Kansas Geological Survey, Open-File Rept. 93-1
Annual Report, FY92--Page 18 of 20
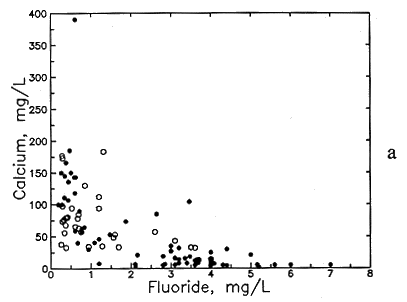
Figure 49--Association of (a) calcium and fluoride concentrations and (b) sodium and fluoride concentrations in ground waters from the Dakota aquifer. Open circles are for the Dakota outcrop or subcrop; filled circles are for the Dakota aquifer where confined by Upper Cretaceous strata.

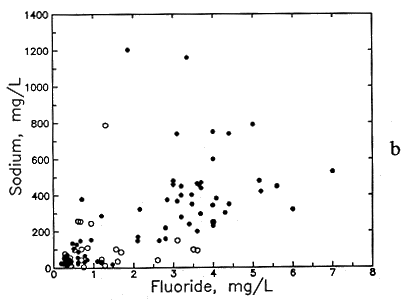
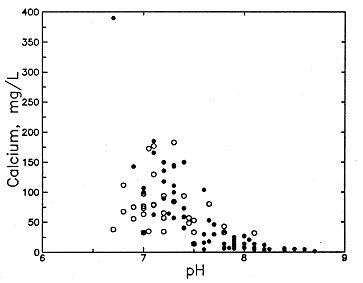
Fluoride concentration in Dakota aquifer water is correlated with the concentrations of certain major dissolved constituents. Fluoride is generally inversely related to dissolved calcium and magnesium. Although low fluoride content (<1 mg/l) exists in a wide range of calcium concentrations, the calcium is always greater than 30 mg/l (Figure 49a). Moderately high fluoride levels (>2 mg/l) nearly always occur in waters with calcium contents of less than 100 mg/l and very high fluoride (>4 mg/l) are found only when dissolved calcium is less than 30 mg/l. The association with magnesium is similar, although not as distinct. Fluoride is generally directly related to dissolved sodium and bicarbonate concentrations and pH (Figures 49b and 50). Although the correlation with sodium and bicarbonate is poor as a whole, the lower and upper parts of the point distributions in Figures 49b and 50a form better linear relationships such that the sodium-fluoride distribution forms a fan starting from low sodium and fluoride and the bicarbonate-fluoride distribution forms a band extending to higher values.
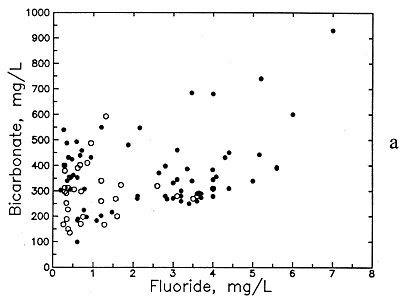
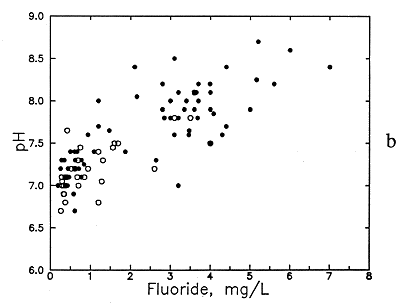
Figure 50--Association of (a) bicarbonate and fluoride concentrations and (b) pH and fluoride concentrations in ground waters from the Dakota aquifer. Open circles are for the Dakota outcrop or subcrop; filled circles are for the Dakota aquifer where confined by Upper Cretaceous strata.
The calcium-fluoride, sodium-fluoride, and bicarbonate-fluoride relationships can be expressed as the common association of low fluoride concentration with Ca-HCO3 waters that are found in the outcrop and subcrop areas of the Dakota aquifer. Higher fluoride concentrations occur in Na-HCO3 waters or in mixed cation-anion type waters with lower calcium and magnesium and appreciable percentages of sodium and bicarbonate. These waters tend to occur in the part of the aquifer confined by Upper Cretaceous rocks. The relationship of the fluoride concentration with the aquifer type is illustrated in Figures 49 and 50. Low to moderately high fluoride concentrations occur in ground waters in both the outcrop and subcrop and confined regions of the aquifer, whereas high fluoride concentrations exist only in the confined aquifer.
Data for wells and boreholes in central Kansas indicate that in the confined region the dissolved fluoride concentration is low in the Upper Cretaceous aquitard and increases with depth from the top of the Dakota and then decreases with depth at the bottom of the Dakota aquifer into Permian strata. The calcium and magnesium concentrations of ground waters in the same sequence of strata in central Kansas follow an inverse pattern, first decreasing and then increasing from the top to the bottom of the Dakota aquifer system. Dissolved sodium and chloride contents generally increase throughout the aquifer, although the changes occur at different rates and depend on the permeability of the strata. Sulfate either increases or first decreases and then increases from the top to the bottom of the Dakota aquifer.
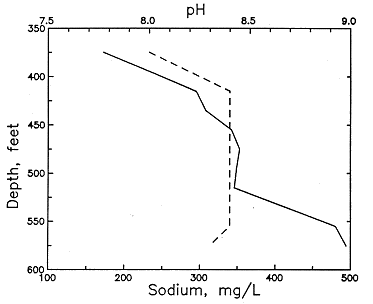
An example of the changes with depth and pH in these dissolved constituents in the Dakota Formation is illustrated in Figures 51 and 52 for a test well in Ellis County. The decrease in calcium (and magnesium) and the increase in sodium content is marked in the upper part of the aquifer. The increase in dissolved sodium is greater than that of chloride in the upper part of the formation, whereas the increase in chloride is greater toward the bottom. Cation exchange of calcium and magnesium for sodium on clays in the strata explains the changes. The exchange positions on the clays in the Dakota sediments were probably first saturated with more sodium than calcium and magnesium as a result of the presence of marine water in the sediment pores followed by later diffusion and dispersion of Na-Cl waters from the underlying Permian rocks. After uplift and erosion of much overlying strata, recharge with Ca-HCO3 waters from southeastern Colorado and southwestern Kansas flowed through the Dakota aquifer to dilute and flush saltwater. The throughflow increased as discharge became greater near the boundary of the confined and outcrop and subcrop parts of the system as a result of erosion of Dakota strata. The calcium plus magnesium to sodium ratios in the throughflow water and in the recharge containing moderate to high calcium and magnesium flowing from the Upper Cretaceous rocks to the top of the Dakota Formation were appreciably higher than the ratio in the water in equilibrium with the clays. The resultant replacement of much sodium by the doubly charged cations caused decreases in calcium and magnesium to below several milligrams per liter and increases in sodium to produce molar sodium/chloride ratios of greater than 1 in some Dakota waters.
Figure 51--Changes in (a) calcium and fluoride concentrations and (b) sulfate and chloride concentrations with depth in ground waters from the Dakota Formation in a test well in Ellis County.
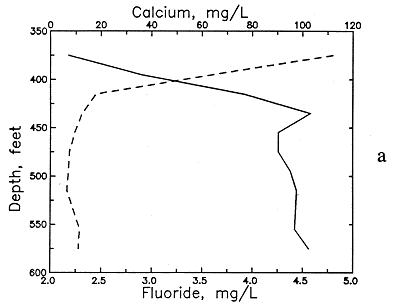
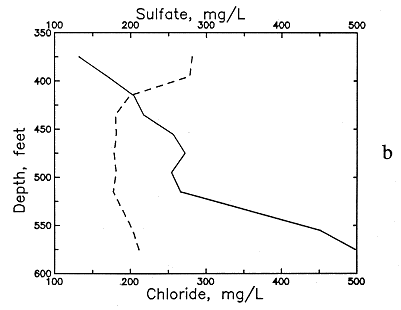
Figure 52--Changes in pH and sodium concentration with depth in ground waters from the Dakota Formation in a test well in Ellis County.
The upper limits of the point distribution for observed fluoride and calcium concentrations in Figure 49a form a hyperbolic curve that suggests that solubility of calcium- and fluoride-containing minerals controls the concentration. Activity products were calculated for chemical species in solution in Dakota waters and were compared with the solubility products of different minerals using the geochemical model SOLMINEQ.88. The computations for Dakota ground waters containing higher fluoride concentrations indicate that the waters are usually somewhat undersaturated (a potential to dissolve more of a mineral in water) with respect to fluorite. The waters are undersaturated to supersaturated (a potential to precipitate a mineral from water) with respect to hydroxyapatite and supersaturated with respect to fluorapatite. The results and the expected mineralogy of the aquifer sediments suggest that hydroxyapatite, in which some of the hydroxyl positions are occupied by fluorine, could be a major mineralogical source of the dissolved fluoride. Precipitation of fluorite might limit fluoride concentration in zones where Permian waters with high calcium concentrations mix with high-fluoride waters in the lower part of the Dakota aquifer. The diffusion and dispersion of high-calcium waters from the Permian certainly would limit the dissolution of apatites in the lower Dakota aquifer.
A source of additional fluoride at higher pH (Figure 50b) could be the exchange of hydroxyl ions for fluoride ions in labile positions on fluorine-containing hydroxyapatite and in phyllosilicates, mainly clays and weathered micas, such as biotite, that are present in the fine- grained sediments of the Dakota aquifer. pH values of greater than 8.5 are probably necessary for this process to be a substantial fluoride source relative to dissolution of apatites because the hydroxyl ion concentration would be too low at lower pH values. Alkaline pH can be generated by the dissolution of carbonate minerals, which could occur after cation exchange decreased calcium and magnesium concentrations to give solutions undersaturated with respect to calcite and dolomite. The general increase in pH with the decrease in dissolved calcium is illustrated in Figure 53. The highest pH's occur in the confined portion of the aquifer, as expected from the greater cation exchange and the concomitant carbonate dissolution in that aquifer environment. No pH's higher than 9 appear to occur in the Dakota aquifer based on available analyses.
Figure 53--Association of calcium concentration and pH in ground waters from the Dakota aquifer. Open circles are for the Dakota outcrop or subcrop; filled circles are for the Dakota aquifer where confined by Upper Cretaceous strata.
Dissolution of carbonates is a major contributor to higher bicarbonate concentration in the Dakota aquifer. The association of higher bicarbonate and lower calcium with dissolution of apatite minerals explains the correlation of fluoride and bicarbonate (see Figure 50a). Again, the highest bicarbonate and fluoride contents occur in the confined aquifer. Another contributor to bicarbonate in the confined portions of the aquifer is oxidation of organic matter to produce dissolved carbon dioxide. Formation of bicarbonate from the carbon dioxide produces acidity, which further drives the dissolution of carbonate minerals or buffers the pH increase from carbonate dissolution caused by the decrease in calcium and magnesium from cation exchange. The wide band of increasing bicarbonate with increasing fluoride in Figure 50a is probably related to both processes.
Although geochemical equilibria computations indicate that some Dakota waters are in equilibrium with respect to calcite and dolomite (i.e., they would not dissolve or precipitate these minerals), other portions of the aquifer contain waters moderately undersaturated with respect to these minerals. The undersaturation could occur in those strata where essentially all available calcite and dolomite have been dissolved. The strata either could have had low initial contents of the carbonate minerals or are more permeable sediments that have allowed a greater throughflow of waters that have been able to dissolve and remove existing carbonate minerals. Greater oxidation of organic matter in portions of the aquifer sediments could also have increased the rate of carbonate dissolution in the past, especially where throughflow could more readily transport dissolved oxygen in the recharge areas.
Another contributor to the range in the constituent concentrations correlated with fluoride at a given fluoride value is the effect of ionic strength on mineral solubilities. As the total concentration of charged species dissolved in water increases in the range from freshwater to saltwater, the solubility of a mineral increases because of the interaction of the charged ions. Although much of the correlation between sodium and fluoride in Figure 49b is related to the increase in sodium with cation exchange and the associated decrease in calcium and dissolution of apatites, some of the correlation could be caused by the ionic strength effect. The sodium, along with the chloride, from the underlying Permian is the main contributor to increasing ionic strength in Dakota aquifer waters. However, the Na-Cl water from the Permian strata also contains relatively high calcium and magnesium contents which limit the cation exchange and dissolution of fluoride-containing apatites in the lower Dakota aquifer. The combined result of all the processes is the fanlike appearance of the point distribution in the sodium and fluoride graph.
Finally, another contribution to the range in calcium and bicarbonate concentrations at a given dissolved fluoride value is the effect of sulfate on calcite and dolomite solubility. Sulfate forms the ion pairs CaSO40 and MgSO40 dissolved in water, thereby decreasing the free concentrations of dissolved Ca2+ and Mg2+ that enter into the solubility equations for calcite and dolomite. A larger sulfate concentration has the indirect result of increasing the solubility of calcite and dolomite. The concentration of sulfate varies in the upper Dakota aquifer because of differences in the composition of recharge and oxidation of sulfide in pyrite within the Dakota and increases in the lower Dakota from the upward diffusion and dispersion of Permian saltwater. The source of the sulfate in the recharge is largely dissolution of gypsum formed during pyrite weathering in the presence of high calcium water. The sulfate source in the Permian saltwater is dissolution of anhydrite in Permian strata. Some areas of lower sulfate content in the Dakota aquifer could be related to reduction of some sulfate to sulfide during the oxidation of organic matter. Such waters would be expected to have a hydrogen sulfide odor, as observed in a few well waters.
The present distribution of fluoride in the Dakota aquifer is controlled by chemical processes and by the relative rates of recharge and discharge in different parts of the system. The chemical processes have had an appreciable time to operate during the recharge of water into the Dakota in southeastern Colorado and southwestern Kansas and operate in the discharge area along the eastern outcrop and subcrop area. Local recharge and discharge in southeastern Colorado and southwestern Kansas and in the outcrop zone in central Kansas are more rapid than the regional flow and have flushed out past high-fluoride Na-HCO3 type water, which probably existed in that area when the aquifer was covered by confining sediments. The faster flow rates have also removed the high sodium content of the sediments by faster attainment of equilibrium with the higher calcium and magnesium to sodium ratio of the recharge. The highest fluoride concentrations in the aquifer occur in the confined aquifer in west-central Kansas, where regional flow is slowly flushing saline water from the aquifer, but the processes have not yet had time to substantially reduce the high sodium content of the clays. Continued cation exchange and concomitant dissolution of fluoride-containing minerals and an increase in pH from carbonate dissolution and concomitant exchange of hydroxyl ions for labile/exchangeable fluoride ions on clays and micas can occur in this area because the capacity of the sediments and the time for the processes to occur is sufficient, and because the amount of regional flow and recharge from overlying rocks is sufficient to supply waters with a relatively high calcium plus magnesium to sodium ratio without being so high that flushing removes and dilutes the Na-HCO3 water at a substantial rate.