Prev Page--Discharge || Next Page--Regions
Chemical Character of Water
The chemical character of the ground water in Elk County is indicated by the 24 analyses of water given in Table 5. The water samples were collected from typical wells and springs in the principal aquifers and were taken from places spaced as evenly as practicable within the county.
The samples were analyzed by Howard A. Stoltenberg, chemist, in the Water and Sewage Laboratory of the Kansas State Board of Health in Lawrence.
Dissolved Solids
The residue left after a natural water is evaporated consists of rock minerals and minor amounts of organic material and water of crystallization.
Water containing less than 500 parts per million of dissolved solids is generally regarded as satisfactory for domestic use, except for difficulties resulting from hardness or excessive iron content. Water containing more than 1,000 ppm of dissolved solids may contain enough of certain constituents to cause a noticeable taste or to make the water unsuitable for use in some other respects. The amount of dissolved solids in the 24 samples collected in Elk County is given in Table 6.
Table 6--Dissolved solids in water samples from wells and springs in Elk County.
| Dissolved solids (parts per million) |
Number of samples |
|---|---|
| Less than 300 | 1 |
| 300 to 499 | 9 |
| 500 to 749 | 6 |
| 750 to 999 | 4 |
| 1,000 or more | 4 |
The water from 10 wells of a total of 24 that were sampled in Elk County had less than 500 ppm of dissolved solids and is suitable for most domestic uses. Ten samples had between 500 and 1,000 ppm of dissolved solids, and the water from 4 wells contained more than 1,000 ppm. Well 28-11-24cd had the highest concentration of dissolved solids, 5,130 ppm.
Hardness
Hardness is the property of water that generally receives the most attention, because of its effect when soap is used with the water. Nearby all the hardness in water is caused by calcium and magnesium, which also cause most of the scale in boilers.
In addition to total hardness, Table 5 gives the carbonate and noncarbonate hardness. The carbonate hardness is due to the presence of calcium and magnesium bicarbonates and may be almost completely removed by boiling. The noncarbonate hardness is caused by the presence of sulfates and chlorides of calcium and magnesium. The carbonate hardness is sometimes called temporary hardness, and the noncarbonate hardness is called permanent hardness.
Water having a hardness of 50 ppm or less is soft; hence, treatment for softening water of this type is not ordinarily necessary. Water having a hardness of 50 to 150 ppm is suitable for most uses, but does increase the use of soap; therefore industries such as laundries, which are large users of soap, find it profitable to soften the water. The fact that hard water causes scale in boilers is an additional reason for industry to do this. Hardness of more than 150 ppm can be noticed by almost anyone, and when the hardness reaches 200 ppm, it requires reduction for most uses. Where municipal water supplies are softened, the hardness is generally reduced to 80 to 100 ppm. The improvement of the water by further softening is not generally thought to be worth the increased cost.
Of 24 samples of water collected from wells in Elk County, two had a hardness of less than 50 ppm, one had a hardness between 150 and 300 parts, and 14 had a hardness of more than 300 parts, including two that had a hardness of more than 1,000 ppm. The two samples in which the water had a hardness of less than 50 ppm were of the sodium bicarbonate type; that is, they had a very large percentage of sodium and bicarbonate and carbonate but a small percentage of calcium and magnesium. The relation between sodium, calcium, and magnesium content and hardness is shown in Figure 10, which is a diagram of the analyses of two soft and two hard waters. The hardness of the 24 samples of water collected in Elk County is given in Table 7.
Figure 10--Graphical representation of analyses of water from wells in principal water-bearing formations in Elk County.
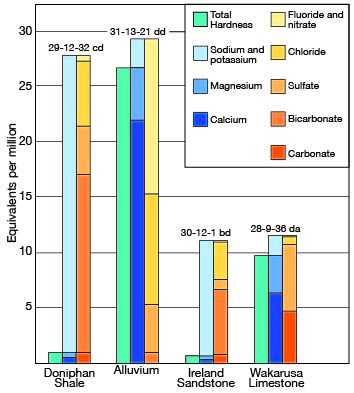
Table 7--Hardness of water from typical wells in Elk County.
| Hardness as CaCO3 (parts per million) |
Number of samples |
|---|---|
| Less than 50 | 2 |
| 50 to 149 | 1 |
| 150 to 299 | 7 |
| 300 to 499 | 10 |
| 500 to 999 | 2 |
| 1,000 or more | 2 |
Iron
Iron is a common troublesome constituent of ground water. The quantity of iron in the water may vary greatly from place to place, even within the same formation. If iron is present in ground water in quantities in excess of about 0.3 ppm, the excess iron will precipitate upon exposure to air. Generally where iron is present in sufficient quantity to cause a disagreeable taste or to stain cooking utensils and textiles, it may be removed by aeration and filtration. In some waters the addition of other chemicals is necessary to remove the iron. Table 8 indicates the iron content in the 24 samples of water from wells and springs in Elk County.
Table 8--Iron content of water samples from wells and springs in Elk County.
| Iron (parts per million) | Number of samples |
|---|---|
| Less than 0.1 | 6 |
| 0.10 - 0.29 | 8 |
| 0.30 - 0.99 | 3 |
| 1.0 - 1.9 | 3 |
| 2.0 or more | 4 |
Fluoride
Generally only a small amount of fluoride is present in ground water. Fluoride in water consumed by children is closely related to dental health. Water containing more than about 1.5 ppm of fluoride may cause mottling of tooth enamel, the severity of the mottling increasing with the fluoride content. Small quantities of fluoride, not sufficient to cause mottled enamel, may reduce tooth decay (Dean, 1936'; Dean, Arnold, and Elvove, 1942).
In Elk County only one sample of water (well 29-12-32dc) had a fluoride content greater than 1 ppm; this had a fluoride concentration of 9 ppm.
Nitrate
The range of nitrate content in waters in Elk County is great. The source of nitrate in well water in Kansas is not definitely known. One possible source is nitrate-bearing rocks within the aquifers, although such rocks have not been found in Kansas in association with well waters containing excessive nitrate. Another source is contamination of the well by surface water containing high concentrations of nitrate. These surface waters may derive the nitrate from barnyards, artificial fertilizers, or nitro-biological activity associated with certain legumes that increase the nitrate in the soil.
Dug wells are more susceptible to contamination than drilled wells, because they are more difficult to seal at and just below the surface than drilled wells, in which the casing generally serves as a good seal against contamination. Table 9 compares nitrate contents in 9 dug and 14 drilled wells sampled in Elk County.
Table 9--Comparison of nitrate in dug and drilled wells in Elk County.
| Nitrate (parts per million) |
Number of samples | |
|---|---|---|
| Dug wells | Drilled Wells | |
| Less than 25 | 1 | 8 |
| 25 to 49 | 2 | 3 |
| 50 to 99 | 0 | 0 |
| 100 to 199 | 3 | 1 |
| 200 or more | 3 | 2 |
Excessive nitrate in well water may cause infant cyanosis ("blue baby") when the water is used in the preparation of formulas. The Kansas State Board of Health regards water containing more than 90 ppm (as NOR) as unsafe for use in infant feeding and regards water containing less than 50 ppm as safe. The nitrate in well water cannot be removed by boiling, as that only concentrates the nitrate; and its removal by chemical means cannot be done practically.
In Elk County 14 of the 23 samples of water contained less than 50 ppm of nitrate and were in the safe range. Nine samples had a nitrate content of more than 100 ppm. The highest nitrate content was in water from well 31-13-21dd in alluvium. This water contained 885 ppm of nitrate (Table 5).
Chloride
Chloride is abundant in sea water and oil-field brines and is dissolved in small quantities from many rock materials. Chloride where concentrated is corrosive to steam boilers.
In Elk County only four samples of water had more than 150 ppm of chloride. The highest, 565 ppm, was in the sample from well 28-11-24cd. Water containing 550 ppm of chloride tastes salty to most persons; as little as 250 ppm can be detected by some persons.
Sanitary Considerations
The analyses of the water from wells in Elk County (Table 5) indicate only its mineral constituents and do not show its sanitary condition. Concentrations of certain minerals, however, such as chloride or nitrate, may indicate possible pollution.
In Elk County nearly all the rural population is dependent on wells for a water supply. In construction of wells, therefore, care should be taken to prevent pollution. A well should not be constructed near possible sources of pollution, such as barnyards, cesspools, or privies. The well should be so constructed that surface water drains away from the well rather than toward it.
If it is necessary to drill a well where drainage will be toward it, the well should be finished with earth mounded around it as a barrier against surface water. Drilled wells are generally protected by the casing which forms a seal, but in some areas, wells must be dug to obtain adequate storage capacity.
Prev Page--Discharge || Next Page--Regions
Kansas Geological Survey, Geology
Web version July 2002. Original publication date July 1958.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Elk/04_gw4.html