Kansas Geological Survey, Open-file Report 2012-21
by
Donald O. Whittemore
KGS Open File Report 2012-21
a report for the
Kansas Corporation Commission
Groundwater pumped from an irrigation well in north-central Stafford County was found to be saline during 2012. The well operator contacted the Kansas Corporation Commission (KCC) concerning whether the salinity could be related to oil and gas operations because the well is located within the general area of an oilfield. The KCC contacted the Kansas Geological Survey (KGS) for determining the source of salinity by geochemical identification. The KGS suggested that samples be collected from the well at different times after the start of pumping to determine whether the salinity changed during pumping. The KCC met with the irrigation well operator to start the irrigation well and collected three samples: one after the start of pumping, a second after 5 minutes, and a third after 15 minutes of pumping (Table 1). The KCC sent the samples to the KGS for analysis and interpretation.
The irrigation well is located in NE NW SE sec. 5, T. 21 S., R. 13 W., about 7 miles south of Great Bend. A WWC-5 well log exists for the well, which was drilled in 1990 to a depth of 108 ft. The well is screened at a depth interval of 37-107 ft. The gravel pack extends from a depth of 20 ft to 108 ft below the surface. The lithology between 38 ft and 106 ft is primarily sand and gravel of the High Plains aquifer. The bottom 2 feet of the borehole extend into clay with a few pieces of broken sandstone, which is bedrock of the Cretaceous Dakota aquifer. The location is about 3 miles west of where Cretaceous bedrock that underlies the High Plains aquifer and overlies Permian strata pinches out.
The chemical data for the water samples are listed in Table 1. The salinity of the water pumped from the irrigation well decreased with time after the start of pumping as indicated by the specific conductance and concentrations of total dissolved solids and major dissolved constituents. The chloride concentration decreased from 648 mg/L to 350 mg/L during the pumping. The samples changed in major constituent character from sodium-chloride type for the first two samples to sodium, calcium-chloride type for the last sample.
Table 1--Information and chemical data for water samples from the irrigation well located in NE NW SE sec. 5, T. 21 S., R. 13 W. The samples were collected by Steve Pfeifer of the Kansas Corporation Commission in 1 L plastic cube containers on November 9, 2012; shipped to the KGS by U.S. Post; and received at the KGS on November 19, 2012. The well was pumped at about 1,000 gal/min. The samples were filtered through membrane filters before analysis; the constituent concentrations represent dissolved quantities.
| KGS lab no. | Sample description |
Lab Sp.C., µS/cm |
Lab pH | SiO2 mg/L |
Ca mg/L |
Mg mg/L |
Na mg/L |
K mg/L |
HCO3 mg/L |
SO4 mg/L |
Cl mg/L |
F mg/L |
NO3-N mg/L |
Br mg/L |
TDS mg/L |
Br/Cl x 10,000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012144 | Start of pumping |
2,540 | 6.84 | 15.7 | 147 | 22.4 | 348 | 5.9 | 232 | 118 | 648 | 0.33 | 6.87 | 0.244 | 1,450 | 3.77 |
| 2012145 | 5 min after start up |
2,240 | 6.95 | 16.5 | 138 | 18.3 | 282 | 5.9 | 233 | 102 | 522 | 0.38 | 5.69 | 0.263 | 1,225 | 5.04 |
| 2012146 | 15 min after start up |
1,674 | 7.01 | 16.8 | 137 | 16.4 | 166 | 5.1 | 226 | 75.2 | 350 | 0.32 | 8.19 | 0.202 | 915 | 5.78 |
| Notation for properties of and chemical constituents in water, and units: Sp.C.--specific conductance (in micro-Siemen per centimeter at 25 deg. Celsius), SiO2--silica, Ca--calcium, Mg--magnesium, Na--sodium, K--potassium, HCO3 --bicarbonate (equal to 1.219 times the alkalinity for this water), SO4--sulfate, Cl--chloride, F--fluoride, NO3-N--nitrate-nitrogen, Br--bromide, TDS--total dissolved solids (sum of dissolved constituents, HCO3 multiplied by 0.4917), mg/L--milligram per liter (essentially the same as ppm or parts per million for a water with TDS less than a couple thousand mg/L) |
||||||||||||||||
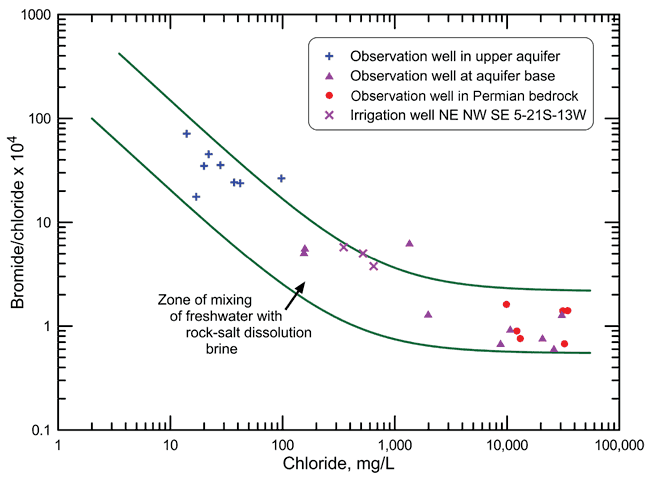
Figure 1--Bromide/chloride weight ratio versus chloride concentration with mixing curves and points for water samples.

The salinity source was identified using the sample data and mixing curves on a plot of bromide/chloride versus chloride concentration using the procedure of Whittemore (1984, 1995). The bromide/chloride plot (Figure 1) contains points for the data in Table 1 and data for water samples collected from the observation well network of Big Bend Groundwater Management District No. 5 (GMD5) during 1983-1987 as part of a study by the KGS, in cooperation with GMD5, on the saltwater intrusion in the region (Whittemore, 1993). The points shown for the GMD5 network are for those wells at the nine network sites within a 16-mile radius of the irrigation well. Each of the nine network sites includes observation wells screened at the aquifer base and within the upper aquifer. Six of the sites include observation wells screened in Permian bedrock underlying the High Plains aquifer. The three network sites without a bedrock well are the westernmost of the nine sites and are in the region where Cretaceous strata separate the base of the High Plains aquifer from the Permian rock.
The samples from the observation wells in the upper High Plains aquifer for which points are displayed in Figure 1 all contained chloride concentrations less than 100 mg/L. Samples from the observation wells screened at the base of the High Plains aquifer for which points are shown in Figure 1 contained chloride concentrations from 155 mg/L to 30,800 mg/L. The chloride concentrations of the samples from the observation wells screened in Permian strata were 9,880 mg/L to 34,800 mg/L. The chloride concentration at the base of the aquifer at the network site about 2 miles to the west of the irrigation well was 158 mg/L and at the network site approximately 4 miles to the east was 26,200 mg/L. The source of the saltwater observed in nearly all of the observation wells in the network is the intrusion of natural saltwater derived from the dissolution of halite (rock salt) in the Permian strata (Whittemore, 1993).
Figure 1 also contains two mixing curves, each of which was calculated from the conservative mixing of two end-member waters. A freshwater end member was selected for the low-chloride end of each of the two mixing curves based on the range in the bromide/ chloride ratios of the water samples with the lowest chloride concentrations observed in observation wells at the 52 network sites that existed by 1987 in GMD5. A saltwater end member was chosen for the high- chloride end of each of the two mixing curves based on the range in the bromide/chloride ratios for the most saline samples collected from the network sites. The two mixing curves define a mixing zone between freshwaters and natural halite-solution brines.
One of the points for a sample from an observation well in the upper High Plains aquifer lies above the upper mixing curve (chloride concentration 98 mg/L, bromide/chloride 0.00265). The water at this location (about 12 miles to the southeast of the irrigation well) was most likely affected by evapotranspiration concentration of water at the surface that infiltrated into the upper aquifer (Whittemore, 1993). The site is not near an oil or gas well. The water sample from the observation well contained 5.9 mg/L nitrate-nitrogen, which is at least twice that expected for the natural level of nitrate in the aquifer. This supports the premise that a surface source or mechanism not related to oil brine contamination, and probably related to agricultural activities, affected the location.
One of the points for a sample from a well screened at the base of the High Plains aquifer lies above the upper mixing curve (chloride concentration 1,350 mg/L, bromide/chloride 0.00064). The water at this location (about 6 miles to the south-southwest of the irrigation well) was determined to be affected by a small amount of oil brine contamination; 7-12% of the total chloride concentration of 1,350 mg/L was estimated to be derived from oil brine and the rest (88-93%) of the chloride was contributed by Permian saltwater intrusion (Whittemore, 1993). A few operating or plugged and abandoned oil wells were located within a half mile of the site. Oil brines in Stafford County typically have a bromide/chloride ratio within the range 0.0030 to 0.0050 (30-50 after multiplication by 10,000 as for the y-axis in Figure 1), and a chloride concentration from over 18,000 mg/L to 100,000 or more (Whittemore, 1993). The water sample from the observation well contained less than 0.1 mg/L nitrate-nitrogen.
The points for the three samples from the irrigation well lie either within the zone of mixing of freshwater with natural saltwater or on the upper boundary of that zone in Figure 1. The bromide/ chloride ratio for the samples decreases with increasing chloride concentration and fits that trend for mixing of fresher with more saline water from the natural Permian source. This indicates that the source of the salinity in the irrigation well waters is natural Permian saltwater. The location of the points close to or on the upper boundary of the mixing zone suggest that water produced by the well could be affected by a small amount by evapotranspiration concentration of the water distributed over the land surface for irrigation, followed by infiltration to the groundwater, especially through the gravel pack in the annulus of the well between the casing and the borehole. The nitrate-nitrogen concentration of 5.7-8.2 mg/L, which is greater than expected for the natural range, supports this premise.
The source of saline groundwater pumped from the irrigation well located in NE NW SE sec. 5, T. 21 S., R. 13 W. is natural intrusion of saltwater from Permian strata that enters the Dakota bedrock underlying the High Plains aquifer at the location and then intrudes into the base of the aquifer. The well is located where some natural salinity is expected at the base of the High Plains aquifer as indicated by the west to east gradient of increasing chloride concentration in the area shown by GMD5 observation network wells. The irrigation well is completed to the base of the High Plains aquifer where sand and gravel directly overlie the Dakota bedrock without any clay separating the aquifer from the bedrock. The well is also completed two feet into the Dakota strata, which could potentially allow a little additional movement of saline water into the well in comparison to a well completed only to the bottom of the High Plains sediments.
Whittemore, D. O., 1984, Geochemical identification of salinity sources; in, Salinity in Watercourses and Reservoirs (Proceedings of the International Conference on State-of-the-Art Control of Salinity), R.H. French, ed.: Stoneham, MA, Ann Arbor Science, Butterworth Publishers, p. 505-514.
Whittemore, D. O., 1993, Ground-water geochemistry in the mineral intrusion area of Groundwater Management District No. 5, south-central Kansas, Kansas Geological Survey, Open-File Report 93-2, 107 p. [available online]
Whittemore, D. O., 1995, Geochemical differentiation of oil and gas brine from other saltwater sources contaminating water resources: Case studies from Kansas and Oklahoma: Environmental Geosciences, v. 2, p. 15-31.
KGS-OFR-2012-21.pdf (848 kB)
To read this file, you will need the Acrobat PDF Reader, available free from Adobe.
Kansas Geological Survey
Updated Dec. 21, 2012
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Hydro/Publications/2012/OFR12_21/index.html