor constituents, such as magnesium, calcium, and sodium, are not concentrated near the top of Nodaway underclay, because the molecules contained in the vegetal debris do not release these elements as readily as potassium ions are released from the plant cells.
Percentages of several constituents in the presumably detrital sand-size fraction of underclay differ markedly from those in the shale next below. Quartz grains 0.06 to 0.125 mm in size are more abundant in the shale, whereas pellets of the same size are more profuse in the Nodaway underclay. Feldspars in the shale are less altered than those in the underclay, and micas are distributed unevenly, both geographically and stratigraphically.
Variation of quartz content--Angular quartz grains of the 0.06- to 0.125-mm fraction are not as abundant in the Nodaway underclay as in the shale below. Figure 5 shows that in Greenwood and Chautauqua counties the silica/alumina ratios for the shale and for the underclay are almost the same, but farther north the ratios indicate much more silica in the shale than in the underclay. This relationship of ratios supports the petrographic observations and clearly means that some condition hindered deposition of detrital quartz during the final stages of deposition of the underclay-shale. Presumably, after the vegetative mantle was formed, only the finer fractions were moved in suspension to the shallow basins.
Angular quartz from underclay has been described by Chapman (1914). In Allen's study (1932) of Pennsylvanian underclays of Illinois, he does not mention the shape of quartz grains, but he does dwell briefly on "flattened pellets, up to one-eighth inch in length, composed of beidellite enclosing quartz and other minerals." Further reference will be made to this in the discussion of pellets of the Nodaway underclay. Grim and Allen (1938) state that in the Lower Pottsville underclays in the Goose Lake area, Grundy County, Illinois, the quartz grains range from 0.06 mm to 0.12 mm, the finest grains being more abundant in the top part of the underclay.
Variation of feldspar content--Feldspars in the shale are less altered than those in the overlying Nodaway underclay. This can be accounted for by (1) greater weathering in the underclay, or (2) more plentiful altered feldspars in the source material of the underclay than in the source material for the shale.
Feldspars are almost totally absent from the underclays of Ohio (McCaughey, 1923). Those found in Pennsylvanian underclays of Illinois (Allen, 1932) have sharp boundaries, in contrast to diffuse boundaries of feldspars in Kansas gumbotils.
Variation of muscovite and biotite content--Muscovite is not an important mineralogical constituent of Nodaway underclay or underlying shale between the Oklahoma-Kansas border and a point approximately 45 miles northward, in southern Greenwood County. Most samples from Chautauqua County lack muscovite in the size fraction studied. Unsuccessful attempts were made to correlate this lack of muscovite in the southern part of the state to other data. Large amounts of muscovite recorded in the northern part of Greenwood County, however, correlate with relatively coarse underclay. The suggestion that these variations of muscovite content in the underclays are reflections of the source of sediments or the weathering history may be true, but data in this report do not substantiate the idea.
Chapman's thin-section study (1914) reveals that much of the muscovite in Carboniferous underclay is wrapped around quartz grains. McCaughey (1923) compares muscovite of coal-measure underclays of Ohio with that of present-day soils. Muscovite occurs in the underclays of coal number 6, West Frankfort, Illinois, and coal number 8, along Salt Fork Creek, Illinois (Allen, 1932; Grim and Allen, 1938). The fact that muscovite is given only a word or two in mineralogical discussion in the last two citations indicates it is uncommon in Illinois underclays.
Biotite is restricted to the shale next below the Nodaway underclay of the northern part of Kansas and is consistently accompanied by chlorite. Evidence has already been presented for the belief that biotite is altered to chlorite and, in well developed underclay, to mixed-layer illite-montmorillonite. The absence of biotite from the shale next below the Nodaway underclay in localities other than those listed in Table 3 cannot be explained. The suggestion that the absence is a reflection of source cannot be supported by correlation with other data.
Concerning biotite, Chapman (1914, p. 11) reports that the Coal Measure clays of Yorkshire contain minor amounts of "small, irregular and very ill-defined pieces, and may be of secondary origin." Stout (1923, p. 499) states that biotite is present in most coal formation clays, but McCaughey (1923, p. 519), in the discussion of mineralogy in the same volume, does not mention it at all. Some underclays of Illinois contain green biotite, which mutually correlates with the highest percentage of heavy minerals and with sandy texture (Allen, 1932). Allen makes the point that biotite alters to anauxite under tropical weathering, but in Illinois underclays the leaching has not been sufficient to remove the greenish color.
Origin of pellets--Pellets (described previously in this report) comprise as much as 95 percent of the 0.06- to 0.125-mm size fraction of much Nodaway underclay. The composition is not accurately known, but probably includes quartz, sericite, and clay minerals. Their occurrence in underclay is postulated by Allen (1932, p. 571) to be due to water as "the sole agent capable of fashioning these pellets." He does not describe any mechanism for the process, however.
McCaughey (1923) discussed the presence of pellets in the underclays of Ohio. He reports that these "non-disintegrated clay aggregates" act like sand grains, and that they are made of quartz, sericite, and clay. Pennsylvanian underclays of Illinois contain elongated pellets as much as 1/8 inch long (composed of beidellite enclosing quartz grains), which are assumed to be detrital (Allen, 1932).
Two other hypotheses offered here can account for pellets in underclay; one inorganic, the other organic. (1) It is conceivable that unreacted colloidal silica in the swamp fused the constituents into pellets, (2) sand- and mud-eating worms deposited the material as faecal pellets at the bottom of the swamp; mucous-like organic compounds perhaps bound the components of the pellets until diagenetic processes fossilized them (Moore, 1939). Imprints of worms or any other kind of animals are lacking in the Nodaway underclay, but it is logical to assume that wormlike organisms lived on the bottom of freshwater swamps and ate vegetal debris mixed with clay.
Literature concerning allochthonous and autochthonous peats usually dwells briefly on the sticky, plastic substratum called gley. [Note: Gley is defined as an intrazonal hydromorphic soil developed under a temperate climate; vlei is the term applied to the subtropical or tropical counterpart (Robinson, 1949, p. 450-452). The spelling used in this report conforms with Webster's International dictionary and British spelling, but in much of the literature on soils in the United States "glei" is used.] Because coal is believed to originate with peat, the idea that underclay and gley are similar is considered. Data assembled in previous pages support a new theory for the origin of underclay.
Most coal originates as peat. "Anyone questioning this conclusion has but to observe the transition from peat to lignite and from lignite to bituminous coal, with a gradual decrease in the distinctness of the plant remains in passing from the lower ranks of coal, to be convinced of this matter" (Moore, 1940, p. 136). Information concerning the origin of peat, then, is important in shedding light on the origin of the material below the vegetal layer. Two main theories for the accumulation of vegetal debris giving rise to coal are supported by two schools of geologists. One holds that organic matter accumulated in place to form autochthonous coal. The other school believes that organic matter drifted into shallow basins to form allochthonous coal (Moore, 1940). It will be shown that the vegetal layer, regardless of origin, has the same effect on the mineral layer below, provided it is submerged beneath the water table for the time prior to transformation to coal.
Autochthonous peat--Bennett and Allison (1928) discuss the subsoil beneath the Zapata Peninsula region, "middle" Cuba, but they do not state clearly whether most of the peat is allochthonous or autochthonous. In mangrove swamps, most of the subsoil is bluish-black clay or bluish and greenish-brown clay with some reddish-brown splotches near the surface, grading downward to an olive color. The plastic and sticky organic muck is 3 to 5 inches thick, at most (Bennett and Allison, 1928, p. 127). In the Royal Palm Swamp (in "middle" Cuba) 3 to 4 inches of peat or muck covers 6 to 16 inches of brown clay, commonly mottled rusty brown, which overlies about 3 feet of greenish-yellow, sticky, plastic clay. In places blue mottling characterizes the upper subsoil and red mottling the lower zones.
In his book on tropical soil-forming processes, Mohr (1930) discusses how the macroflora synthesizes organic materials, and how the microflora breaks down the associated macroflora to form humus, which in turn forms peat, and eventually coal. If the peaty soil is almost continuously under water, humic acids in the warm water cause subaqueous weathering, which produces pale-gray or bluish-gray soils composed of little else than kaolinite and silicic acid. These subhydrogens (permanently water-saturated soils) occur, according to Mohr, on the bottom of marshes, on the sea floor, and along large rivers.
Kubiena (1953, p. 99) described a turf peat moor, situated on the North Sea Coast, which overlies an "impermeable gley layer, rich in colloidal material." Under the moor, the chemical weathering and clay genesis are increased sharply.
The three preceding examples illustrate the almost universal occurrence of gley below peat that is clearly autochthonous in origin.
Allochthonous peat--Concerning mangroves in Florida, Davis (1940, p. 357) has briefly described plastic (allochthonous) peat, which he believes accumulated in the quieter-water parts of swamps, especially around lakes and ponds. Furthermore, he has found fibrous (autochthonous) and plastic (allochthonous) peat soils covering many different types of soil or substratum.
The Florida Everglades have received considerable attention from botanists and soil scientists, but little reference is made to the subsoil of allochthonous peat in this area. Florida is entirely within the Coastal Plain physiographic province, and the area of Everglades is underlain by Vicksburg beds, or later limestone covered with Pliocene clay or Pleistocene sand (Forsaith, 1916). The very gently undulating country is characterized by numerous depressions that extend below the permanent water table, to form more than 2,000 lakes, from many of which Forsaith has described allochthonous peat. Lake Newman, for example, is surrounded by a sand plain on which upland vegetation extends to the water's edge. Peat is not present near shore, but in the center of this 16-square-mile body of water there is an allochthonous layer of peat about 2 feet thick. Lake Weir, which is 30 feet deep, has no lacustrine peat within 0.25 mile of shore, but in its central part allochthonous peat is 5 feet thick. North of Lake Harris, autochthonous peat (nonplastic, fragments readily identifiable) overlies allochthonous peat (plastic, fragments microscopically indistinct, contains other detrital material), which rests on a bluish-gray clay. Forsaith writes, "When one considers the probable amount of lacustrine peat, both from the standpoint of the number of lakes and the extent of deposits as revealed by the probings in the chosen regions, one must infer that the allochthonous type of peat in these 2,000 lakes and surrounding marshes must be enormous, and, when compared with those of an autochthonous nature, their relative superiority is very striking."
In 1917 Forsaith presented additional evidence showing that allochthonous and autochthonous peats can be distinguished microscopically, and that coals in general show organization characteristic of the allochthonous type of peat. Also he says (p. 199), "Probings in several localities showed about 15 feet of peat resting upon a stratum of bluish clay (the initial stage of 'fireclays' usually found under coal beds)." He makes the point that dense entanglements of trees growing in very shallow water never yield accumulations of more than a few inches of humus, although in such environment one might expect peat deposits to be thickest.
Allison and Dachnowski-Stokes (1932) wrote about the same area with no more than brief comments on the soft, marly to sandy substratum. They published a cross section along a line from South Bay on Lake Okeechobee to a point 15 miles south, which shows a persistent finger of plastic (allochthonous) peat throughout this distance.
Significance--The foregoing examples of the occurrence of gley beneath allochthonous or autochthonous peats show that a gray, bleached zone of clayey texture occurs almost everywhere, regardless of the way in which the peat is deposited. The only requirements are the presence of humus material covered with water while the gley is being formed, accompanied by poor aeration, which insures reducing conditions.
Joffe (1936, p. 328) defines gley (after Vuisotzkii) as a "more or less compact, sticky loam or clay parent material, which is not, however, as sticky as the usual loam or clay, frequently with more or less clearly pronounced light greenish-blue tinge." It is typical of marshes and areas in which the water table is high, and because of the water-logged environment, anaerobic conditions favor reducing reactions and minimize leaching effects. There is little organic matter in the gley, but its percentage of soluble humus is greater than in the overlying peat. Modern gleys are slightly lower in content of calcium than their parent materials, but the overall chemical composition is almost exactly the same. In regions where ground water is rich in potassium and sodium the gley is more sticky. Although gley "horizons" below peat occur in almost every latitude, the tropical analogue (called vlei) is not well known, because less research has been devoted to it (Robinson, 1949, p. 365).
Recently, at the Rothamsted Experimental Station, England, "gleying" has been reproduced in the laboratory by use of various clays and organic compounds as source material (Bloomfield, 1950; 1951). Bloomfield emphasizes the fact, which Zavalishin first found, that gley and its parent substances have essentially the same chemical composition; in gley, however, the iron has been translocated a few inches to cracks and tiny fissures so as to give the gley an overall gray color. Bloomfield (1950) indicated, by using glucose solutions, that gley formation under reducing conditions is due to the removal of the masking effect of amorphous ferric compounds. Also, the amount of dissolved iron varies directly with the concentration of calcium carbonate. When the soluble ferrous compounds are reoxidized, they form ferric oxide and organic complexes.
Later, Bloomfield (1951), using ground-up humus, peat, leaves, and grass in order to duplicate natural processes more closely, concluded that the "gleying" is due to action of organic compounds formed from the decay of plant parts. The role of bacteria still is not defined, but seems to influence the "gleying" by altering the physico-chemical relationship of the environment.
New data on the clay minerals of some Scottish soils, but pertaining also to gley, have been published recently (Michell, 1955). Mitchell analyzed clays from seven soils formed from magnesium-rich parent materials. Four of these soils are well drained, and the other three are gleys. The gleys contain more montmorillonite than vermiculite, in contrast to three of the four well-drained soils, which have less montmorillonite than vermiculite. Mitchell states (p. 96), "This influence of drainage is clearly seen in soils developed on norite, where the change from vermiculite to montmorillonite can be traced on going up the moderately gleyed profiles." It has been explained previously in this report that a montmorillonite-type clay (mixed-layer illite-montmorillonite) is associated with the topmost portions of the best-developed Nodaway underclay, whereas a 14Å clay (vermiculite-type or chlorite type) has been left unaltered in the poorly developed equivalent.
To class the Nodaway coal and underclay as an example of autochthonous coal and "fossil" soil from which certain salts have been extracted is to ignore the facts. Where the Nodaway coal is thickest, it would be reasonable to assume that the soil was most fertile, but this relationship does not seem to be true. The underclay overlain by thick coal in the Forest City basin is not judged to have a higher base-exchange capacity than underclay overlain by thin coal on the Bourbon arch. In fact, on the basis of larger kaolinite content in the underclay of the Forest City basin, it can be argued that the base-exchange capacity there is lower (Crim, 1954, p. 129, Table 14). Furthermore, nutrients such as magnesium and phosphorus are not plentiful in the underclay in the Forest City basin (Fig. 4). Fossil roots (Stigmaria) are absent from the underclay, except at one place described.
Stout's concept (1923) that underclay is due to oxidizing conditions does not apply to the Nodaway underclay. Oxidizing conditions would not allow the preservation of numerous carbonized twigs, stalks, and leaves; neither would sulfides be formed.
The discussion presented in this report concerning Stout's theory can be applied to the idea that underclay is a buried gumbotil-like clay developed under atmospheric weathering (Weller, 1930; Allen, 1932).
Grim and Allen (1938) postulated that the mineralogical assemblages in the underclays of Illinois have not changed character since deposition. They believe that underclay was deposited as it is found, and reflects climatic conditions affecting the source material. For instance, Grim and Allen (1938, p. 1508) consider that kaolinite is a weathering product formed in quantities under certain climatic conditions, and that when carried away, will retain its kaolinitic aspect where deposited. This seemingly is not true. Ross (1943) states that "in reality there is only one fundamental factor in clay formation--the chemical character of the reacting system." Shawarbi (1952, p. 233) reports that kaolin, montmorillonite, and mica-like clays have been found in almost all types of soils, regardless of the kind of parent material or climate where the soils are formed. Further, Shawarbi states that kaolinite occurs in well-leached soils of both humid temperate and humid tropical climates. It is improbable that climatic variations accounted for the lateral differences in the Nodaway underclay.
The process of underclay formation is believed to be analogous to the process that forms modern gley. It is not within the scope of this report to analyze the factors involved in the genesis of gley, but is is appropriate to point out that the facts pertaining to the Nodaway underclay correspond to facts known about gley under peat.
The relationship between gley and peat has been explained previously. There are four striking similarities between gley and the Nodaway underclay.
The conclusion that the Nodaway underclay is a "fossil" or buried gley is more acceptable than other postulates, which require that the coal-forming peat be deposited only in situ or only by allochthonous processes. Examples have been given on previous pages to prove that gley develops under either allochthonous or autochthonous peat. Formation of gley is due to action of organic compounds, which move downward by diffusion from the peat to the shale. In some places studied (e.g., sec. 21, T. 5 S., R. 19 E.) soft, sticky, gray clay occurs above the Nodaway coal. This relationship is interpreted as the result of upward diffusion of organic compounds after the peat has been covered by marine shale.
Lack of laminae in underclay may be explained by alteration of biotite to chlorites and mixed-layer illite-montmorillonite.
According to the gley hypothesis, thick underclay can develop if sufficient organic compounds are available to react with the parent material. If a thick deposit of peat contains only small amounts of reactive organic compounds, it is reasonable to postulate that the underlying material will be altered very little. In contrast, if a thin deposit of peat has a high content of reactive organic compounds, a thick gley may develop. In general, then, the peat is only important as a supply of reactive organic compounds. A realization of the preceding statements may help to explain thick coals overlying no underclay and thick underclay overlain by little or no coal. Because useful information regarding bacterial activity as an agent of weathering is almost lacking, this factor remains obscure.
It is known with certainty that chemical environment ultimately determines the composition and structure of clay minerals (Ross, 1943; Grim, Dietz, and Bradley, 1949; Shawarbi, 1952), but the study of processes that render certain elements available has only started. Zobell (1939) summarized contributions of workers who have revealed the presence of bacteria endowed with the ability to modify chemical composition or physical characteristics of marine bottom deposits. Bacteria do this, according to Zobell, by first altering the hydrogen ion concentration and redox potential, which in turn causes the inorganic constituents to react and become stable in the new environment.
It is reasonable to suppose that anaerobic bacteria were active below peat in Pennsylvanian time as they are today (Kubiena, 1953, p. 96). Why chlorite in one area does not yield its magnesium to form mixed-layer illite-montmorillonite, as it does in another area, is not known. Such features as a slightly greater amount of calcium accompanying the stable chlorite probably have an indirect effect. The condition of peat as it is deposited on a lake bottom also probably affects the activity of organic compounds and bacteria.
An appraisal of the similarities of underclay and gley could be conducted easily. The ideal situation would be a permanently saturated peat bog on biotite-bearing shale that contains a coal seam and underclay. The underclay, presumably formed from the shale, could be compared with the gley (formed from the same shale) below the peat in the bog.
Allen, V. T. (1928) Anauxite from Ione formation of California: Am. Mineralogist, v. 13, p. 145-152.
Allen, V. T. (1932) Petrographic and minerological study of the underclays of Illinois coal: Am. Ceramic Soc. Jour., v. 15, p. 564-573, fig. 1-9.
Allison, R. V., and Dachnowski-Stokes, A. P. (1932) Physical and chemical studies upon important profiles of organic soils in the Florida Everglades: Second Internat. Cong. Soil Sci., v. 6, p. 222-245.
Bennett, H. H., and Allison, R. V. (1928) The Soils of Cuba: Tropical Plant Research Found., Washington, D. C., p. 1-410, fig. 1-101, maps.
Bloomfield, C. (1950) Some observations on gleying: Jour. Soil Sci., v. 1, p. 203-211, fig. 1-3.
Bloomfield, C. ((1951) Experiments on the mechanism of gley formation: Jour. Soil Sci., v. 2, p. 196-211, fig. 1-9.
Bradley, W. F., Grim, R. E., and Clark, G. L. (1937) The behavior of montmorillonite upon wetting: Zeitschr.-Kristallographie, ser. A, v. 97, p. 216-222.
Broadhead, G. C. (1873) Part II, Nodaway County; in, Pumpelly and others, Preliminary Report on Iron Ores and Coal Fields: Missouri Geol. Survey, p. 388-402, illus. 1-190.
Brown, G., and MacEwan, D. M. C. (1950) The interpretation of X-ray diagrams of soil clays, II. Structures with random interstratification: Jour. Soil Sci., v. 1, p. 239-253, fig. 1-11.
Chapman, S. E. (1914) Some petrological characteristics of underclays: Leeds Geol. Assoc. Trans., pt. 17, p. 8-15.
Davis, J. H. (1940) The ecology and geologic role of mangroves in Florida: Papers Tortugas Lab., v. 32; Carnegie Instit. Wash, Pub. 517, p. 303-412.
Dietz, R. S. (1941) Clay minerals in recent marine sediments: Ph.D. thesis (unpub.), Illinois Univ., Urbana, p. 1-68.
Duparque, Andre (1948) Sur la formation de la houille et sur le presence de sols de vegetation fossiles sous les couches de houille allochtones: Soc. géol. Nord Annales, t. 68, p. 127-162.
Duparque, Andre (1949) Sur les houilles schisteuses et sur l'interstratification des houilles et des schistes dans certaines veines de charbon: Soc. géol. Nord Annales, t. 69, p. 237-269, pl. 8-9.
Forsaith, C. C. (1916) A report on some allochthonous peat deposits of Florida, Part I, Topographical: Bot. Gazette, v. 62, p. 32-52.
Forsaith, C. C. (1917) A report on some allochthonous peat deposits of Florida, Part II, Morphological: Bot. Gazette, v. 63, p. 190-208.
Frederickson, A. F. (1952) The genetic significance of mineralogy: Am. Inst. Min. Met. Eng. Symposium, Problems of clay and laterite genesis, p. 1-11, fig. 1-4.
Gresley, W. S. (1887) Notes on the Formation of Coal-Seams, as suggested by evidence collected chiefly in the Leicestershire and South Derbyshire Coal-Fields: Geol. Soc. London, Quart. Jour., v. 43, p. 671-674.
Grim, R. E. (1953) Clay mineralogy: McGraw-Hill, New York, p. 1-384, fig. 1-121.
Grim, R. E., and Allen, V. T. (1938) Petrology of the Pennsylvanian underclays of Illinois: Geol. Soc. America, Bull., v. 49, p. 1485-1514, pl. 1-2.
Grim, R. E., Dietz, R. S., and Bradley, W. F. (1949) Clay mineral composition of some sediments from the Pacific Ocean off the California coast and the Gulf of California: Geol. Soc. America, Bull., v. 60, p. 1785-1808, fig. 1-22, pl. 1-5.
Harrington, B. J. (1883) Life of Sir William E. Logan; Kt. Sampson Low, Marston, Searle, & Rivington, London, p. 1-432, illus.
Haworth, Erasmus (1898) Special report on coal: Kansas Univ. Geol. Survey, v. 3, p. 1-347, fig. 1-54, pl. 1-70.
Hopkins, T. C. (1901) A short discussion of the origin of the coal measures fireclays: Am. Geologist, v. 28, p. 47-51.
Hutchings, W. H. (1894) Notes on the composition of clays, slates, etc., and on some points in their contact-metamorphism: Geol. Mag., dec. 4, v. 1, p. 36-45.
Jewett, J. M. (1951) Geologic structures in Kansas: Kansas Geol. Survey, Bull. 90, pt. 6, p. 107-172, fig. 1-2. [available online]
Joffe, J. S. (1936) Pedology: Rutgers Univ. Press, Rutgers, New Jersey, 1st ed., p. 1-575, fig. 1-4, pl. 1-41.
Krumbein, W. C., and Pettijohn, F. J. (1938) Manual of sedimentary petrography: Appleton-Century-Crofts, New York, p. 1-549, fig. 1-265.
Kubiena, W. L. (1953) The soils of Europe: Thomas Murby & Co., London, p. 1-317, fig. 1-11, pl. 1-26.
Lee, Wallace (1943) The stratigraphy and structural development of the Forest City basin in Kansas: Kansas Geol. Survey, Bull. 51, p. 1-142, fig. 1-22. [available online]
Logan, W. E. (1842) On the character of the beds of clay lying immediately below the coal seams of South Wales: Geol. Soc. London Proc., v. 3, p. 275-277.
Lyell, Charles (1845) Travels in North America: Wiley & Putnam, New York, v. 1, p. 1-251, fig. 1-6, pl. 1.
MacEwan, D. M. C. (1951) The montmorillonite minerals (montmorillonoids); in, Brindley, G W., X-ray identification and crystal structures of clay minerals: Mineralogy Soc. London, p. 86-137, fig. 1-18.
McCaughey, W. J. (1923) Mineralogical examination of coal formation clays; in, Stout, Wilber, and others, Coal formation clays of Ohio: Ohio Geol. Survey, 4th ser., Bull. 26, p. 516-522.
Millot, Georges (1949) Relations entre la constitution et la genèse des roches sedimentaires argileuses: Géol. Appliquée et Prospection Minière, Assoc. ing. Géologues Univ. Nancy Bull., t. 2, nos. 2, 3, 4, p. 1-352, fig. 1-20, pl. 1-7.
Millot, Georges (1952) Prospecting for useful clays in relation with their conditions of genesis: Am. Inst. Min. Met. Eng. Symposium, Problems of clay and laterite genesis, p. 107-114.
Milner, H. B. (1952) Sedimentary petrography: Thomas Murby & Co., London, p. 1-666, fig. 1-100, pl. 1-52.
Mitchell, W. A. (1955) A review of the mineralogy of Scottish soil clays: Jour. Soil Sci., v. 6, p. 94-98.
Mohr, E. C. (1930) Tropical soil forming processes and the development of tropical soils: China Geol. Survey, Peiping, 2nd ed. (Translated from Dutch by Robert L. Pendleton, 1933).
Moore, E. S. (1940) Coal: John Wiley & Sons, New York, 2nd ed., p. 1-473, fig. 1-151, pl. 1-20.
Moore, H. B. (1939) Faecal pellets in relation to marine deposits; in, Trask, P. D., Recent marine sediments: Am. Assoc. Petroleum Geologists, p. 516-524.
Moore, R. C. (1935) Stratigraphic classification of the Pennsylvanian rocks of Kansas: Kansas Geol. Survey, Bull. 22, p. 1-256, fig. 1-12.
Moore, R. C. (1949) Divisions of the Pennsylvanian system in Kansas: Kansas Geol. Survey, Bull. 83, p. 1-203, fig. 1-37. [available online]
Murray, H. H. (1954) Genesis of clay minerals in some Pennsylvanian shales of Indiana and Illinois; in, Clays and Clay Minerals: Nat. Acad. Sci. Nat. Research Council, Pub. 327, p. 47-66, fig. 1-4, pl. 1-2.
Potonie, H. (1910) Die Entstehung der Steinkohle und der Kaustobiolithe überhaupt: Gebrüder Borntraeger, Berlin, 5th ed.
Powers, M. C. (1954) Clay diagenesis in the Chesapeake Bay area; in, Clays & Clay Minerals: Nat. Acad. Sci.-Nat. Research Council, Pub. 327, p. 68-80, pl. 1-2.
Rankama, Kalvero, and Sahama, T. G. (1949) Geochemistry: Univ. Chicago Press, p. 1-911.
Robinson, G. W. (1949) Soils, their origin, constitution and classification: Thomas Murby & Co., London, 3rd ed., p. 1-573, fig. 1-22, pl. 1-9.
Ross, C. S. (1943) Clays and soils in relation to geologic processes: Washington Acad. Sci. Jour., v. 33, p. 225-235.
Schoewe, W. H. (1946) Coal resources of the Wabaunsee group in eastern Kansas: Kansas Geol. Survey, Bull. 63, p. 1-144, fig. 1-35, pl. 1-5. [available online]
Schultz, L. G. (1954) The petrology of underclays: Ph.D. thesis (Illinois Univ.), Public. No. 9136, University Microfilms, Ann Arbor, Mich.
Shawarbi, M. Y. (1952) Soil chemistry: Chapman & Hall, London, p. 1-4207 fig. 1-18.
Stainer, X. (1934) Matériaux pour 1'étude de la formation des gisements houillers: Soc. belge Géologie, t. 44, p. 51-273.
Stainer, X. (1935) Études sur le mur des conches de charbon (1re Note): Soc. Sci. Bruxelles Annales, t. 55 B, p. 196-206.
Stainer, X. (1937) Études sur le mur des couches de charbon (2me Note): Soc. Sci. Bruxelles annales, Ser. II, Sci. Nat. Med., t. 57, p. 175-189.
Stainer, X. (1940) Veines de houille d'origine marine: Soc. Sci. Bruxelles Annales, Ser. II, Sci. Nat. Med., t. 60, p. 37-45.
Stephen, I. (1952) A study of rock weathering with reference to the soils of the Malvern Hills, Part I. Weathering of biotitite and granite: Jour. Soil Sci., v. 3, p. 20-33.
Stout, Wilber (1923) Origin of coal formation clays; in, Stout, and others, Coal formation clays of Ohio: Ohio Geol. Survey, 4th ser., Bull. 26, p. 533-568.
Stutzer, Otto, and Noe, A. C. (1940) Geology of coal: Univ. Chicago Press, p. 1-461, fig. 1-188.
Takahashi, Jun-Ichi (1939) Synopsis of glauconitization; in, Trask, P. D., Recent marine sediments, Am. Assoc. Petroleum Geologists, p. 503-512, fig. 1-5.
Thiel, G. A. (1928) A summary of the activity of bacterial agencies in sedimentation: Natl. Research Council, Rept. Comm. Sedimentation 1927-1928, Circ. 85, p. 61-77.
Verville, G. J. (1951) Pennsylvanian and Permian stratigraphy of Elk County, Kansas: Ph.D. thesis (unpub.) Wisconsin Univ., Madison.
Walker, G. F. (1949) The decomposition of biotite in the soil: Mineralog. Mag., v. 28, p. 693-703, fig. 1-2.
Weller, J. M. (1930) Cyclical sedimentation of the Pennsylvanian period and its significance: Jour. Geology, v. 38, p. 97-135, fig. 6.
White, David, and Thiessen, Reinhardt (1913) The origin of coal: U. S. Bur. Mines Bull. 38, p. 1-390.
Worthen, A. H. (1866) Geology: Illinois Geol. Survey, v. 1, p. 1-504, pl. 1-8, vertical section.
Zobell, C. E. (1939) Occurrence and activity of bacteria in marine sediments; in, Trask, P. D., Recent marine sediments: Thomas Murby & Co., London, p. 416-427.
Descriptions of localities where sections were measured and samples were collected from Nodaway underclay and shale directly below.
1. SW SE sec. 15, T. 35 S., R. 9 E., Chautauqua County; 0.45 mile west of SE corner of section, on S line; measured in road ditch.
2. Center sec. 15, T. 35 S., R. 9 E., Chautauqua County; 0.55 mile north of S line; measured on steep slope in road ditch.
3. NW NE sec. 29, T. 34 S., R. 9 E., Chautauqua County; 0.1 mile east of center of N line; measured in road ditch on gentle hill.
4. SE SW sec. 3, T. 34 S., R. 9 E., Chautauqua County; 0.4 mile east of SW corner of section on north side of U.S. Highway 166.
5. SW SW sec. 26, T. 33 S., R. 9 E., Chautauqua County; 0.1 mile north of SW corner of section; measured in road ditch.
6. SE NE sec. 18, T. 32 S., R. 10 E., Chautauqua County; 0.3 mile south of NE corner of section; measured in road ditch.
7. NE NW sec. 4, T. 32 S., R. 10 E., Chautauqua County; 0.4 mile east of NW corner of section; measured in road ditch.
8. NE NE sec. 21, T. 31 S., R. 10 E., Elk County; 0.15 mile south of NE corner of section; measured in road ditch.
9. N2 sec. 9, T. 30 S., R. 10 E., Elk County; 0.1 mile south of N line; measured 40 feet northwest of north end of low-water bridge.
10. SE SE sec. 26, T. 29 S., R. 10 E., Elk County; 0.2 mile north of SE corner of section; measured in road ditch.
11. SE sec. 10, T. 29 S., R. 10 E., Elk County; 0.25 mile north, of SE corner of section; measured at southwest corner of steel bridge.
12. SE SE sec. 12, T. 28 S., R. 10 E., Greenwood County; section measured on creek at back of red barn.
13. SE sec. 17, T. 27 S., R. 11 E., Greenwood County; on west side of State Highway 99 at a bend on a hill.
14. Center NW sec. 36, T. 26 S., R. 10 E., Greenwood County; 0.15 mile south of N line from cross-roads at 0.3 mile east of NW corner of section; section measured in road ditch at south end of steel bridge across Honey Creek.
15. N2 sec. 30, T. 25 S., R. 11 E., Greenwood County; 0.3 mile south of N line; section measured along creek 500 feet eastward from road.
16. SW sec. 33, T. 24 S., R. 11 E., Greenwood County; 0.22 mile north of SW corner of section, 0.1 mile east on south side of road; measured in ditch.
17. SE SW sec. 1, T. 24 S., R. 11 E., Greenwood County; 0.45 mile east of SW corner of section; measured in road ditch.
18. NW NW sec. 9, T. 23 S., R. 12 E., Greenwood County; 0.15 mile south of NW corner of section, 100 feet eastward in cow pen behind barn.
19. Center sec. 17, T. 22 S., R. 12 E., Greenwood County; 200 feet south of bridge crossing Verdigris River on State Highway 57; measured on east bank of river.
20. SE SW sec. 9, T. 21 S., R. 13 E., Lyon County; 0.45 mile east of SW corner of section; measured in small stream channel about 300 feet north of culvert.
21. NE SW sec. 6, T. 21 S., R. 13 E., Lyon County; 0.4 mile north of S line on north-south side road just west of center of section; measured in road ditch at south end of steel bridge.
22. SW SW sec. 32, T. 18 S., R. 14 E., Osage County; 0.2 mile east of SW corner of section in road ditch.
23. S2 sec. 34, T. 17 S., R. 14 E., Osage County; measured in a gully 200 feet north of center of S line of section
24. SE SE sec. 25, T. 16 S., R. 14 E., Osage County; 0.2 mile north of SE corner of section; measured in road ditch.
25. NW NW sec. 29, T. 15 S., R. 15 E., Osage County; 0.15 mile east of NW corner of section.
26. Center sec. 19, T. 14 S., R. 16 E., Osage County; measured in road ditch just east of Carbondale.
27. SE SE sec. 16, T. 13 S., R. 15 E., Shawnee County; measured on road embankment 256 feet west of SE corner of section.
28. NE sec. 27, T. 11 S., R. 15 E., Shawnee County; measured on south bank of Kansas River under bridge of U.S. Highway 40 about 1000 feet west of E line of section.
29. NW sec. 12, T. 11 S., R. 15 E., Shawnee County; about 400 feet east and 200 feet south of wooden bridge at NW corner of section; measured on bank of creek.
30. Center sec. 23, T. 10 S., R. 16 E., Shawnee County; measured on road embankment approximately 0.05 mile north of center of section.
31. SW sec. 22, T. 9 S., R. 17 E., Jefferson County, measured just west of wooden bridge on S line at SW comer of section.
32. Center S line sec. 3, T. 9 S., R. 17 E., Jefferson County; measured on embankment of State Highway 4 just east of concrete railed bridge.
33. NE sec. 26, T. 8 S., R. 18 E., Jefferson County; 0.15 mile west of center of E line of section; measured directly west of bridge on S line of quarter.
34. NE sec. 7, T. 8 S., R. 18 E., Jefferson County; 0.1 mile west of E section line on good gravel road; measured in road ditch on north side of road.
35. SE SW sec. 28, T. 7 S., R. 18 E., Jefferson County; 0.4 mile east of SW corner of section, about 300 feet north of steel bridge, on stream bank.
36. NW sec. 21, T. 5 S., R. 19 E., Atchison County; measured on vertical stream bank at junction of two eastward-flowing streams about 600 feet east of cement bridge on W line of section.
37. NW sec. 34, T. 4 S., R. 19 E., Doniphan County; measured on west bank of stream 300 feet east of W line at back of chicken house.
38. SW SE sec. 29, T. 3 S., R. 19 E., Doniphan County; 0.3 mile west of SE corner of section; measured on side of road.
39. SW SW sec. 15, T. 3 S., R. 19 E., Doniphan County; 0.1 mile east of SW corner of section; measured in road ditch.
Mechanical analyses of samples of Nodaway underclay (udc) and shale next below (sh)
| Sample Locality No. |
Depth below coal, inches |
Type | Cumulative percent coarser than | Median size, microns |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250µ | 62.5µ | 31.2µ | 15.6µ | 7.8µ | 3.9µ | 2.0µ | 1.0µ | 0.5µ | ||||
| Chautauqua County | ||||||||||||
| 1 | 0-9 | udc | 4.3 | 11 | 52 | 57 | 5.0 | |||||
| 2 | 0-7 | udc | 5.7 | 6.4 | 11 | 22 | 32 | 41 | 49 | 56 | 65 | 1.8 |
| 3 | 0-5 | udc | 1.1 | 3 | 12 | 26 | 44 | 63 | 1.5 | |||
| 4 | 0-3 | udc | 1.6 | 6.4 | 17 | 24 | 37 | 51 | 1.0 | |||
| 3-8 | udc | 3.5 | 5.0 | 12 | 19 | 27 | 37 | 52 | 1.0 | |||
| 8-14 | udc | 2.0 | 3.7 | 11 | 22 | 31 | 35 | 57 | 1.0 | |||
| 14-18 | sh | 1.0 | 3.0 | 8.5 | 15 | 29 | 47 | 52 | 2.0 | |||
| 5 | 0-3 | udc | 0.7 | 2.9 | 48 | 65 | 2.1 | |||||
| 6 | 0-3 | udc | 3.0 | 4.2 | 17 | 29 | 45 | 60 | 72 | 2.9 | ||
| 7 | 0-5 | udc | 3.9 | 4.0 | 6.5 | 19 | 32 | 44 | 52 | 1.2 | ||
| Elk County | ||||||||||||
| 8 | 0-5 | udc | 4.1 | 6.4 | 39 | 48 | 1.7 | |||||
| 9 | 0-5 | udc | 0.5 | 5.9 | 11 | 22 | 38 | 51 | 64 | 73 | 4.0 | |
| 10 | 0-4 | udc | 0.4 | 9.2 | 21 | 32 | 50 | 62 | 73 | 79 | 7.8 | |
| 11 | 0-5 | udc | 1.0 | 4.3 | 4.7 | 17 | 38 | 54 | 67 | 77 | 4.3 | |
| Greenwood County | ||||||||||||
| 12 | 0-6 | sh | 2.2 | 8.7 | 18 | 27 | 43 | 57 | 69 | 79 | 4.9 | |
| 6-11 | sh | 1.0 | 20 | 32 | 45 | 58 | 70 | 81 | 88 | 13.0 | ||
| 13 | 0-5 | udc | 1.7 | 7.2 | 11 | 22 | 43 | 58 | 72 | 82 | 5.5 | |
| 14 | 0-3 | udc | 3.9 | 10 | 22 | 36 | 53 | 66 | 75 | 83 | 8.3 | |
| 3-8 | sh | 11 | 26 | 42 | 59 | 66 | 75 | 83 | 11 | |||
| 15 | 0-3 | udc | 0.6 | 16 | 44 | 57 | 69 | 77 | 84 | 90 | 24 | |
| 16 | 0-5 | udc | 0.4 | 10 | 20 | 33 | 50 | 64 | 74 | 82 | 8.0 | |
| 5-11 | sh | 0.2 | 4.1 | 13 | 26 | 42 | 55 | 66 | 76 | 5.0 | ||
| 17 | 0-4 | udc | 1.2 | 17 | 42 | 55 | 67 | 75 | 83 | 87 | 19 | |
| 4-7 | sh | 1.4 | 15 | 32 | 45 | 57 | 67 | 75 | 81 | 11 | ||
| 18 | 0-5 | udc | 0.3 | 16 | 35 | 60 | 70 | 77 | 84 | 89 | 21 | |
| 19 | 0-2 | udc | 0.5 | 17 | 62 | 68 | 75 | 80 | 86 | 89 | 42 | |
| Lyon County | ||||||||||||
| 20 | 0-3 | udc | 1.3 | 10 | 26 | 40 | 56 | 61 | 70 | 79 | 10 | |
| 21 | 0-5 | udc | 1.1 | 7.0 | 36 | 50 | 2.0 | |||||
| 5-10 | udc | 4.2 | 30 | 62 | 68 | 74 | 78 | 85 | 88 | 50 | ||
| 10-18 | sh | 0.9 | 19 | 46 | 58 | 66 | 74 | 81 | 85 | 25 | ||
| Osage County | ||||||||||||
| 22 | 0-7 | udc | 1.6 | 4.6 | 17 | 45 | 60 | 69 | 86 | 6 | ||
| 7-20 | sh | 2.5 | 8.7 | 28 | 63 | 80 | 86 | 91 | 94 | 20 | ||
| 23 | 0-4 | udc | 3.0 | 3.6 | 3.9 | 17 | 36 | 53 | 65 | 74 | 4.2 | |
| 4-8 | udc | 15 | 31 | 61 | 79 | 86 | 94 | 94 | 20 | |||
| 8-16 | sh | 5.4 | 17 | 54 | 74 | 82 | 88 | 91 | 16 | |||
| 16-24 | sh | 6.8 | 22 | 58 | 78 | 85 | 90 | 92 | 95 | 17 | ||
| 24 | 0-7 | udc | 0.8 | 1.2 | 3.0 | 3.2 | 6.6 | 23 | 41 | 57 | 1.3 | |
| 7-14 | udc | 1.3 | 2.8 | 6.5 | 22 | 52 | 73 | 83 | 4 | |||
| 14-24 | sh | 3.8 | 13 | 33 | 55 | 69 | 77 | 84 | 87 | 3.5 | ||
| 25 | 0-7 | udc | 0.1 | 1.0 | 6.5 | 25 | 44 | 59 | 70 | 2.9 | ||
| 26 | 0-6 | udc | 1.2 | 1.8 | 5.3 | 18 | 41 | 58 | 68 | 78 | 2.6 | |
| Shawnee County | ||||||||||||
| 27 | 0-7 | udc | 0.6 | 1.9 | 5.1 | 18 | 39 | 71 | 76 | 1.4 | ||
| 28 | 0-5 | udc | 0.2 | 1.0 | 5.8 | 26 | 46 | 59 | 70 | 79 | 2.9 | |
| 5-9 | trans. | 5.0 | 25 | 51 | 70 | 79 | 86 | 90 | 8.3 | |||
| 9-14 | sh | 19 | 44 | 63 | 78 | 85 | 89 | 92 | 97 | 25 | ||
| 29 | 0-6 | udc | 1.2 | 4.2 | 9.8 | 25 | 41 | 54 | 67 | 77 | 2.2 | |
| 30 | 0-5 | udc | 1.9 | 9.0 | 23 | 51 | 63 | 76 | 83 | 88 | 8 | |
| Jefferson County | ||||||||||||
| 31 | 0-7 | udc | 2.3 | 2.5 | 5.0 | 13 | 29 | 43 | 80 | 1.6 | ||
| 7-14 | udc | 2.0 | 6.4 | 7.0 | 8.8 | 19 | 27 | 48 | 56 | 1.7 | ||
| 14-20 | sh | 3.7 | 10 | 12 | 14 | 27 | 45 | 69 | 3.3 | |||
| 32 | 0-4 | udc | 0.1 | 3.1 | 8.0 | 27 | 49 | 61 | 68 | 73 | 4.2 | |
| 33 | 0-4 | udc | 0.4 | 3.1 | 8.3 | 27 | 45 | 58 | 67 | 74 | 5.9 | |
| 34 | 0-6 | udc | 0.4 | 2.2 | 4.0 | 9.5 | 20 | 34 | 48 | 60 | 1.7 | |
| 35 | 0-5 | udc | 0.4 | 2.0 | 2.1 | 5.7 | 14 | 36 | 58 | 70 | 2.4 | |
| Atchison County | ||||||||||||
| 36 | 0-6 | udc | 0.2 | 1.5 | 2.4 | 4.3 | 11 | 24 | 42 | 54 | 1.3 | |
| Doniphan County | ||||||||||||
| 37 | 0-3 | udc | 1.1 | 1.1 | 6.5 | 26 | 52 | 67 | 76 | 4 | ||
| 38 | 0-7 | udc | 0.6 | 2.7 | 5.2 | 11 | 22 | 36 | 48 | 0.9 | ||
| 39 | 0-4 | udc | 0.2 | 1.0 | 6.1 | 9.3 | 18 | 32 | 44 | 0.6 | ||
| 4-12 | sh | 2.5 | 3.2 | 5.0 | 15 | 34 | 51 | 65 | 80 | 2.1 | ||
Kansas Geological Survey, Geology
Placed on web Jan. 13, 2009; originally published in Nov. 1956.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/119_6/index.html