Kansas Geological Survey, Open-File Rept. 93-1
Annual Report, FY92--Page 16 of 20
Field measurements of specific conductance, temperature, and pH were made and water samples were taken during the pumping test of the 5-in., 494-ft-deep (151-m-deep) well conducted on June 1-2, 1992, at the Finney County site. The specific conductance was monitored throughout the test and was found not to vary based on values that remained within the instrument measurement error of ±2%. The concentrations of major and minor dissolved constituents determined for water samples taken at 4.2 hr and 21.7 hr after the start of pumping were within analytical error, also indicating no detectable change in chemistry with time. The waters appear to have been pumped from within the more permeable strata horizontally with no detectable amounts of more saline water drawn from either the greater or the shallower depths during the test. This suggests that less permeable layers in the Dakota aquifer can restrict movement of overlying or underlying ground water at the site. The water quality in the Dakota Formation at the Finney County site is very fresh, with a specific conductance of 476 microS/cm and a total dissolved solids (TDS) content of 288 mg/l. The sulfate concentration (74 mg/l) was appreciably higher than the chloride concentration (6.1 mg/l), and the nitrate and ammonium ion contents were less than detection (0.1 mg/l as nitrogen). The site lies in the Arkansas River floodplain at the edge of an irrigated field where corn was being grown. The high sulfate/chloride ratio with undetectable nitrogen suggests that waters from the overlying alluvium of the Arkansas River that received sulfate-rich floodwaters in the past may have affected the chemistry over recent geologic time and that infiltration of water passing through the soils during agricultural use has not yet affected the water quality.
Water samples were collected soon after development from the three wells drilled by a private contractor for the KGS at the Hodgeman County site. The wells include an observation well and the pumping-test well screened in the Dakota Formation and a deeper observation well screened in the Cheyenne Sandstone underlying shale of the Kiowa Formation. The specific conductances of the waters from the Dakota wells (1,240-1,300 microS/cm) indicate that the waters were fresh with TDS contents less than 900 mg/l. The water collected from the Cheyenne well after development was saline. Field measurements were made and water samples were collected from the pumping well during the pumping test of June 3-4, 1992. The specific conductance rose from 1,200 to 7,300 microS/cm during the pumping. Extensive field studies were conducted to determine the cause of the salinity increase, and then the Cheyenne well was plugged. Field tests are continuing to examine the status of the site.
Alan Dutton of the Texas Bureau of Economic Geology has focused his research on the paleohydrology of aquifers in the Great Plains. His research is designed (1) to determine whether certain ground waters beneath the Great Plains are "fossil" (ancient) water as a result of landscape evolution, (2) to determine whether a paleorecharge model for the southern Great Plains applies to other parts of the Great Plains, including western Kansas, and (3) to evaluate the implications of the results to paleoclimatology. A joint sampling of selected Dakota aquifer and High Plains wells was conducted in early November 1991. The KGS collected water samples for determination of dissolved major, minor, and trace inorganic and radiochemical constituents; Dutton collected samples for measurement of isotopic and selected dissolved inorganic constituents. The isotopes of interest include carbon-13, carbon-14, chlorine-36, and the stable isotopes oxygen and deuterium for estimating the climatic characteristics and the age of the recharge.
A few staff members of the Lawrence Livermore National Laboratory (LLNL) of Livermore, California, are interested in applying measurement of several isotopic and dissolved trace gas and inorganic constituents to improve methods for characterizing the paleohydrology, especially recharge, and the hydrogeochemistry of aquifer systems. The LLNL has facilities that have been used mainly in examining nuclear testing sites and present and proposed locations of nuclear waste disposal. The laboratory wishes to expand the application of these facilities to aid in resource and environmental studies of the United States. The research being conducted on the Dakota aquifer by the KGS provides a valuable field test for these methods. In turn, the state of Kansas will obtain additional data that will assist in assessing the recharge and hydrology of the Dakota aquifer system.
Staff members of the KGS and LLNL planned for sampling well waters in the Dakota aquifer system along a flow path from a recharge area in southeastern Colorado through the confined aquifer in southwestern and central Kansas to a discharge area along the eastern outcrop band. The flow path is shown in Figure 4. A geologic cross section along the flow path is illustrated and discussed in the FY91 Annual Report (Macfarlane Et al., 1992, Figure 39, p. 66). The first joint sampling was conducted in May 1992 in southeastern Colorado and southwestern Kansas. The second sampling took place in July 1993 from southwestern to central Kansas. Most of the sample locations of the joint study with Alan Dutton are in the vicinity of the same flow path being studied in cooperation with the LLNL, whereas two of the wells sampled are along the southern flow path or cross section illustrated in Figure 38 of the FY91 annual report (Macfarlane et al., 1992, p. 65). Results from both the Texas and LLNL cooperative studies will be discussed n the FY93 annual report.
In north-central and central Kansas the ground-water chemistry data indicates that ground water in the Dakota aquifer is a mixture of waters from the Permian Cedar Hills Sandstone, regional flow within the aquifer from the west, and local surface recharge. The Cedar Hills Sandstone is part of the Permian red bed evaporite sequence. In western Kansas the Cedar Hills Sandstone is cemented by halite and other minerals, such as anhydrite, magnesite, and dolomite. Along the east margin of the Permian salt basin, halite cement is not present in the Cedar Hills Sandstone because it was removed by dissolution during subaerial exposure in pre-Cretaceous time and by ground-water recharge and discharge after it had been deposited. Na-Cl water from halite dissolution is the dominant water type in the Cedar Hills aquifer.
Computations based on the geochemical model SOLMINEQ88 were made to determine the state of saturation of Dakota ground water relative to major minerals. The geochemical model results show that ground water in the Cedar Hills Sandstone is only slightly undersaturated with respect to anhydrite (CaSO4), which means that some dissolution of anhydrite could occur in the formation. The model also indicates that the Cedar Hills water is saturated with magnesite (MgCO3) when the water is forced to be saturated with calcite by allowing the pH to be adjusted by the program. The resultant computed pH values were between 7 and 6.3. Magnesite has been observed in cores of the Cedar Hill Sandstone in western Kansas. Recharge of water from overlying units in parts of the Cedar Hills Sandstone causes dissolution of magnesite and elevation of magnesium and bicarbonate concentrations. High magnesium and bicarbonate water also occurs near the contact of the Dakota and Cedar Hills aquifers where the Cedar Hills discharges to the base of the Dakota aquifer.
The Dakota water immediately west of the Cedar Hills subcrop is characterized by Na-HCO3 type water, which is a result of cation exchange of calcium and magnesium for sodium. The typical range of ground-water TDS concentration in this area is 900-1800 mg/l. Based on water samples obtained from Trego County and the western part of Ellis County, typical concentrations of calcium and magnesium are in the ranges 8-12 mg/l and 3-5 mg/l, respectively, and the Ca/Mg equivalent ratios are in the range of 1-1.7. The depletion of calcium causes dissolution of calcite, which increases the ground-water pH to as high as 8.5.
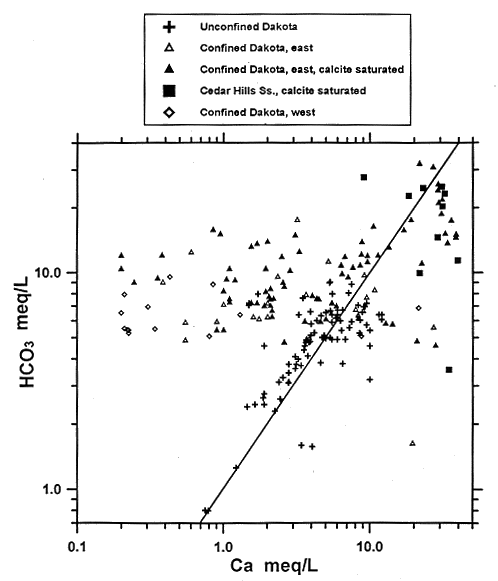
The geochemical model shows that dissolution/precipitation of calcite is a major factor controlling ground-water chemistry throughout the entire system. Precipitation of calcite keeps the bicarbonate concentration low in the Cedar Hills water. In the confined Dakota aquifer cation exchange keeps the calcium concentration low while equilibrium with calcite maintains the bicarbonate concentration at a relatively high level (Figure 43).
Figure 43--Bicarbonate versus calcium equivalent concentrations for ground waters in the confined and unconfined Dakota aquifer and the Cedar Hills Sandstone in central and north-central Kansas. The line indicates the 1:1 relationship of the two constituents.

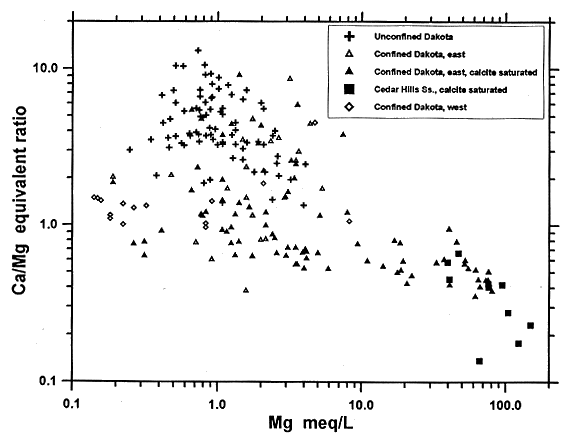
Analysis using theoretical mixing curves of (calcium and magnesium versus sodium) indicates that factors other than simple mixing of Cedar Hills and confined Dakota waters occur in the system. The data indicate that after intrusion of Cedar Hills water into the Dakota aquifer, the magnesium concentrations in the mixed water are lower than the conservative mixing curve. If the concentrations of sodium and chloride in the high-TDS water are assumed to have come from dissolution of halite only, then the mole concentrations of sodium and chloride should be about equal. However, the difference of absolute mole values between the sodium and the chloride shows that the sodium exceeds the chloride by about 47 meq/l for the water near the contact of the Cedar Hills and Dakota aquifers. Normally, water samples from the confined Dakota aquifer have sodium exceeding the chloride concentration only by about 5 meq/l. The difference is believed to be a result of cation exchange. The most extensive cation exchange occurs in the zone of mixing of Cedar Hills and Dakota waters because of the extremely high magnesium concentration from the Cedar Hills water. The intensity of cation exchange decreases along the ground-water flow downstream to the east. This feature can be illustrated by the difference between the sodium and chloride concentrations in the ground-water samples. Figure 44 shows that the cation exchange process not only decreases the calcium and magnesium concentrations in the Dakota aquifer but also increases the calcium/magnesium ratio from less then 0.2 to about 1.5 because of greater exchange of magnesium for sodium in the early stage of ion exchange. The quantity of cation exchange capacity and the selectivity coefficients to be used in the coupled flow and chemical reaction model will be estimated using sensitivity analysis in the coupled hydrochemical model.
Figure 44--Calcium/magnesium equivalent ratio versus magnesium equivalent concentration for ground waters in the confined and unconfined Dakota aquifer and the Cedar Hills Sandstone in central and north-central Kansas. .
Some lenses of calcite or high-magnesian calcite cemented sandstone found near the western portion of the eastern band of outcropping Dakota strata may indicate that the mixing of confined Dakota water with surface recharge in the outcrop area causes precipitation of calcite in the chemical transition zone. This point will be examined further during simulations of the coupled hydrochemical model.