
Kansas Geological Survey, Bulletin 102, part 3, originally published in 1953

Originally published in 1953 as Kansas Geological Survey Bulletin 102, part 3. This is, in general, the original text as published. The information has not been updated.
Detailed analyses of uranium-bearing phosphate nodules from 11 localities in eastern Kansas are reported. Seven Pennsylvanian shales were sampled. Graphs showing relationships between phosphate content and several constituents, including uranium, are presented. Combined evidence suggests that the nodules consist of collophane and dahllite with varying mixtures of impurities, primarily quartz and clay. The average composition of the 11 samples was 30.2 percent P2O5 0.017 percent U3O8 and 3.2 percent fluorine.
During the last 5 years the scope of the studies on eastern Kansas black shales by the State Geological Survey has been expanded from investigations of their possible utilization as fertilizers to the determination of their shale oil content, and more recently to the long-range search for uranium reserves. According to releases to the public press the Atomic Energy Commission has developed an economically feasible method for extraction of uranium from rock phosphate during the manufacture of superphosphate fertilizer. If these phosphatic nodules from black shales can be utilized, another large reserve of uranium will become available. Detailed analyses of the different nodules were required as a first step.
In 1949 the State Geological Survey of Kansas published a report on phosphatic shales in eastern Kansas (Runnels, 1949), and in 1952 a report on oil shale (Runnels and others, 1952) treating many of the same stratigraphic units was published. This has indicated several possible uses of the shale in bulk without special beneficiation or separation. During the course of these studies, samples of the phosphatic nodules that occur in the shales were collected and this paper reports the results of analyses of these concentrated nodules freed from the shale matrix that contains them. Metallurgical studies directed toward the development of economically feasible methods of separation of nodules from shale are now being conducted (summer 1953) by Kenneth E. Rose in our laboratories. Although the potential future value of the uranium content of these nodules is not known, the fact that their phosphate content is roughly comparable to that of commercial rock phosphate makes them of interest as a potential fertilizer source.
The 11 locations reported here include samples from seven different shale beds of Pennsylvanian age. The samples were selected to cover possible changes in composition or mineralogy with changes in stratigraphic positions. Some thin shale beds from coal stripping areas were studied because of availability, whereas other shale beds were selected because of the large reserves they contained.
In ascending order the shales examined are described below (Moore and others, 1951).
The shale above the Mulky coal was tested. This shale occurs uniformly below the Fort Scott limestone a few feet above the Mulky coal. It is black fissile shale with abundant round to oval phosphate concretions throughout. Because of the general availability of this shale, samples were collected from several locations in Crawford and Labette Counties (Table 1, lab nos. 5022, 50545, and 50546) .
Nodules from the Little Osage shale member of the Fort Scott limestone were collected because of the general uniformity of the shale as well as availability of the unit due to quarrying of the Fort Scott limestone. Shale oil analyses from this unit were uniformly high. One sample of nodules was obtained from this shale (Table 1, lab no. 50547).
The Anna shale member of the Pawnee limestone, which occurs just below the Myrick Station limestone member, was sampled because of the abundance of the nodules. In general, this shale is not readily accessible; however, the general purity of the nodules (34 percent P2O5) and the rather high value of uranium (0.02 percent U3O8) prompted its consideration.
Two samples were obtained from the Lake Neosho shale member of the Altamont limestone. In the sample from Linn County (Table 1, lab no. 5023) the nodules were collected from a weathered remnant of shale. As the nodules were somewhat anomalous chemically, a fresh sample was obtained from Crawford County (Table 1, lab no. 50548) for comparison.
The Pleasanton group contains a shale which has a consistent black fissile bituminous facies and which crops out from northern Neosho County to southern Labette County. This bituminous facies gradually increases in thickness from less than 15 feet in the northern part of Neosho County to about 30 feet in the vicinity of Parsons, Kansas. Most of this thickness was exposed in a county quarry 6 miles south and 4 miles west of Parsons (Table 1, lab no. 5021). The top 18 feet is barren of nodules but the next 10 feet, and possibly more of the unit, shows numerous if not abundant nodules. These nodules are somewhat different than many of the others in that they often have a core of iron sulfide. The shale itself contains a higher than average amount of iron sulfide. The nodule-bearing portion of this shale crops out over a wide area which is generally favorable for stripping and as such represents a very large reserve.
Nodules from the Muncie Creek shale member of the Iola limestone were the first to be examined in this study. The Wyandotte County location (Table 1, lab no. 5020) yielded nodules that averaged 37.1 percent P2O5 and 0.03 percent U3O8. There is much evidence that these nodules have been reworked, probably being redeposited during final deposition and initial compaction of the shale. Another sample from the Muncie Creek shale taken in Wilson County (Table 1, lab no. 52331) about 150 miles south showed well-formed nodules with no evidence of reworking. These nodules seem to reflect the general silty nature of the shale.
The Heebner shale member of the Oread limestone is a persistent black shale occurring between the Plattsmouth and Leavenworth limestone members. The shale is well known to oil men because of its very large gamma ray emission. The nodules collected from a fresh outcrop in Douglas County (Table 1, lab no. 52263) were generally well formed and numerous. The abundance was about the same as in other shales. However although the phosphate content was fairly high (32 percent P2O5), the uranium content was not anomalous (0.017 percent U3O8).
Table 1--Location and description of shales sampled.
| Graph no. |
Lab no. |
County | Location | Stratigraphic horizon |
Thickness feet |
|---|---|---|---|---|---|
| 1 | 5020 | Wyandotte | 12-11-24E | Muncie Creek shale member, Iola limestone |
3 |
| 2 | 5021 | Labette | SE SE 17-32-19E | Pleasanton shale | 6 ft. zone, possible 10 ft. additional |
| 3 | 5022 | Labette | SE NE 16-33-21E | Shale above Mulky coal, Cherokee shale |
3 |
| 4 | 5023 | Linn | NE NE 8-22-24E | Lake Neosho shale member, Altamont limestone |
weathered remnant |
| 5 | 50545 | Crawford | SW SE 16-31-23E | Shale above Mulky coal, Cherokee shale |
3 |
| 6 | 50546 | Labette | NE NW 2-35-20E | Shale above Mulky coal, Cherokee shale |
3 |
| 7 | 50547 | Labette | NW SW 9-34-20E | Little Osage shale member, Ft. Scott limestone |
4 |
| 8 | 50548 | Crawford | SW NW 30-29-21E | Lake Neosho shale member, Altamont limestone |
4 |
| 9 | 50549 | Labette | NW SW 3-33-20E | Anna shale member, Ft. Scott limestone |
4 |
| 10 | 52263 | Douglas | SW 25-12-19E | Heebner shale member, Oread limestone |
3 |
| 11 | 52331 | Wilson | NE SW 29-29-17E | Muncie Creek shale member, Iola limestone |
3 |
All samples of nodules were separated manually from the shale matrix. Usually this necessitated only removing a few pieces of shale clinging to some of the rougher nodules. Sometimes a tumbling action in the sample bag was sufficient. In a few instances the nodules were rinsed quickly in distilled water and allowed to dry in air. The clean nodules were then crushed in a small jaw crusher and split in a Jones riffle splitter until two samples of approximately 50 grams remained. This laboratory sample was ground by hand to pass an 80-mesh screen, placed in small paper sample bags, and stored in the chemistry laboratory.
Chemical analyses for phosphate, fluorine, uranium oxide, calcium oxide, silica, alumina, total iron as ferric oxide, magnesium oxide, sulfate sulfur, sulfide sulfur, potassium oxide, sodium oxide, and loss on ignition were made (Table 2). Uranium oxide was the most critical component and because of its small percentage was one of the more difficult analyses.
Table 2--Chemical analyses of nodules.
| Lab no. |
P2O5 | U3O8 | F | CaO | L.O.I.* | SO3 | S | SiO2 | Al2O3 | MgO | Fe2O3† | TiO2‡ | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5020 | 37.34 | 0.03 | 4.03 | 52.46 | 4.55 | nil | 0.30 | 0.93 | 2.99 | 0.64 | 0.30 | 0.21 | 0.09 | 0.07 |
| 5021 | 27.22 | 0.011 | 2.84 | 42.32 | 11.71 | 0.94 | 2.53 | 6.93 | 2.57 | 0.11 | 6.65 | 0.21 | 0.24 | 0.25 |
| 5022 | 28.62 | 0.024 | 2.76 | 39.78 | 8.97 | 0.11 | 0.15 | 12.29 | 6.75 | 0.77 | 1.26 | 0.24 | 0.08 | 0.05 |
| 5023 | 28.23 | 0.007 | 3.51 | 44.27 | 7.19 | nil | 0.21 | 11.33 | 6.18 | 0.81 | 1.72** | |||
| 50545 | 24.72 | 0.010 | 2.45 | 35.02 | 9.68 | 0.17 | 0.96 | 18.94 | 6.12 | 0.05 | 3,42 | 0.57 | 0.29 | 0.22 |
| 50546 | 29.01 | 0.010 | 2.99 | 42.12 | 9.71 | 0.13 | 0.09 | 10.37 | 6.03 | 0.08 | 1.04 | 0.37 | 0.03 | 0.05 |
| 50547 | 30.88 | 0.029 | 3.40 | 44.71 | 11.14 | 0.15 | 0.63 | 6.93 | 2.64 | 0.32 | 1.77 | 0.54 | 0.31 | 0.21 |
| 50548 | 30.44 | 0.021 | 3.43 | 43.74 | 10.39 | 0.12 | 0.70 | 8.86 | 2.59 | 0,20 | 2.38 | 0.43 | 0.03 | 0.04 |
| 50549 | 34.10 | 0.020 | 3.62 | 47.77 | 6.81 | nil | 4.07 | 1.25 | 0.11 | 2.47 | 0.37 | 0.03 | 0.05 | |
| 52263 | 31.95 | 0.017 | 3.24 | 46.38 | 6.29 | 0.44 | 0.05 | 6.73 | 5.08 | 0.35 | 0.48 | 0.36 | 0.26 | 0.36 |
| 52331 | 29.93 | 0.007 | 2.94 | 42.11 | 9.51 | 1.05 | 0.03 | 7.10 | 3.94 | 0.37 | 4.02 | 0.45 | 0.30 | |
| *Loss on ignition 105°C to 1000°C. †Total iron expressed as ferric oxide. ‡Precipitated gravimetrically with cupferron. **TiO2 not separated. |
||||||||||||||
Since expensive equipment for so few samples did not seem to be justified, a gravimetric method described by Hillebrand (Hillebrand and Lundell, 1946 ) was selected. This method is based upon the fact that sexivalent uranium is not precipitated by cupferron reagent thus allowing iron, vanadium, titanium, zirconium, tin, and copper to be separated. The sexivalent uranium can then be passed through mercury amalgamated zinc in a Jones reductor to reduce it to quadravalent uranium which can be collected by cupferron. The only disadvantage to this method is the relatively large blank determination obtained from the reagents used, primarily nitric and sulfuric acids even of "reagent grade."
Fluorine was determined by steam distillation of fluosilicic acid collected in water and titrated with thorium nitrate using an indicator composed of alizarin red and zirconium nitrate. This method as described in Scott (1939) gave precise results with fluorine contents ranging from more than 4 percent to as low as a few tenths of 1 percent. Duplicate results were obtained with Bureau of Standards samples 56b and 120.
The other determinations made were by methods usually used for rock and mineral analysis. General references are Kolthoff and Sandell (1946), Hillebrand and Lundell (1946), Scott (1939), and other comparable texts.
X-ray diffraction was used on three of the nodule samples. Ada Swineford, petrographer and clay mineralogist for the Geological Survey, prepared spectrogoniometer records of three nodule samples, one specimen of crystalline fluoapatite, and the U. S. Bureau of Standards sample 56b. Nickel-filtered copper radiation was used.
Differential thermal analysis was performed on two nodule samples by Norman Plummer, geologist in charge of the ceramics division, using a Leeds and Northrup instrument.
The x-ray diffraction analyses showed two major facts: (1) the d-values for the (231) and (004) reflections and the spacings between them from the phosphate compounds agree with the values given by Silverman, Fuyat, and Weiser (1951) for a carbonate-fluoapatite and (2) the largest impurity is quartz. The presence of quartz is not surprising as the shales themselves contain a large percentage of silt. It is surprising, however, that clay and possibly small amounts of iron and aluminum phosphates were not found. A small percentage of kaolin mineral was noted in sample 52263. Figure 1 shows the x-ray diffraction data on the five samples.
Figure l--Smoothed traced spectrogoniometer record of x-ray scattering distribution. A, 5020, nodules from Muncie Creek shale; B, 52263, nodules from Heebner shale; C, 5021, nodules from Pleasanton shale; D, Bureau of Standards 56b, Tenn. brown rock phosphate; E, crystalline fluoapatite. Abbreviations: Ap, apatite; Q, quartz; and K, kaolin. Records prepared by Ada Swineford using nickel-filtered copper radiation.
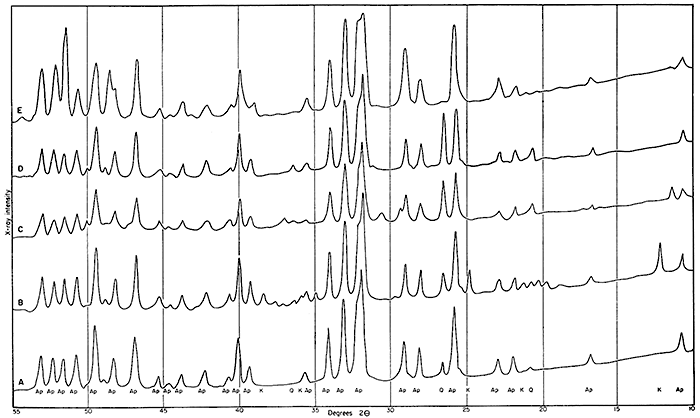
Other observations that can be made from Figure 1 are: (a) there are very few differences between the nodules and the "phosphorite" 56b; (b) the Heebner shale sample (52263) is the only sample which shows any clay, and the kaolin reflections are very sharp, suggesting large well-defined crystals; (c) there are almost no unexplained peaks. This is of interest since the chemical analyses show sufficient impurities in all the sedimentary phosphates (both nodules and 56b) to be detected by x-ray diffraction if combined in one or two compounds.
Because of the impurities reported by chemical analysis and not detected by x-ray diffraction techniques, two samples of nodules were subjected to differential thermal analysis techniques. No extraneous compounds were found, and in general the method substantiated previous conclusions that the primary mineral present is a carbonate-bearing fluoapatite. The small amount of kaolin in sample 52263 was detected.
In an effort to determine more closely the mineralogical form of the phosphate mineral (or minerals) in the nodules, the average composition of the 11 samples was calculated for comparison with the theoretical value of the various phosphate minerals. Table 3 shows this average composition. It also shows theoretical composition of fluoapatite and dahllite. An approximate recast of the major compounds in the average nodule analysis was made by assuming 3.2 percent carbon dioxide (Silverman, Fuyat, and Weiser, 1951). It is of interest to note that the calculated recast shows higher fluorine than theoretical apatite, while phosphate is definitely lower than theoretical, and calcium is about the same.
Table 3--Calculated vaLues from nodule composition.
| Element | Average composition |
Calculated composition of major constituents* |
Theoretical fluoapatite |
Theoretical dahllite |
||||
|---|---|---|---|---|---|---|---|---|
| P2O5 | 30.2 | 37.6 | 42.22 | 41.32 | ||||
| CaO | 43.7 | 54.4 | 54.01 | 52.86 | ||||
| F | 3.2 | 3.98 | 3,77 | |||||
| U3O8 | 0.017 | 0.021 | ||||||
| L.O.I** | 8.7 | (CO2) | 3.9 | (CO2) | 5.82 | |||
| *Using 3.2 percent CO2 to obtain a total of 80.34 percent major constituents. **Loss on ignition 105°C to 1000°C. |
||||||||
Rankama (in Rankama and Sahama, 1949, p. 217) states: "Collophane, the microcrystalline carbonate-fluoapatite, and dahllite (oxyfrancolite) are the chief phosphate minerals present in marine phosphate nodules." This author states (p. 591) that modern nodules collected on the coast of Southern California average 67 percent Ca3(PO4)2 with a fluorine content of 2.47 to 3.36 percent.
It was necessary to establish a uniform method for comparing the various samples. The most logical method seemed to be a series of graphs (Figs. 2 and 3). These graphs plot phosphate content against uranium oxide (Fig. 2A), fluorine (Fig. 2B), and calcium oxide (Fig. 2C). These graphs verified that the nodules have essentially the same mineral composition. They indicate also that several of the analyses for uranium content were too high. Thus the average value of 0.017 U3O8 (Table 3) seems high when compared to values obtained from Figure 2A. On the other hand, there is evidence that with a higher phosphate content more uranium will precipitate, under the general limitations of marine deposition. If a location where the phosphate concentration in the nodules is 35 percent were selected, then slightly more than 0.02 percent U3O8 could be expected. Also, one could expect that if the maximum phosphate content of 42 percent were obtained, 0.04 percent or more U3O8 would be present.
Figure 2--Graphs showing relationship between phosphate content and other constituents. A, Percent U3O8 vertically, P2O5 horizontally; B, percent F vertically, P2O5 horizontally; C, percent CaO vertically, P2O5 horizontally.
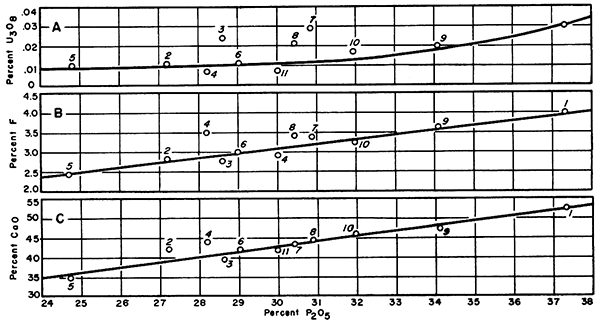
Figure 3 further shows the relationship between uranium oxide and phosphate content. In this graph uranium oxide is plotted vertically and fluorine is plotted horizontally. The result is a curve very similar to the curve obtained in Figure 2A.
Figure 3--Graphs showing relationship between A, fluorine and uranium, B, silica and alumina, and C, alumina and phosphate.
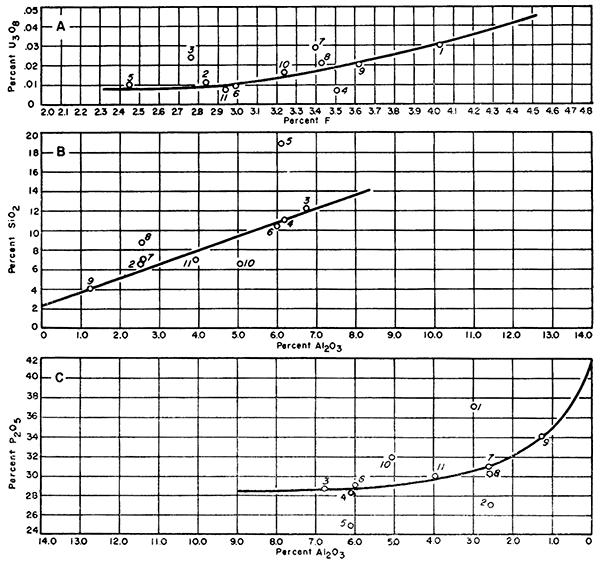
It is possible that the curve in Figure 2A could result if there were no relationship between uranium oxide and phosphate content. However, the curve obtained in Figure 3A using fluorine suggests a valid relationship. Although the fluorine is dependent upon phosphate, it varies over a short range compared with phosphate. If the uranium were present in a random manner, it would be shown by plotting it against a more sensitive component such as fluorine.
Figures 3B and 3C were prepared to see if any relationship could be established concerning silica and alumina. Figure 3B plots silica against alumina. The general grouping along a straight line suggests that aluminum silicates of some nature exist in the nodules. The values above the line indicate quartz is present. The values which are below the line indicate an excess of alumina. In Figure 3C, which plots phosphate and alumina, samples indicating an excess of alumina in Figure 3B are now above the curve. The obvious conclusion would be that the analysis for aluminum is wrong, however; sample 52263 is the sample showing kaolin and sample 5020 was rechecked several times. No final explanation for this particular constituent is apparent at this time.
The phosphatic nodules in Pennsylvanian black shales collected from 11 localities in eastern Kansas have an average composition of 30.2 percent P2O5 0.017 percent U3O8 and 3.2 percent F. These are combined in a form tentatively identified as a carbonate-bearing fluoapatite mineral. Chemically the percentages lie between those of fluoapatite and dahllite. The sedimentary origin of the nodules tends to emphasize the possibility of the presence of dahllite (carbonate apatite) but the x-ray diffraction patterns agree with previous work suggesting a single carbonate-fluoapatite mineral.
Much additional detailed work with x-ray diffraction is needed to prove definitely whether or not an intimate mixture of carbonate apatite (dahllite) and fluoapatite would appear as a separate mineral. Rankama (Rankama and Sahama, 1951) refers to collophane as a carbonate-fluoapatite but he also assumes that dahllite, a definite carbonate apatite, is usually present in sedimentary phosphates. Silverman, Fuyat, and Weiser (1951) refer to carbonate-bearing fluoapatite as a separate mineral without reference to collophane or dahllite.
For this paper we are assuming that the carbonate-bearing apatite reported by the x-ray diffraction studies is "microcrystalline collophane" and that dahllite is probably present although not definitely established.
Hillebrand, W. G., and Lundell, G. E. F. (1946) Applied inorganic analysis: 9th printing, pp. 1-929, John Wiley & Sons, Inc., New York.
Kolthoff, I. M., and Sandell, E. B. (1946) Textbook of quantitative inorganic analysis: rev. ed., pp. 1-794, Macmillan Co., New York.
Moore, R. C., and others (1951) The Kansas rock column: Kansas Geol. Survey, Bull. 89, pp. 1-132. [available online]
Rankama, Kalervo, and Sahama, Th. G. (1950) Geochemistry: Univ. Chicago Press, pp. 1-912.
Runnels, R. T. (1949) Preliminary report on phosphate-bearing shales in eastern Kansas: Kansas Geol. Survey, Bull. 82, pt. 2, pp. 37-48. [available online]
Runnels, R. T., and others (1952) Oil shale in Kansas: Kansas Geol. Survey, Bull. 96, pt. 3, pp. 157-184. [available online]
Scott, W. W. (1939) Standard methods of chemical analysis: vol. 1, 5th ed., pp. 1-1234 (N. H. Furman, editor), D. Van Nostrand Co., Inc., New York.
Silverman, S. R., Fuyat, R. K., and Weiser, J. D. (1951) The quantitative determination of calcite associated with carbonate-bearing apatites: U. S. Geol. Survey (Atomic Energy Comm.), TEI-118, pp. 1-23.
Kansas Geological Survey, Uranium-bearing Phosphate Nodules from Kansas Shales
Placed on web Feb. 5, 2013; originally published in October 1953.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/102_3/index.html