Kansas Geological Survey, Open-File Rept. 93-1
Annual Report, FY92--Page 17 of 20
Ground water discharges from the Dakota aquifer to alluvial aquifers along the eastern outcrop-subcrop belt. The discharge mixes with direct recharge from precipitation on the alluvium, with recharge from rock units younger and older than the Dakota Formation and with recharge from Dakota rocks cropping out at higher elevations where river valleys have cut deeply into the aquifer strata. The mixture of recharge in the alluvium then discharges to the river and is the main control on the major dissolved constituents in the river water during moderate to low flows. Ground water in the Dakota to the west of the outcrop-subcrop boundary of the aquifer in central Kansas is saline. Where the saline water has not been diluted by local freshwater recharge to outcropping Dakota strata, the saline discharge contributes to the salinity of stream and river waters.
Four river valleys cross the eastern edge of the confined Dakota aquifer where saline ground water is present: the Republican, Solomon, Saline, and Smoky Hill rivers. Saline Dakota discharge enters the alluvium of the Republican River valley in southwestern Republic and northern Cloud counties, the Solomon River valley in Mitchell and southwest Cloud counties, the Saline River valley in Russell and Lincoln counties, and the Smoky Hill River valley in southeast Ellis, southern Russell, and northwest Ellsworth counties. The most saline water generally discharges several miles or more downstream of where the outcrop-subcrop boundary first crosses the river valley. The impact on the river-water quality depends not only on the amount of the saline ground-water discharge but also on the river flow that dilutes the discharge.
Appreciable portions of the areas of natural saline discharge to the Smoky Hill and Saline River valleys are also within oil and gas fields. Although oil fields are not present in the area of saline Dakota discharge to the Solomon River valley, they do occur upstream in the South and North forks of the river. A water-quality concern is the possible contribution of oil-field brine pollution to the total salinity of the alluvial aquifer and hence the river. The Kansas Water Office (KWO) and the KGS conducted a study of selected portions of the Solomon, Saline, and Smoky Hill rivers to assess whether oil brine is currently contributing to the total salinity of the river water. The KWO collected river-water samples during low flows in October 1991 and January 1992 (Table 5) and gave them to the KGS for geochemical identification of salinity sources. The KGS had previously collected samples in 1988 from the Saline River above and below the Haberer salt marsh in northwestern Russell County; these samples are included in the discussion. During the sampling in January 1992, the KWO also collected municipal waste water discharged from the cities of Stockton and Hayes and saltwater from a well in the Cedar Hills Sandstone in north-central Ellis County to provide waters that could be used to assist in the differentiation of natural and anthropogenic salinity.
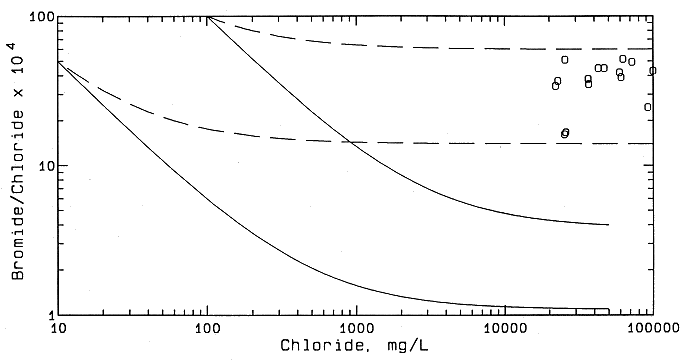
The chloride sources were identified using mixing curves and background data for halite solutions in Permian strata, ground waters in the Dakota aquifer system, and oil brines in central Kansas. The method is based on the occurrence of different bromide/chloride ratios in saltwaters of different origin and the conservative behavior of chloride and bromide in surface and ground waters. Sulfate/chloride ratios are also useful for complementing interpretations based on the bromide/chloride data. Halite solutions have low bromide/chloride ratios, whereas oil brines in Kansas typically have ratios more than 20 times greater. Saline ground waters in the Dakota aquifer have low bromide/chloride ratios, indicating intrusion of underlying Permian saltwater, although the ratios are slightly elevated over most halite dissolution waters given the same chloride concentration. These relationships are illustrated in Figure 45, which contains a zone of mixing between freshwaters and saltwaters in the Dakota aquifer and points for oil-field brines in central Kansas. Each curve on the diagram represents the conservative mixing between two end-point waters. Points for Dakota waters are not plotted in Figure 45 but would plot between the two solid curves that form the boundaries of the Dakota mixing zone. The zone of mixing of freshwaters with chloride contents less than 100 mg/l with oil brines is bounded by the two dashed curves. The estimation of chloride concentrations from a mixture of two salinity sources can be estimated using intersecting mixing curves on a bromide/chloride versus chloride graph. The method has been successfully used at the KGS for saltwater source identification in many different locations.
Figure 45--Zone of mixing of freshwaters with natural saltwaters in the Dakota aquifer and zone of mixing of Dakota freshwaters with oil brines in central Kansas based on bromide/chloride mass ratio versus chloride concentration. The open circles are oil brines.

Table 5.--Location Description and Flow for River Water Samples.
| Location description | Location | Sample date | Flow (cfs) |
|---|---|---|---|
| South Fork Solomon River | |||
| 1 mi east of Graham County at gage above Webster Reservoir, stage 1.09 | 8S 20W 07AD | 1-08-92 | <1 |
| 3 mi west of Stockton, river pool | 7S 18W 33BC | 1-08-92 | <1 |
| Just south of Stockton | 7S 18W 25AC | 1-08-92 | 1 |
| South of Woodston at gage, stage 4.03 | 7S 16W 16DD | 1-08-92 | 1.5 |
| South of Alton, beaver dam upstream | 7S 15W 12CA | 1-08-92 | 9 |
| South of Bloomington | 7S 13W 18DD | 1-08-92 | 12 |
| Osborne at gage, beaver dam nearby | 7S 12W 19DA | 1-08-92 | 12 |
| North of Corinth | 7S 11W 22BB | 1-08-92 | 13 |
| Saline River | |||
| 1 mi upstream of Haberer salt marsh | 12S 15W 15ABBB | 7-07-88 | Nb |
| 100 ft below stream from Haberer salt marsh, north side of river | 12S 15W 14CAA | 7-07-88 | N |
| Bridge 1 mi downstream of Haberer salt marsh | 12S 15W 13CBA | 7-07-88 | N |
| At gaging station near bridge of US-281, 4 mi north of Russell | 12S 14W 34ADDD | 10-29-91 | <1 |
| Bridge north from Bunker Hill | 12S 13W 25BCCC | 10-29-91 | <1 |
| Smoky Hill River and Big Creek | |||
| 1 mi east of Pfeifer, ponded, no flow | 15S 17W 25DD | 1-07-92 | 0 |
| 3 mi west of Russell County line, north side of river | 15S 16W 27BD | 1-09-92 | 0.5 |
| 1 mi east of Ellis County line, ponded, beaver dams, south side of river | 15S 15W 20BB | 1-07-92 | N |
| 4 mi east of Ellis County line, south side of river | 15S 15W 10DDA | 1-07-92 | N |
| Above junction with Big Creek, north side of river, flow measured 1-09-92 | 15S 14W 06BC | 1-07-92 | 1.6 |
| Big Creek near junction with Smoky Hill River, flow measured | 14S 15W 31BCC | 1-07-92 | 5.0 |
| 2 miles west of US-283, north side of river | 14S 14W 33BBB | 1-07-92 | 11 |
| Bridge of US 281, 6.5 miles south of Russell, north side of river | 15S 14W 02BCCC | 10-30-91 | <2 |
| As above | 1-07-92 | N | |
| Bridge 4.5 miles south, 1 mile east of Homer, north side of river | 14S 13W 32ADAD | 10-30-91 | 2.5 |
| As above, stage 2.21 | " | 1-07-92 | 12 |
| Bridge 5 miles south of Bunker Hill, north side of river | 14S 13W 36ADDA | 10-30-91 | 3 |
| As above | " | 1-07-92 | N |
| Bridge 3 miles south of Dorrance, north side of river | 14S 11W 31BADC | 10-30-91 | 3 |
| As above, flow measured | " | 1-07-92 | 15.5 |
The samples are listed in downstream order for each river. The first three samples from the Saline River were collected by the Kansas Geological Survey; the other samples were collected by the Kansas Water Office. Flows are estimated except where indicated as measured.a. Township, range, section, quarter sections from largest to smallest quarter, A = NE, B = NW, C = SW, D = SE.
b. Flow not estimated.
Waters from the South Fork Solomon River in Rooks and Osborne counties contain a greater concentration of sulfate (in mg/l) than chloride (Table 6). The main influence on the major dissolved constituents is probably mineralized ground-water discharge from Upper Cretaceous rocks. In general, the waters become fresher in the downstream direction as the river flow increases. The chloride concentration remains below the recommended limit for drinking water of 250 mg/l. No oil brine could be conclusively identified as contributing substantially to the chloride content of waters from the South Fork Solomon River.
Table 6.--Specific Conductance and Dissolved Constituent Concentrations for the River Waters.
| Sample description | Sample date | Sp. C. (microS/cm) | Cl (mg/l) | SO4 | F (mg/l) | Br (mg/l) |
|---|---|---|---|---|---|---|
| South Fork Solomon River | ||||||
| 1 mi east of Graham County line | 1-08-92 | 1,645 | 157 | 281 | 0.40 | 0.28 |
| 3 mi west of Stockton | 1-08-92 | 1,380 | 138 | 318 | 0.39 | 0.21 |
| Just south of Stockton | 1-08-92 | 1,650 | 234 | 260 | 0.30 | 0.16 |
| South of Woodston at gage | 1-08-92 | 1,450 | 148 | 298 | 0.30 | 0.28 |
| South of Alton | 1-08-92 | 1,165 | 101 | 255 | 0.29 | 0.20 |
| South of Bloomington | 1-08-92 | 1,135 | 89.4 | 229 | 0.28 | 0.18 |
| Osborne at gage | 1-08-92 | 1,125 | 87.5 | 229 | 0.27 | 0.18 |
| North of Corinth | 1-08-92 | 1,145 | 88.4 | 232 | 0.26 | 0.18 |
| Saline River | ||||||
| Upstream of salt marsh | 7-07-88 | 2,430 | 405 | 531 | 0.5 | 0.52 |
| At salt marsh | 7-07-88 | 3,600 | 735 | 595 | 0.6 | 0.69 |
| Downstream of salt marsh | 7-07-88 | 3,650 | 747 | 601 | 0.6 | 0.70 |
| North of Russell | 10-29-91 | 16,150 | 4,650 | 1,431 | 0.75 | 1.70 |
| North of Bunker Hill | 10-29-91 | 25,300 | 7,720 | 2,100 | 0.72 | 1.97 |
| Smoky Hill River and Big Creek | ||||||
| 1 mi east of Pfeifer | 1-07-92 | 1,790 | 134 | 633 | 0.28 | Int.b |
| 3 mi west of Russell County line | 1-09-92 | 2,440 | 408 | 422 | 0.90 | 0.24 |
| 1 mi east of Ellis Co. line | 1-07-92 | 3,310 | 724 | 399 | 0.80 | 0.32 |
| 4 mi east of Ellis Co. line | 1-07-92 | 3,500 | 760 | 401 | 0.89 | 0.31 |
| Above jct. with Big Creek | 1-07-92 | 6,810 | 1,781 | 556 | 0.75 | 0.50 |
| Big Creek near junction | 1-07-92 | 1,800 | 330 | 203 | 0.59 | 0.58 |
| 2 mi west of US-281 | 1-07-92 | 3,800 | 910 | 340 | 0.64 | 0.69 |
| US-281 south of Russell | 10-30-91 | 7,020 | 1,879 | 530 | 0.56 | 2.69 |
| " | 1-07-92 | 3,600 | 843 | 328 | 0.63 | 0.74 |
| South of Homer | 10-30-91 | 6,450 | 1,702 | 538 | 0.55 | 2.29 |
| " | 1-07-92 | 3,310 | 790 | 293 | 0.58 | 0.98 |
| South of Bunker Hill | 10-30-91 | 4,590 | 1,184 | 293 | 0.56 | 2.14 |
| " | 1-07-92 | 3,200 | 746 | 268 | 0.53 | 1.04 |
| South of Dorrance | 10-30-91 | 4,230 | 1,061 | 313 | 0.55 | 1.92 |
| " | 1-07-92 | 3,700 | 902 | 292 | 0.51 | 1.16 |
| Wastewater discharges | ||||||
| Stockton | 1-23-92 | 3,300 | 638 | 371 | 0.38 | 0.18 |
| Hays | 1-23-92 | 1,690 | 220 | 299 | 1.02 | 0.16 |
| Cedar Hills Sandstone | ||||||
| 11S 18W 26CAA | 1-07-92 | 52,600 | 18,420 | 4,937 | 0.94 | 7.9 |
Samples are listed in the same downstream order as in Table 5. Additional samples include waste water discharge that enters the South Fork Solomon River below Stockton and Big Creek below Hays and ground water from a well yielding saltwater from the Cedar Hills Sandstone in north-central Ellis County.a. Specific conductance in micro-Siemens or micromho/cm at 25°C.
b. Interference; sample was collected from a ponded location without flow.
One sample site, that just south of Stockton, appears to have an anomalously high chloride concentration compared with the other sites. The substantial increase in chloride in the river water at Stockton accompanied by decreases in the bromide/chloride and sulfate/chloride ratios fits the chemistry of the municipal waste-water discharge, which has a low bromide/chloride ratio for the chloride content present. Some waste waters from homes and municipalities have been found to be saline from the discharge of brine from water softeners. The bromide/chloride ratio for the waste-water discharge is low because the salinity source is halite, which is used to regenerate the capacity of a water softener to reduce hardness by exchanging calcium and magnesium for sodium. An estimated 90 mg/l of the chloride in the river at Stockton is probably derived from the mixture of the waste-water discharge and the river water (Table 7). Downstream, the river water chemistry returns to a trend more consistent with predominantly natural sources of major dissolved constituents. The next site downstream of Stockton (south of Woodston site) probably does not contain more than 20 mg/l chloride, which could be attributed to the sewage effluent.
Both chloride and sulfate concentrations increased greatly in the Saline River from northwest Russell to central Russell County, where the highest values are observed for any rivers in central and north-central Kansas (see Table 6). The waters from the Saline River have decreasing bromide/chloride ratios that reflect conservative mixing with a halite-dissolution brine (Figure 46) the bromide/chloride ratios are somewhat elevated relative to samples obtained from the Cedar Hills Sandstone and other Permian strata in the Nippewalla Group in Stafford and Pratt counties. The mixing curve for the Saline River waters (the dashed curve in Figure 46) falls within the band of points for naturally saline Dakota ground waters in central Kansas (the area between the two solid lines). The amount of any oil-field brine in the river waters, if present, would contribute less than a few percent to the chloride concentration. Although the sulfate concentrations increase appreciably, the sulfate/chloride ratios of the Saline River waters decrease from upstream of the Haberer salt marsh to the downstream site north of Bunker Hill. The sulfate/chloride ratio at the site north of Bunker Hill is still much greater than the low ratios for most oil brines in central Kansas. The sulfate/chloride ratio reflects the input of the sodium-chloride water with high sulfate contents in the saltwater-containing portions of the Dakota aquifer. The high sulfate is principally derived from anhydrite dissolution in underlying Permian strata.
Figure 46--Mixing of freshwater and saline water I n the Saline River in Central Kansas based on bromide/chloride mass ratio versus chloride concentration. The solid curves are the boundaries of the mixing zone for freshwaters and natural saltwaters in the Dakota aquifer showin in Figure 45. "S" indicates Saline River water.
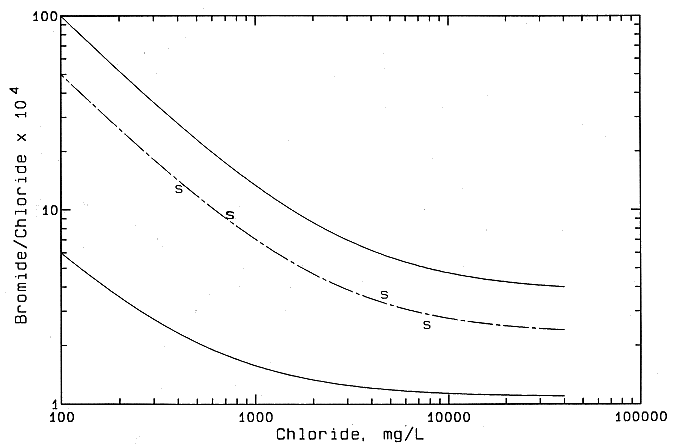
Table 7.--Estimate of the Natural, Oil-Brine, and Waste Effluent Contributions to the Chloride Concentration of the Water Samples from the Solomon River and Smoky Hill Rivers.
| Chloride (mg / l) | |||||
|---|---|---|---|---|---|
| Sample Description | Total | Natural | Waste effluent | Oil brine | Oil Brine % |
| South Fork Solomon River | |||||
| 1 mi east of Graham County line | 157 | 157 | c | <10 | <10 |
| 3 mi west of Stockton | 138 | 138 | c | <10 | <10 |
| Just south of Stockton | 234 | 140 | 90 | <10 | <5 |
| South of Woodston at gage | 148 | >130 | <20 | <10 | <10 |
| South of Alton | 101 | >90 | <10 | <10 | <10 |
| South of Bloomington | 89.4 | >80 | <10 | <10 | <10 |
| Osborne at gage | 87.5 | >80 | <10 | <10 | <10 |
| North of Corinth | 88.4 | >80 | <10 | <10 | <10 |
| Smoky Hill River samples collected October, 1991 | |||||
| US-281 south of Russell | 1,879 | 1,340 | b | 540 | 29 |
| South of Homer | 1,702 | 1,250 | b | 450 | 26 |
| South of Bunker Hill | 1,184 | 730 | b | 450 | 38 |
| South of Dorrance | 1,061 | 670 | b | 390 | 37 |
| Smoky Hill River and Big Creek Samples collected January, 1992 | |||||
| 1 mi east of Pfeifer | 134 | 134 | c | d | d |
| 3 mi west of Russell County line | 408 | 408 | c | <20 | <5 |
| 1 mi east of Ellis County line | 724 | 724 | c | <30 | <5 |
| 4 mi east of Ellis County line | 760 | 760 | c | <40 | <5 |
| Above jct with Big Creek | 1,781 | 1,781 | c | <90 | <5 |
| Big Creek near junction | 330 | 230 | b | 100 | 30 |
| 2 mi west of US-281 | 910 | 810 | b | 100 | 11 |
| US-281 south of Russell | 843 | 740 | b | 100 | 12 |
| South of Homer | 790 | 640 | b | 150 | 19 |
| South of Bunker Hill | 746 | 560 | b | 190 | 25 |
| South of Dorrance | 902 | 700 | b | 200 | 22 |
The estimated error in the chloride concentration estimates for the oil brine contribution is ±20% which is equivalent to an error of 5-6% in the oil-brine percentage for the October 1991 Big Creek samples and 2-5% for the January 1992 samples from the Smoky Hill River.
a. Percentage of total chloride that is attributed to an oil-brine source.
b. Amount contributed probably less than a few percent.
c. Amount contributed unknown but probably small.
The salinity source in the Smoky Hill River waters upstream from Big Creek is essentially all natural ground-water discharge based on the chemistry. Figure 47 includes the same solid curves as Figure 46, which bound the regional zone of freshwater-saltwater mixing in the Dakota aquifer. The points for Smoky Hill River waters above Big Creek can be fitted well with a mixing line (long and short dashed curve) with a saltwater end-point having a bromide/chloride ratio lower than that for the saltwater end-member for the Saline River curve. The lower end-point ratio fits the trend to even lower ratios for saltwater in Permian strata farther to the south in Stafford and Pratt counties. Oil brine, if present, does not contribute more than a few percent to the chloride concentration. In Ellis County the Smoky Hill River waters are characterized by a higher sulfate content (in mg/l) than the chloride content (see Table 2), probably reflecting the influence of Upper Cretaceous rocks. The amount of chloride from waste-water discharges from towns is unknown but is probably small. As the waters approach the Ellis-Russell county line, the sulfate concentration decreases and the chloride concentration increases. Downstream from the Ellis-Russell county line the chloride content of the river increases and the sulfate content decreases slightly to a relatively constant value, then the chloride content increases substantially because a large influx of saline water enters just above the junction with Big Creek. A comparison of the higher flow and lower salinity for river waters collected farther downstream in January 1992 with the values for the same sites in October 1991 suggests that the salinity of the river before the Big Creek junction was greater during the previous October. The increase in chloride content with a relatively moderate decrease in sulfate/chloride ratio in the river water above Big Creek also fits the chemistry of saltwater in the Dakota as derived from underlying Permian strata.
Figure 47--Mixing of freshwater, natural saline water, and oil brine in the Smoky Hill River in central Kansa based on change in bromide/chloride mass ratio with chloride concentration. The solid curves are the boundaries of the mixing zone for freshwaters and natural saltwaters in the Dakota aquifer shown in Figure 45. H, uncontaminated Smoky Hill River; o contaminated Smoky Hill River, October 1991; X contaminated Smoky Hill River, January 1992; B Big Creek; W Haus municipal waste water.
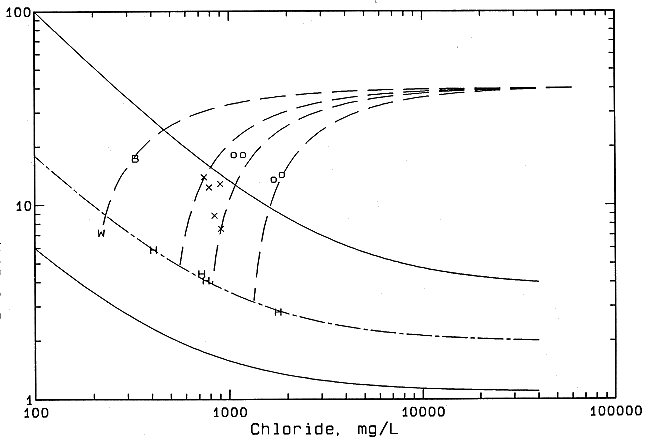
The water from Big Creek just above the confluence with the Smoky Hill River is slightly saline but contains a much lower chloride concentration than in the Smoky Hill River 1 mi (0.6 km) directly south (see Table 6). The bedrock channel of the Smoky Hill River at this location probably cuts deeper into the Dakota aquifer, where the water is more saline, than the bedrock valley of Big Creek. The Big Creek sample is more saline than the waste water from Hays. The waste-water discharge is expected to contribute a significant amount of flow to Big Creek during low flow periods. The bromide/chloride ratio is appreciably greater (see Figure 47) and the sulfate/chloride ratio is lower in the Big Creek sample than in the waste water and, given an equivalent chloride content, in the Smoky Hill River water in southwest Ellis County.
The curves with equal-length dashes in Figure 47 represent the mixing of average oil brine with Dakota ground waters of different salinities. Each curve was calculated such that it extends from the oil-brine end-point through a river water with an elevated bromide/chloride ratio to the mixing curve for a natural saltwater source for the Smoky Hill River. The chloride concentration at the intersection of the oil-brine mixing curve with the curve for natural saltwater represents the natural source in the polluted water. The intersection value for Big Creek water is listed in Table 7 along with the contribution from oil brine calculated as the difference between the natural source and total concentration of chloride. Thus Big Creek water appears to be contaminated by oil brine; the oil-brine contribution to the chloride concentration is estimated as 100 mg/l, which is 30% of the total chloride in the sample. The extension of the dashed curve for the Big Creek sample meets almost exactly the point for the waste water from Hays, suggesting that the waste water might be the main contributor to the flow at the sampling time. Thus the chemistry of the Big Creek sample appears to fit the mixing of waste-water discharge and oil brine diluted by freshwater. The larger the contribution of waste water to the chloride, the smaller the natural contribution and the larger the oil brine contribution to the chloride content based on mixing curve computations.
The true oil-brine contribution to the Big Creek water and to the other samples with elevated bromide/chloride ratios depends on the bromide/chloride ratio of the oil brines that actually cause the contamination. A higher oil-brine ratio would give a mixing line with a greater curvature, resulting in an interpretation of a higher contribution of natural chloride and a lower percentage of oil-brine contamination; the lower the oil-brine ratio, the higher the oil-brine percentage.
The water in the Smoky Hill River below the junction with Big Creek becomes less saline as a result of the dilution with Big Creek water and, possibly, with additional fresh discharge from the alluvium of the river valley. The river water continues to drop in salinity, although the salinity increased at the site south of Dorrance for the January 1992 sampling in comparison with the continued decrease at the Dorrance site for the October 1991 sampling. The salinity was lower for the higher flows in January 1992 compares with the approximately five-times lower flow in October 1991.
The bromide/chloride ratios for the Smoky Hill River waters below the junction with Big Creek are appreciably higher than the ratios for the mixing of freshwater and natural saltwater discharge from the Dakota aquifer (see Figure 47). The chemistry indicates that the source of salinity in Smoky Hill River waters downstream from Big Creek is a mixture of freshwater, Dakota aquifer saltwaters, and oil brine. Curves illustrating the mixing of average oil brine with naturally saline river water are shown in Figure 47 for three of the river samples.
The contribution of oil brine to the chloride content in the section of the Smoky Hill River from below Big Creek to south of Dorrance ranged from 390 to 540 mg/l in October 1991 and from 100 to 200 mg/l in January 1992. The percentage of oil brine in the total chloride content was also greater during October than in January. The oil-brine pollution appears to contribute not only higher concentrations of chloride during lower flows but also a greater proportion of the total salinity. The changes in oil-brine contamination downstream from the junction with Big Creek exhibited both similarities and differences between the October and January samplings. At both sampling times the percentage of oil-brine pollution first increased to south of Bunker Hill and then leveled out. This suggests that the main entrance of oil brine is within the Big Creek to Bunker Hill section. Both the total chloride concentration and the oil-brine chloride content decreased downstream from Big Creek in October; the total chloride decreased to south of Bunker Hill and then increased to Dorrance and the oil-brine chloride concentration increased to Bunker Hill and was about the same at Dorrance in January. The river flows in this section generally increased downstream, giving a mass flow of oil-brine chloride that was relatively constant from Big Creek to south of Dorrance in October in contrast to an increasing mass of oil-brine chloride downstream in January. Therefore different stream-aquifer interactions in changing climatic conditions are important in controlling both the quantities and the percentage of contamination at each location along the river.
Sulfate concentrations generally decrease and sulfate/chloride ratios remain relatively constant downstream in the Smoky Hill River below the junction with Big Creek. If the oil-brine contribution to the chloride content were subtracted from the Smoky Hill River samples, the sulfate/chloride ratios would be higher and closer to the range for the Saline River waters. The sulfate concentration in the oil brines is typically so low compared with the chloride concentration that it contributes little, if at all, to the sulfate concentration in polluted water.
Most of the Smoky Hill River from Big Creek to south of Dorrance passes through oil and gas fields, specifically, the southern parts of the large Gorham and Hall-Gurney fields. The source of the oil-field pollution in the river water could be from saline ground water discharging from the alluvial aquifer contaminated by surface disposal ponds and/or discharge of brine from the Dakota aquifer into the alluvium along the bedrock channel underlying the river valley. Frye and Brazil (1943) indicate that in the early 1940's some oil brines from these fields were being disposed in wells in Cretaceous sandstones of the area and in deep disposal wells. Surface disposal ponds were probably more prevalent before subsurface disposal become the general practice in the area. A map in Frye and Brazil's (1943) bulletin shows that a few of the shallow disposal wells were located near the Smoky Hill River valley from Big Creek to south of Homer.
The fluoride concentrations are lower in the waters from the South Fork Solomon, Saline, and Smoky Hill rivers than in ground water in the Dakota aquifer near the sampling sites. This reflects dilution of the Dakota aquifer water that enters the base of the alluvium with freshwaters in the alluvium derived from direct recharge or recharge through outcropping strata overlying the Dakota Formation. Although fluoride concentrations in the river waters generally increase with increasing salinity, the total range is relatively small and is not as useful as the bromide, chloride, and sulfate concentrations for saltwater source identification.
The well-water sample from the Cedar Hills Sandstone collected in January 1992 has a salinity close to that of seawater. A point for the water would plot slightly above the mixing zone for freshwater and natural saltwater in the Dakota aquifer shown in Figures 45-47. A few percent of the chloride content could be from oil brine, especially because the well is in an oil field. An oil-brine source is also suggested because the bromide/chloride ratio is higher than the ratio calculated for the monitoring well in the Cedar Hills Sandstone drilled in northern Ellis County (NENE sec. 30, T. 12 S., R. 18 W.) for previous Dakota studies. However, there is not enough data available to rule out the possibility that the composition is natural and may reflect a few percent of seawater trapped in low-permeability shales that is still diffusing into the more permeable sandstone, increasing the bromide/chloride ratio. A comparison of the bromide/chloride ratios with geographic location for the Dakota aquifer and for the KGS monitoring well in the Cedar Hills Sandstone in northern Ellis County are considered further in the next section. Saltwater Sources in Test Wells of the City of Hays
As a result of growing demands for water supplies, limits on increasing the amount of ground water from Smoky Hill River alluvium, and problems in obtaining good-quality surface waters, the city of Hays investigated the potential use of the Dakota aquifer as a supplemental water source. Several test wells were drilled recently in an attempt to determine where and at what depth sufficient quantities of usable water could be found in Dakota sandstones in Ellis County, preferably relatively close to Hays. Slightly saline water was considered usable because it could be mixed in small quantities with fresher water from the current supply. In addition, the city was considering methods of treatment to lower salinity, such as electrochemical dialysis and reverse chemical osmosis.
Several test wells were drilled in the spring of 1992 in the Dakota aquifer a few miles southwest of Hays. Production wells were also later installed in parts of the area. Oil fields are present in the test and production well area; therefore a concern of the Kansas Corporation Commission and of the city was whether oil-brine contamination is present in the Dakota aquifer either from brine migration upward along the annular space in oil production or saltwater disposal wells or from past injection into the Cedar Hills Sandstone underlying the Dakota aquifer. If oil-field brine were not present, another concern was whether the oil and disposal wells have allowed additional Permian saltwater to enter the Dakota aquifer. Samples of the test wells were sent to the KGS for geochemical identification of salinity source, using the same method as that described in the previous section for river waters. The chemical character of the test-well water was also examined for anomalous concentration relationships relative to other data for central Kansas.
Thirty-six samples from 13 boreholes drilled into the Dakota aquifer were sent to the KGS for analysis. The multiple samples for some holes were collected at different depths in the Dakota aquifer. One other sample was collected from a borehole drilled as a test well into a plugged oil well to within the depth of Dakota strata and then perforated. The chloride concentration for the test-well samples ranged from 445 to 2,344 mg/l as shown in Figure 48, and the chloride in the water from the perforated oil well was 3,230 mg/l (shown as a circle in Figure 48).
Figure 48--Mixing curves for ground waters from test and monitoring wells in the Dakota aquifer in Ellis County based on change in bromide/chloride mass ratio with chloride concentration. The solid curves are the boundaries of the mixing zone for freshwaters and natural saltwaters in the Dakota aquifer shown in Figure 45. The dashed curve above the Hays test-well curve is for the Saline River as shown in Figure 46; the dashed curve below the Hayes test-well curve is for the Smoky Hill River, as shown in Figure 47. +, Hays test well; o drilled and perforated oil-well plug; M, KGS monitoring well in northern Ellis County.
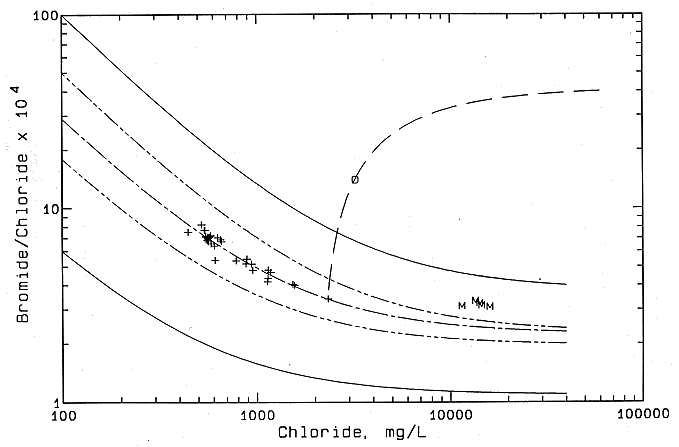
The points for the test-well waters follow a curve (one long and two short dashes) for the mixing of freshwater and natural saltwater in the Dakota aquifer that was derived from the underlying Permian strata through geologic time. The mixing curve falls between the mixing lines (alternating long and short dashes) for natural salinity that discharges from the Dakota aquifer to the Saline and Smoky Hill rivers. The two solid curves are the boundaries for regional data that show mixing of freshwaters and natural saltwaters in the Dakota aquifer. These curves and those for the Saline and Smoky Hill rivers are also shown in Figures 46 and 47. The results indicate that no detectable contamination from oil brine is present in the test-well waters.
The location of the mixing curve for the test-well waters between those for the Saline and Smoky Hill rivers fits the geographic location of the test wells between the two rivers. Points for the monitoring wells in a borehole drilled by the KGS in northern Ellis County (NENE sec. 30, T. 12 S., R. 18 W.) for previous Dakota studies are also included in Figure 48. The monitoring wells are screened in the Cedar Hills Sandstone, the Cheyenne Sandstone, and the upper and lower Dakota Formation. The consistency of the bromide/chloride ratios for the monitoring-well waters indicates a Permian source of saltwater to the Dakota and an absence of oil brine. The trend of increasing bromide/chloride ratios for the saltwaters in the direction from the Smoky Hill River curve to the monitoring well suggests that greater amounts of remnant seawater trapped in low-permeability shales and diffusing to coarser parts of the Dakota could be present in northern Ellis and Russell counties compared with southern Ellis and Russell counties. The bromide/chloride ratio of seawater is 0.0035, a value within the range of oil brines in central Kansas, although the average oil brine value is greater.
In the test well area lower chloride concentrations were generally encountered in sandstones of greater permeability. The chloride content tended to increase with depth in the strata with overall finer-grained sediments, whereas decreases in chloride content were observed with depth at selected intervals in some wells. The salinity distribution suggests that the most permeable sandstone units have allowed faster flushing of old saltwater by fresher regional flow from the west. The decreases in salinity with depth could represent penetration into coarser sediments in the sandstone river channels in the aquifer.
The sample from the perforated borehole in the plugged oil well appears to be a mixture of oil brine and natural Dakota aquifer water. The curve with long dashes (see Figure 48) extends from an end-point representing average oil brine in central Kansas through the sample to intersect with the mixing curve for the Hays test-well waters. The intercept of the two curves is the expected chloride concentration in uncontaminated Dakota aquifer water (2,330 mg/l) outside the plugged well. Although the amount of oil brine interpreted in the sample (900 mg/l chloride) is 28% of the total chloride concentration in the sample, the amount is only about 1.5% of the concentration in the average oil brine. The attempt to perforate the well was apparently not successful, as indicated by a small yield of water, probably a result of the thickness of the cement in the annulus. Because less than 2% by volume of oil brine would be needed to mix with Dakota water to produce the observed chemistry, the water sample could be as easily explained by small amounts of remnant oil brine trapped somewhere in the casing and annular zone or by contamination of the Dakota aquifer outside the plugged well.