Prev Page--Introduction || Next Page--Systematic Paleontology
Shell Morphology
Glossary of Shell Terms
It is desirable to define briefly some of the terms employed in the text. The following list is arranged in alphabetical order:
Adductor impressions—In the majority of Mytilacea there are two adductor muscles, an anterior and a posterior adductor, both of which cause distinctive marks at their insertions in the shell. The anterior adductor, always very small, or even absent, is situated beneath the beaks. Under the opposite end of the hinge occurs a large bifid muscle impression produced by the large. posterior adductor, the posterior byssal, and pedal retractors. The ventral part of the bifid impression is produced by the posterior adductor.
Angle α—The angle between the umbonal ridge and the cardinal margin is designated by the conventional Greek letter α. During the ontogeny of an individual, and during the phylogeny of some of the mytiloids, as well as other kinds of pelecypods, there is a notable increase in the value of the angle α. In view of the fact that the umbonal ridge flattens out somewhat at maturity, a precise measurement of this angle seldom can be obtained; nor is a high order of accuraey needed for useful results.
Angle β—The angle between the posterior margin and the cardinal margin, neglecting of course any irregularities that obviously have no phylogentic significance, is designated by the Greek letter β. In juveniles and primitive species this angle is relatively obtuse and often ill defined. During ontogenetic growth or phylogenetic change the angle decreases progressively until it measures in some forms considerably less than 90°.
Anisomyarian—The anterior adductor is highly atrophied in the majority of Mytilacea. In some species of living Mytilus, as well as species in certain other genera, the anterior adductor completely disappears at maturity. The progressive reduction of the anterior adductor has well recognized taxonomic significance, but no great weight is attached to the final disappearance of the adductor in species that otherwise are similar to forms retaining a small anterior adductor at maturity.
Auricle—Primitive mytiloids are not auriculate, but some specialized species, through posterior extension of the hinge, develop a salient that is called a posterior auricle, or sometimes a "wing." Such species have a pronounced homeomorphic resemblance to certain pterioids. Anterior projections are not sufficiently conspicuous to be called auricles, although the term "anterior lobe" is commonly used for an anteroventral emargination of the border in some forms like Volsella.
Byssal sinus—The anteroventral margin of practically all mytiloids is more or less concave at an intermediate point, as viewed laterally. In life, the conchiolinous strands of the byssus extend between the slightly gaping margins of the valves near the middle of the sinus. It is convenient to refer to this indentation as the byssal sinus.
Byssal retractors—Several muscles are attached to the byssal gland at the proximal end of the byssus. They serve to control the orientation and shell movements while anchored by the byssus. A series of insertions occur on the shell just dorsal to the posterior adductor impression and, together with the still more dorsally placed pedal retractor scar, form the posterodorsal prong of the bifid muscle impression, commonly called the "posterior adductor". An anterior byssal retractor muscle is inserted slightly above and behind the anterior adductor. In many Paleozoic forms, two scars occur at the position of the anterior byssal retractor; so, presumably, the muscle was divided in some instances into two parts where it joined the shell.
Cardinal area—In the myalinas, as in the areas, pterias, and some other pelecypods, two flat areas diverge upward from the hinge axis of the two valves. The flattened area of each valve is commonly called a cardinal area. In the above-named groups the cardinal area essentially coincides with the surface occupied by the ligament, in which case the term ligament area is synonymous with cardinal area. The majority of mytiloids, however, do not have cardinal areas.
Cardinal margin—Dorsal edge of the valves. Since the dorsal margin lies above the hinge axis and does not coincide with it in some pelecypods, such as the myalinas, the two terms are not synonymous. In such instances the term hinge margin is ambiguous.
Convexity—Because single valves are more common than bivalved specimens, it is convenient to use this term for the maximum convexity of a single valve, measured from the plane of commissure to the outer surface of the shell. In many instances, two valves of a single individual may have quite different convexities (inequivalve), or one valve may fit inside the margin of the other (discordancy); so that where measurements of both valves in apposition are made, such measurements may not be directly comparable to measurements of individual valves.
Discordancy—Many mytiloids are nearly equivalve, but in some of the more specialized myalinas the right valve is somewhat smaller at the margin than the left. Such a discrepency in the shell margins is called discordancy.
Gill suspensories—Thick-shelled myalinas commonly possess a linear series of fifteen or twenty muscle impressions extending in a flattened are from the rear anterior byssal retractor to the front margin of the posterior adductor. Although such muscle pits are not visible in Volsella or Mytilus, inspection of anatomical structures in these genera reveals that the gills are suspended from the mantle at exactly this position. Presumably the pits alluded to in the myalinas represent the insertion points of small muscle strands that supported the gills.
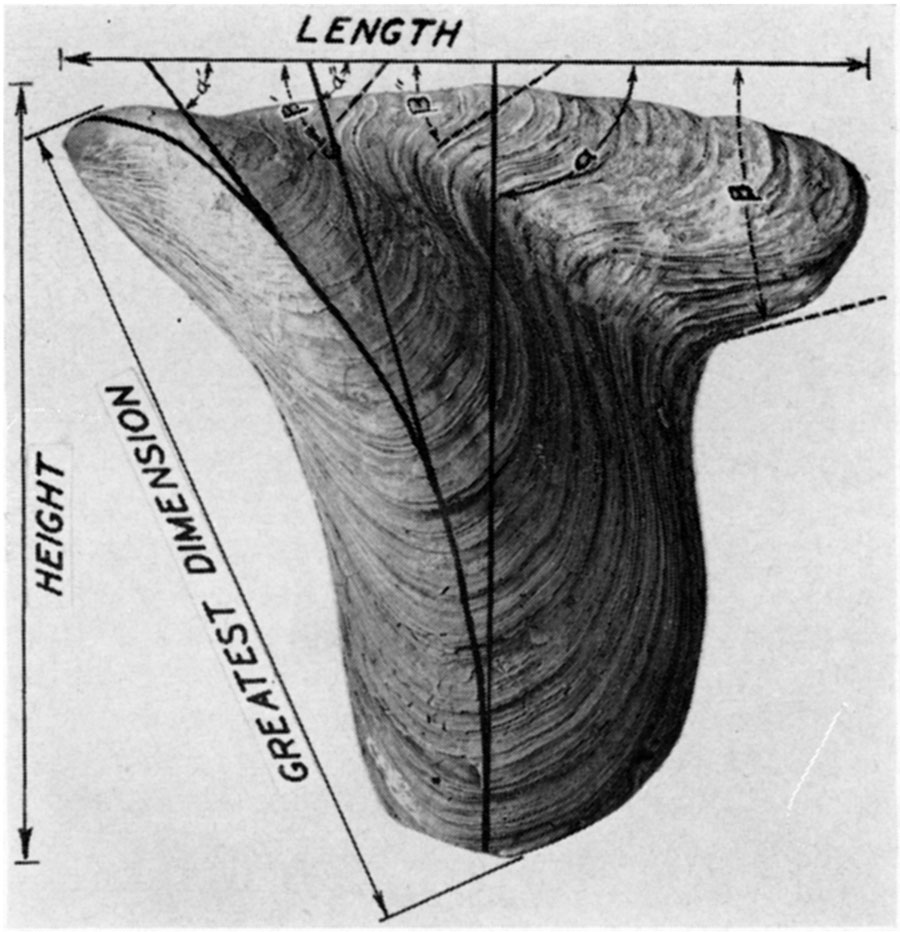
Greatest dimension—Although elongated, the majority of mytiloids are markedly oblique in form, so that the greatest linear dimension is neither the length nor height. Measured from the umbones to the most distant point on the shell margin, this dimension is one of the most useful in describing mytiloids. For want of a better term it will be called the greatest dimension.
Growth lines—It is desirable to distinguish between regular concentric ornamentation and the irregular, less significant lines, on the shell, produced by increments of growth. Ornamentation in the ordinary sense does not occur in Late Paleozoic mytiloids, so there is not much occasion to stress concentric markings on the shell.
Growth lamellae—It is common in pelecypods, particularly mature individuals, for the mantle to withdraw from the shell margin at successive intervals before secreting new shell deposits. The result is likely to be the formation of imbricating shell lamellae that produce a relatively roughened surface where they are exposed. An important generalization for the myalinas is that right valves are almost invariably smoother than left valves. This difference probably has its origin in the seeming fact that the majority of myalinas, perhaps all of them, lie on the right valve when at rest.
Height—Normally the length of a pelecypod is measured along a line drawn through the two adductor muscles, and the height is measured along another line at right angles to it. The animal of the mytiloids has undergone considerable torsion, however, from the primitive condition. In order to use shell terminology that is reasonably consistent, it is preferable to measure the length parallel with the hinge axis and the height at right angles to it.
Hinge axis—The axis of rotation of the two valves is called the hinge axis. Naturally, the hinge axis is invariably a straight line. However, since the axis occurs within the substance of the ligament, and since the ligament is relatively short in some pelecypods, the exact position of the hinge axis is not always easy to locate in fossil specimens.
Hinge teeth and sockets—Teeth and sockets are present in many kinds of Mytilacea. Generally, the dentition occurs at the front of the hinge below the beaks. On the assumption that the dentition was derived from primitive surface ornamentation of the valves, such teeth have been classed as dysodont. Ontogeny of Mytilus edulis and other species, which are normally unornamented, does not indicate the postulated origin of these teeth. Some species of Mytilus and Volsella become edentulous at maturity, but presumably all possess teeth at some stage in the ontogeny. The majority of Myalinidae possess one tooth beneath the beak of the right valve, and a corresponding socket in the left valve. The trace of the tooth and socket during growth produces a more or less conspicuous ridge and furrow at the front edge of the cardinal area of mature individuals. The ridge and furrow do not interlock, however, except at their postero-ventral extremities.
Hypostracum—Muscle tissues secrete calcareous material at their insertion in the shell. Generally, this deposit, the hypostracum, is fibrous, the fibers being elongated normal to the shell surface, Commonly they are composed of the mineral aragonite. So far as is now known, the hypostracum of the myalinas consists of calcite.
Length—The maximum linear distance between the margins of a valve measured parallel with the hinge axis is termed the length.
Ligament—The ligament commonly consists of two unlike kinds of elastic conchiolin. One kind is impregnated with spicules of aragonite, the fibrous ligament, and is elastic only to compressional stresses. This part of the ligament is located mainly below (ventral to) the hinge axis. The other type of material, the lamellar ligament, contains no calcareous material. It is highly elastic to both compressional and tensional stresses, but chiefly functions as a tensional organ because it lies above the hinge axis. The type of C-spring ligament found in Mytilus is called parivincular; the complex ligament of some areas and myalinas may be called duplivincular.
Ligament area—This is the cardinal surface to which the ligament is attached. The character of the ligament insertions in the shell gives a clue to the nature of the ligament itself in fossil shells.
Ligament grooves—The chevron grooves of Arca and the linear grooves of Myalina represent the locus of attachment of the edges of successive bands of lamellar ligament. This type of ligament may be referred to as duplivincular.
Lobe—Shells having a "modioloid" contour exhibit a rounded emargination of the anteroventral part of the shell just in front of the beaks. This salient is commonly called the anterior lobe.
Midumbonal line—The bisectrix of the umbonal ridge is called the midumbonal line. Since the umbonal ridge is not straight, but swings forward during growth, the midumbonal line is arcuate. In some gerontic individuals the midumbonal line swings backward near the ventral margin so that it follows roughly a sigmoidal path across the shell. The angle between the midumbonal line and the cardinal margin is called angle α.
Musculature—The muscle pits that can be observed in well preserved specimens are as follows: one to three pits in or near the umbonal cavity, one or two anterior byssal retractors, one anterior adductor, which may, however, be absent; the pallial line, broken into disconnected pits toward the anterior adductor; the posterior muscle impression, more or less bifid and made up of impressions of the posterior adductor, posterior byssal muscles, and the posterior pedal retractor; a series of disconnected pits, the gill suspensories, extends from the posterior adductor toward the anterior byssal retractors.
Obliquity—Primitive Mytilacea are highly oblique, that is, the angle between the umbonal ridge and the dorsal margin (angle α) is relatively small. During ontogeny and phylogeny this angle steadily increases so that the ventral part of the shell swings forward. Where the angle α is less than 90° the obliquity may be called prosocline, where it is approximately 90° acline, and opisthocline where the angle is obtuse.
Ostracum—The term ostracum is applied to the calcareous shell. In Recent species four component parts of the test are recognized. The outer horny covering is the periostracum; the outer calcareous layer, usually quite thin, is the outer ostracum; the inner ostracum commonly is relatively thick and forms inner features, including the hinge; the hypostracum includes all of the more or less disconnected deposits at the insertions of the muscles.
Pallial line—Radial muscle strands of the mantle are inserted in a narrow band parallel with the shell margin. In the Mytilidae the pallial line is a continuous obscure furrow, but in the Myalinidae the anterior part of the pallial line is broken into a series of distinct pits.
Pedal retractor—In Mytilacea there is a long dorsal prolongation of the rear part of the large posterior muscle impression. Most of this salient is produced by the insertions of several byssal retractors. A single large pedal retractor joins the shell on each side at the distal end of the series, not far from the posterior extremity of the hinge in Mytilus, but more remote from the hinge in some other forms.
Periostracum—The outer corneous covering of the shell of many kinds of pelecypods is termed the periostracum. By analogy with living Mytilacea, it may be supposed that all fossil representatives of the superfamily also possessed a periostracum. Fossil periostracum is extremely rare. Unquestioned examples of it from the Paleozoic are not known to me, although there have been several citations to fossil periostracum, particularly in the so-called fresh-water forms from the Pennsylvanian.
Plane of commissure—The plane which approximately coincides with the valve margins is designated as the plane of commissure.
Pleurothetic—The majority of inequivalve pelecypods owe their lack of symmetry to the habit of lying at rest on one side. Such shells are called pleurothetic, It has been demonstrated in the pterioids, pectinoids, and oysters that any particular group is remarkably consistent as regards the side that normally is undermost. Practically all of the Ostracea rest on the left valve, whereas Pteriacea and Pectinacea rest almost invariably on the right valve. There seems to be far less variation from the normal condition, in this respect, than there is variation from right handedness in human beings or from sinistral coiling in normally dextral snails. The pleurothetic habit may cause a secondarily induced torsion of the body within the shell, with consequent atrophy of certain muscles, as in the Pectinacea. Generally, the principal distortion is found in decreasing convexity and reduction of the area of one of the valves. In Ostracea the upper valve commonly is reduced, in Pectinacea and Pteriacea, the lower one. The smaller valve in Myalinidae is the right one, and presumably this valve was undermost.
Prismatic layer—The outer ostracum of the pelecypod shell in many instances is composed of polygonal prisms of calcite or aragonite, which may be disposed normal to the shell surface (Myalinidae) or nearly parallel with the surface, in which case they are radially disposed in respect to the umbones (Mytilidae). In rare instances the prisms locally give way to homogeneous calcareous material within a single shell. Rarely, the inner ostracum is prismatic. The hypostracum is commonly fibrous, but the fibers are rarely polygonal in section. In pleurothetic pelecypods the outer ostracum of the right valve may be prismatic, while that of the left valve is not (some Pectinacea, some Myalinidae), or both valves may be alike. Examples are unknown to me where the outer ostracum of the right valve is homogeneous while that of the left valve is prismatic.
Resilifer—Triangular pit along the hinge and under the beak for reception of the compressional part of the ligament (resilium) is called the resilifer. Mytilacea possess resilifers only in the prodissoconch and early dissoconch stages.
Shell thickness—Shell material of a pelecypod is thickest near the hinge and thinnest near the ends and ventral margin, with more or less gradation in thickness in the intermediate areas. The thickness is, however, for practical purposes nearly uniform over the central area of both valves, and measurements of shell thickness may be given for the central parts of shells having a stated length and height.
Sinus—Any indentation of the shell margin which may be shown in growth lines is called a sinus.
Umbones—The protuberant area around the beak of a pelecypod valve, in which the early ontogeny of a shell is shown by growth lines, is defined as the umbo. The distinctness of the umbones varies in different genera.
Umbonal cavity—The internal space beneath the umbones is known as the umbonal cavity.
Umbonal ridge—In mytiloids a more or less prominent ridge occurs near the anteroventral margin, extending diagonally across the shell from the beaks to the ventral margin of the shell, being best defined near the beaks. The angular relation between this ridge and the dorsal (cardinal) margin (angle α) is a very critical character in phylogenetic studies.
Umbonal septum—In the majority of mytiloid shells the anterior extremity is extended, or even somewhat acuminate, so that the umbonal cavity is extended far forward under the beaks. In a few of these shells the inner part of the umbonal cavity is partially floored over in the plane of the commissure to produce a small umbonal deck or septum. In some instances the septum supports the anterior adductor muscle. More commonly, the septum seems to represent simply a brace to strengthen a weak part of the shell and to anchor more securely the extension of the animal into the umbonal cavity. It cannot be doubted that an urnbonal septum has been independently acquired in relatively unrelated stocks; yet, in conjunction with other characteristics, the presence or absence of an umbonal septum is a useful generic character.
Figure 3—Measurements in mytiloid shells. The length of the shell is the greatest linear dimension measured parallel with the hinge axis, a line which is invariably straight, generally lying somewhat below (ventral) to the dorsal margin. Height is the greatest linear dimension measured at right angles to the hinge axis. The "greatest dimension" is generally measured along a straight line extending from the beak to the posteroventral margin of the shelL During shell growth the angle between the hinge axis and the crest of the umbonal ridge (angle α) increases in size. This ontogenetic change in form has phylogenetic significance; and the value of the angle α, measured at a mature ontogenetic stage, is a useful specific character. In many instances the angle formed by the intersection of the hinge axis with the posterior margin of the shell (angle β) can only be approximated. However, the form of this posterodorsal angle is a very useful specific character. In many Paleozoic mytiloids, as well as pectinoids and pterioids, the angle β decreases in size during both ontogeny and phylogeny. Some tribes tend to develop a posterior auricle.
Ligament
Seldom are ligament structures preserved in Paleozoic pelecypods. The surfaces of ligament attachment, or ligament areas, of the shell, however, are diverse and in some instances highly distinctive. By analogy with living pelecypod species it is possible to obtain some idea regarding the more or less complicated ligaments of fossil forms, To any student of the pelecypods it is evident that tribes of pelecypods are characterized by distinctive kinds of ligaments. Thus, the pterias, pectens, areas, and unios have markedly different ligaments, as well as other distinctive characters. Neumayr, Bernard, Dall, and others among the pioneer s.udents of pelecypods recognized some of the more striking kinds of ligament and made some use of ligament types in their taxonomic treatment of the bivalve mollusks. Few students of Paleozoic shells, however, have given even eursory attention to ligament characters, thereby more or less ignoring one important clue to obscure phylogenies.
While investigating the Late Paleozoic pectinoids (Newell, 1938, pp. 26-35) I was struck by the diverse appearance of the ligament areas in shells that otherwise are superficially alike. The ontogenetic changes in shell form, as revealed by lines of growth, strongly suggest that these Paleozoic shells of pectinoid habit truly form a phyletic group. Consequently, they were classed in families of the Pectinacea. Perusal of any of the great monographs on Paleozoic pelecypods reveals that there also are several kinds of ligament areas in shells that have distinctly a pterioid aspect or a mytiloid aspect, The problem becomes especially complex when it is noted that mytiloid shells, through rapid evolution, develop pterioid contours, or pterioid shells become modified through minor changes in proportion to the pectinoid form. Perhaps it is not so surprising that the early representatives of the three great superfamilies—Pectinacea, Mytilacea, and Pteriacea—present taxonomic difficulties, because they were just unfolding from a common ancestry, during the Paleozoic era, and had not as yet attained the degree of standardization that characterizes their post-Paleozoic descendants.
Mature consideration of the various types of ligaments possessed by Paleozoic pelecypods leads inevitably to the conclusion that each type thus far recognized represents but a stage in a series of changes undergone by the ligaments in different tribes of pelecypods. Some of these distinctive types of ligament were certainly developed more or less independently in separate families, and therefore do not imply close relationship in shells that otherwise are quite different. A useful analogy might be found in the Ammonoidea, in which the ceratite suture is now known to represent a transition stage between the goniatite and ammonite suture and was acquired independently in several different stocks. The tentative phylogeny of ligament types suggested by me (Newell, 1938, p. 33) incorporates this view. Consideration of Bernard's (1895-1897) studies of the ontogenies of several Cenozoic pelecypods strengthens the belief that any particular type of ligament represents only one of several stages through which the ligament passed in its phyletic development. Further examination of Bernard's evidence, however, forces me to abandon part of the hypothetical sequence alluded to above.
The Paleozoic Mytilidae that I have studied had a ligament like that of modern representatives of the family. The Myalinidae, although clearly members of the Mytilacea, possessed a ligament quite unlike any post-Jurassic representatives of that superfamily. The flat and divergent faces of the ligament area are lined by deep, closely-spaced grooves, or furrows, reminiscent of similar furrows of Arca, Pectunculus, and, among Paleozoic genera, by Pseudaviculopecten, Leiopteria, Ptychodesma, and others. Many authors, unfamiliar with the ligaments of contemporary pelecypods, have failed to appreciate the significance of these ligament furrows, in some instances referring to them as "growth-lines." The ligament grooves in Myalinidae are peculiar in that they are nearly or entirely opisthodetic, in harmony with the fact that the beaks in these, as in the majority of Mytilacea, are terminal. Otherwise, the ligament grooves seem to be entirely homologous with the chevron grooves of some Arcacea. In order to obtain an understanding of the ligaments of Paleozoic Mytilacea I have examined the ligaments of some modern Mytilidae and Arcidae.
Ligament of Mytilidae—Like the ligament of other pelecypods, that of Mytilus and Volsella consists of two parts which are structurally and functionally unlike. A dorsal layer (fig. 4A-C, 2) consists of highly elastic, conspicuously lamellar, conchiolin. Because this lamellar structure lies almost entirely above the axis of rotation of the valves, it is subjected to tensile stresses when the valves are closed. The ventral part of the ligament system consists of a layer of conchiolin (fig. 4A-C, 3) in which are suspended closely packed calcareous needles arranged more or less normal to the ventral or growing surface of the ligament. Petrographic analysis by Dr. R. C. Emmons, University of Wisconsin, reveals that these calcareous fibers have a thickness of approximately one micron and are composed of aragonite. There is a marked tendency for the fibrous ligament to split parallel to the fibers, so that it is very weak to tensile stresses. However, since it lies almost wholly below the hinge axis, it is subjected, normally, only to compression. The fibrous ligament separates the lamellar ligament from the ligament gland of the mantle over the front part of the ligament; but, since the fibrous ligament does not extend to the posterior part of the hinge, the lamellar ligament is in contact with the ligament gland near the rear extremity of the hinge.
Figure 4—Ligament structures in Volsella ("Modiolus") and Arca (D-F). A-C, Series of transverse sections through the ligament of Volsella modiola (Linnaeus), valves closed, near the beaks (A), at the middle of the hinge (B), and at the posterior end of the hinge (C), respectively. D, transverse section cut midway through the ligament of Arca pexata (Say). E-F, transverse sections cut through the ligament of Arca transversa (Say), near the middle of the hinge (E), and near the posterior end along the line a-b, (F). Solid black, shell material; black dot near center, position of axis of rotation; 1, periostracum; 2, laminar ligament; 3, fibrous ligament; 4, secondary calcareous ligament buttress.

The ligament of Mytilidae is of the type commonly called "internal," viz., most of the ligament is concealed beneath the overhanging margins of the dorsal edge of the shell. In order to give the ligament better foothold on the surface of attachment on the shell a ridge of spongy calcareous material is added below the ligament on each side. The resulting depression, or furrow, in which the ligament is lodged is called the ligament groove and, of course, is quite invisible exteriorly, even where the ligament has been stripped from the shell.
Ligament of some Arcidae—In connection with the study of Paleozoic pectinoids I made certain observations, on some pelecypod ligaments (Newell, 1938, pp. 26-35), which now require further comment and correction.
The fibrous ligament of Arca consists of a rather thin sheet covering the two flat and divergent surfaces of the broad ligament area. Both Dall (1895, p. 500) and Bernard (1896, pp. 67, 71) refer to this covering as an epidermal (lamellar) ligament. For the species (A. transversa Say, A. pexata Say) that I have studied, there can be no doubt that this part of the ligament is fibrous and compressional where it joins the two valves at their contact below the axis (fig. 4D,F, 3). My earlier observations on fibrous ligaments must be revised, as follows: In cross sections of the ligament of Arca transversa (fig. 4E,F) , the fibrous ligament exhibits distinct growth lamellae, which I originally mistook for calcareous fibers. The fibrous ligament is iridescent or "pearly" through the outer one-third to one-half of its thickness. When the hinge axis migrates downward, during shell growth, to a position below a given part of the fibrous ligament, that part is subjected to tensile stress and soon parts, or splits, along a vertical plane just above the axis. The reaction of the lamellae after breaking is greater near the surface where there is more space in which to expand than next to the shell surface, and the resulting strain of the lamellae produces the marked iridescence.
The fibers of calcareous material are imbedded in the conchiolin normal to the ventral or growing surface of the ligament, not parallel with it as I formerly thought. Being interested in the characteristics of the calcareous spicules, I referred them to Dr. R. C. Emmons, who has reported that they are composed of acicular fibers of aragonite somewhat less than one micron in diameter. The spicules are relatively long, seemingly extending at least through several growth lamellae.
These observations apply equally well to the fibrous ligament of Arca pexata, Pinctada, Yoldia, Volsella, and Mytilus, and probably characterize pelecypods in general. The errors into which I fell were due to my inability to separate satisfactorily the organic conchiolin from the aragonite fibers; and, consequently, I did not realize how exceedingly fine they are. Specimens of fibrous ligament treated for a day or so in a slightly alkaline solution of H2O2 of 30 per cent concentration are freed of organic material and take on the appearance, at 500 diameters, of a fine camels-hair brush. It seems as though the calcareous spicules in the observed specimens make up more than one-half of the entire volume of the fibrous ligament.
Ontogenetic development of the ligament—Bernard made the extremely important observation that the ligament is invariably internal when it appears first in the young pelecypod, and that it is lodged in a small oblique resilifer just below the beaks. In some genera, such as Pecten, Nucula, Pteria, Lima, and Ostrea, this condition apparently continues to maturity; although, in the last three genera, growth expansion of the two valves at their margins causes the resilifers to become exposed exteriorly, resulting in an "external" ligament. For this type of ligament, Dall (1895, p. 500) suggested the term alivincular. Seemingly, the part of the ligament that is lodged in the resilifers of adults of the above mentioned genera is largely, if not wholly, of fibrous type, although I formerly thought otherwise (1938, pp. 31-35). Whether or not the central ligament of the early dissoconch stage is homologous with the fibrous ligament is not certainly known, but it is invariably compressional in shells having the functional part of the ligament below the hinge axis.
Since the alivincular ligament appears early in the ontogeny of so many genera (Nucula, Pectunculus, Arca, Mytilus, Volsella, Modiolarca, Lasaea, Lucina, Crassatella, Cytherea, Venus, Mactra, Donax), it is reasonable to suspect, with Bernard, that this was the primitive ligament from which other types were derived.
Jackson (1890, pp. 327-333) has shown that the multiple ligament of the Pernidae passes through an alivincular stage in the early ontogeny, where the ligament is like that of Pteria. In this type new resilia are added successively at the rear of the hinge margin. Dall (1895, p. 500) proposed the term multivincular to describe this type of ligament. In both the alivincular and multivincular types the compressional "resilium," composed of fibrous ligament, does most of the work in opening the valves, whereas the lamellar ligament serves chiefly to tie the two margins of the shell together (Newell, 1938, pp. 26-35).
The term parivincular was introduced by Dall (1895, p. 500) to describe the C-spring type of ligament found in Cardium, Tellina, Unio, Mytilus, and Volsella (fig. 4A-C). Some of the areas (fig. 4D) also have a parivincular ligament in the adult stage; probably all of the areas pass through a parivincular stage somewhere in the ontogeny. Bernard (1895-1897) has shown that the prodissoconch and early dissoconch stages are alike in many pelecypods in possessing an "internal" triangular resilium bounded behind and ahead by two series of small interlocking denticulations along the hinge margins of the valves. These denticulations correspond to a sort of taxodont dentition, which in most eases is lost and replaced by other teeth in the later ontogeny. In Mytilus edulis, Bernard (1896, p. 415) found that the posterior row of denticles early becomes divided into two parts separated by a shallow depression, in which is lodged a new part of the ligament destined to become the true ligament of the adult Mytilus. He was not able to determine the relationship between the embryonic ligament and the secondary one. In many of the areas
the primitive ligamentary fossette, occupied by the cartilage, does not remain simple; it is subdivided into two parts, which diverge, forming thus the first two chevron furrows, which are continued to the top of the cardinal edge where they abut against the thin grooves at the cardinal margin. Other grooves for the cartilage appear a little later near the center and are divided in the same manner, and so on. (Bernard, 1896, p. 71).
Bernard's allusion to "cartilage" grooves is unfortunate, for in the areas with which I am familiar the chevron grooves contain the insertions of the lamellar bands of ligament; and the fibrous ligament ("cartilage") covers the general surface of the ligament area (fig. 4D-F).
It is certain that all areas possessing multiple bands of tensional lamellar ligament pass through an ontogenetic stage in which there is only one of these bands; because the ligament bands are added in succession, one at a time, each making its first appearance near the center of the hinge axis, and there is migration during growth towards the hinge extremities in order to make space for new ligament bands. As pointed out by Bernard (1896, pp. 71-72), several species show a delay in differentiation of the parivincular ligament until rather late in the ontogeny. In other species the ligament does not progress beyond the parivincular condition.
It is clear, then, that the multiple ligament of some areas represents a sort of reduplication of the parivincular ligament and has somewhat the same relation to that ligament as the multivincular ligament (as typified by the Pernidae) has to the more primitive alivincular ligament. It seems quite improper to class the multiple ligament of Arca with the multivincular type illustrated by Perna, because the ligaments of the two genera are structurally different and have different ontogenies. The term "arcid ligament" previously used by me seems inappropriate. Therefore, I propose to employ the term duplivincular for ligaments of the type possessed by Arca transversa (fig. 4E,F). All Myalinidae known to me had a duplivincular ligament in the adult stage, differing from modern types principally in being opisthodetic instead of amphidetic. A restoration of a typical myalinid ligament is shown in text figure 5.
Figure 5—Ligament construction in Myalinidae, Liebea squamosa, Permian of Germany, dorsal view, with valves tightly closed. Drawn from the specimen illustrated on plate 15, figure 3. Several conical bands of lamellar ligament, partly invaginated, occur along the hinge line, being inserted in diverging grooves (shown in white) in the ligament area. A thin fibrous ligament occurs (judging from analogy with Arca) in the area between ligament grooves. This type of ligament corresponds to the posterior part of the chevrons of Arcidae.
Musculature
Mytilacea are anisomyarian. That is, through progressive atrophy of the anterior adductor the posterior adductor has assumed most of the function that seems to have been divided originally almost equally between the two adductor muscles. Whether or not the progressive decrease in size of the anterior adductor represents an evolutionary trend that many times independently affected more or less distantly related tribes, or whether this condition had a common origin in a single ancestral stock, cannot yet be positively determined. Of course, it is known that the Tridaenidae have lost the anterior adductor independently of other types of pelecypods. The remaining anisomyaria and monomyaria, such as the oysters, scallops, pterias, mytiloids, and others, commonly are classed together in an order called Dysodonta, on the supposition that they have had a common origin. All of the living members of this order are byssate during part of the life of the animal, and it has been supposed that the adoption of the byssal mode of fixation led to a compression and crowding-out of the organs of the anterior part of the body. In the monomyarian stage the complete disappearance of the anterior adductor was accompanied by a ventral migration of the posterior adductor to a centrally located position to restore a symmetrical relation of the closing muscle to the hinge. There are other factors, however, not yet understood, that affect the placement of the posterior muscles, because there are species of Mytilus in which the anterior muscle is entirely lacking in the adult, yet the posterior adductor remains in its normal place near the rear of the shell, as in anisomyarian forms of the genus. Seemingly, the anterior adductor is not indispensable in Mytilidae, because damage to or complete removal of the anterior adductor in living specimens does not seriously affect the ability of the animal to close its valves tightly.
The musculature of the myalinas, judging from the muscle impressions in fossil shells, was practically identical with that of modern Mytilidae, and rather unlike that of other dysodonts, such as the Pteriidae. It is the similarity of the muscle systems, as well as close likeness of form between primitive myalinas and the Mytilidae, that convinced me that the myalinas are Mytilacea instead of Pteriacea, as supposed by many previous workers.Anterior muscles—Two conspicuous muscle impressions occur near the front end of the shell of living Mytilidae, such as Mytilus and Volsella. One of these, the anterior adductor, occurs at the front terminus of the pallial line, near the anterior extremity of the shell. The other pit lies somewhat behind and dorsal to the adductor, situated very close to the hinge axis. This muscle impression is produced by the anterior byssal retractor, a strong muscle that extends posteroventrally about one-half the length of the shell to the byssal gland at the proximal end of the foot. In some genera (e.g., Septifer) the anterior adductor is attached to the shell on a calcareous shelf, or umbonal septum. In Paleozoic Myalinidae the anterior adductor impression may be located approximately as in Mytilus, in a small pit near the anteroventral margin of the shell (Myalina, Naiadites, Orthomyalina), or on a septum, as in Septifer and Liebea, or underneath a septum with which it has no obvious relationship (Septimyalina). Perhaps the anterior adductor is missing in some species. In all of the better known Myalinidae there are two muscle pits that seem to correspond to the single byssal retractor of the Mytilidae. Presumably, the byssal retractor of the Myalinidae had a bifid insertion at the anterior end.
Figure 6—Musculature in genotypes of Paleozoic and Recent Mytilacea. A Volsella modiola, Recent; internal mold of right valve, X 1; B, Selenimyalina meliniformis, Pennsylvanian, left valve, X 2; C, Septimyalina perattenuata, Pennsylvanian, left valve, X 1; D, Mytilus edulis, Recent, internal mold, right valve, X 1; E, Myalina goldfussiana, Viséan, left valve, X 1; F, Naiadites carbonarius, Pennsylvanian, left valve, X 2; G, Orthomyalina slocomi, Pennsylvania, left valve, X 1.
Musculature essentially the same in both valves. ab, Anterior byssal retractor; a, b, insertions of bifid anterior byssal retractor; c, anterior adductor, seemingly absent in figure C; d, pallial line; e, posterior byssal and pedal retractor; t, posterior adductor; g, gill suspensories, not visible on shell in figures A and D; h, dental socket, or fulcral point, absent in A, D, and F; j, muscle pits of uncertain function; k; umbonal cavity, especially characteristic of Septimyalina, figure C. Small pits over the general interior in figures A, C, and G represent points of attachment of the mantle. They are not visible in all specimens, and their arrangement does not seem to be significant.
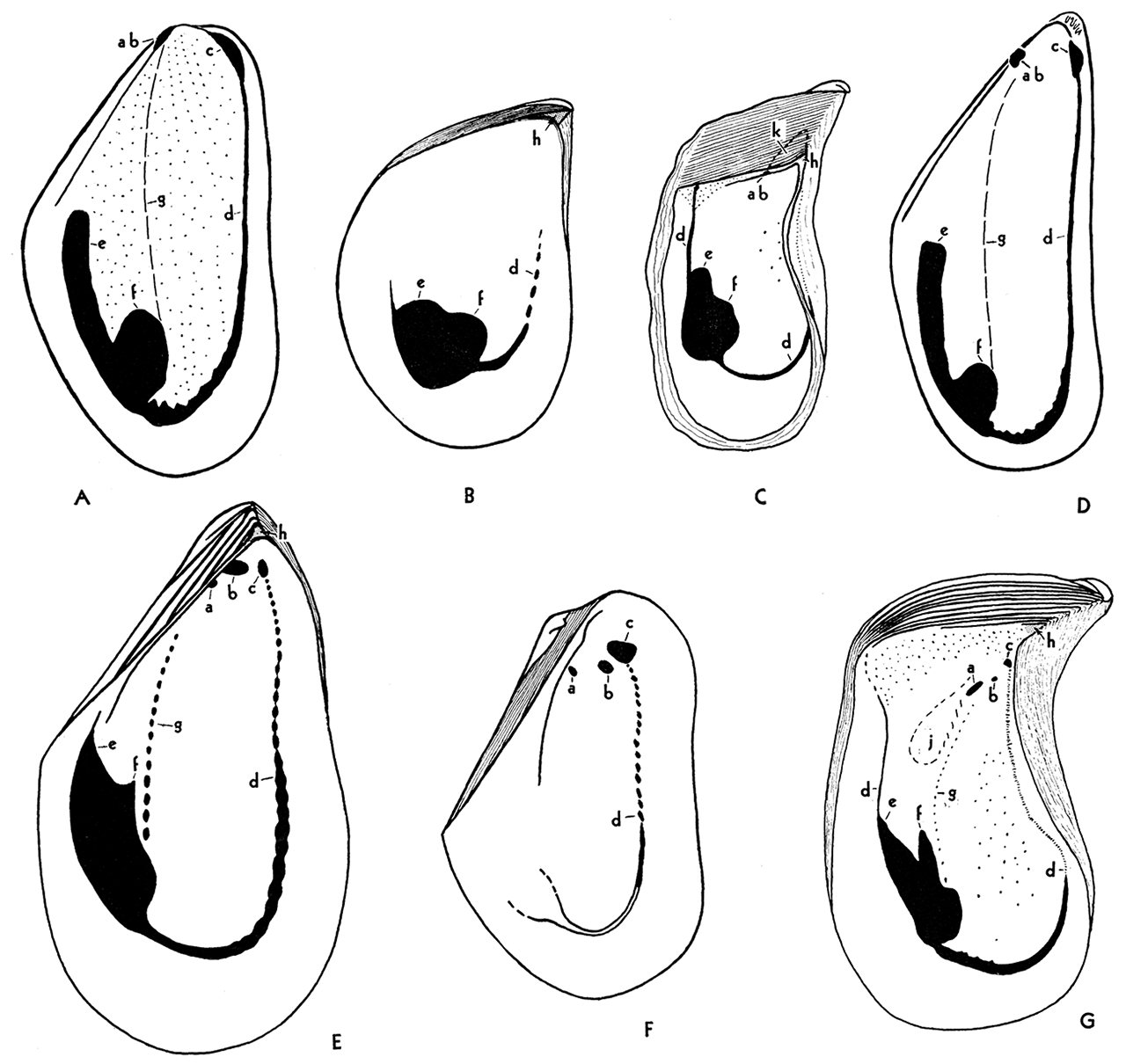
Posterior muscles—In the Mytilacea several muscles are inserted in a small area in the rear part of the shell. Unfortunately, these separate muscles seldom produce discrete spots in the shell so that the general area of insertion cannot readily be differentiated into all of the component parts. The form of the large posterior impression, however, is quite distinctive, and is uniform within a species or genus. The muscle area in the shell is strongly bilobed, divided into two distinct prongs by a well defined dorsal or anterodorsal cleft. The larger ventral lobe, to which the rear end of the pallial line is attached, is the impression of the posterior adductor. The posterior or dorsal lobe is produced by a series of byssal and foot retractors. The pedal retractor muscle, extending from the distal end of the foot, is inserted in the shell at the dorsal extremity of this lobe. Between the pedal retractor and the posterior adductor is a series of six or seven posterior byssal retractors, attached to the byssal gland at the proximal end of the foot, near the middle of the shell. The insertions of the byssal retractors cannot be differentiated in the shell, but they form the main body of the dorsal lobe of the great posterior impression. In the Myalinidae the dorsal lobe seems to be considerably shorter than in the Mytilidae.
Pallial line—The pallial line in Mytilidae is a continuous, rather distinct furrow extending around the margin of the interior of the shell between the two adductor impressions. Examination of the pallial system in the animal of Mytilus or Volsella reveals that the free margin of the mantle is provided with a large number of radial muscles whose function is the withdrawal of the mantle from between the valve margins, preparatory to closing the valves. The insertions of these radial muscles produce the pallial line in the shell.
In the Myalinidae the anterior one-half or two-thirds of the pallial line is composed of discrete pits, which evidently mark the insertions of radial muscles of the mantle. These are spaced more widely than in Mytilus. The rear part of the pallial line, where it joins the posterior adductor impression, is as in the Mytilidae. In a few of the Myalinidae the posterior muscles are withdrawn so far from the hinge line that there is space for a dorsal extension of the pallial line above the muscle impression. In this instance the line is relatively thin and obscure but continuous.
Other muscles of the mantle—Some of the Myalinidae show irregularly disposed fine pits or punctations of the inner surface of the shell inside the limits of the pallial line. Similar pits are common in some of the early Paleozoic Ambonychiidae. Living Mytilidae are relatively thin-shelled, so that distinct muscle marks are commonly lacking in the shells. However, an oceasional Mytilus or Volsella exhibits similar punctations. In some instances they are more or less regularly disposed in radial rows (fig. 6A). Microscopic examination of the mantle of such animals indicates that these punctations represent the insertions of tiny bits of outer surface of the mantle where it is attached to the shell. Mantle attachments of this type are unknown to me outside of the examples mentioned.
Gill attachments—In some of the better known Myalinidae an arcuate row of muscle pits extends from the anterior margin of the posterior adductor impression to a point near the rear impression of the anterior byssal retractor (figs. 6E, g; 6G, g). This line corresponds exactly in position with the location of the attachment of the gills to the mantle in Mytilidae (figs. 6A, g; 6D, g), although the gills seemingly are not directly attached to the shell, and therefore do not produce muscle pits in Mytilus and Volsella. Presumably the gills in Myalinidae were attached at muscle insertions directly to the shell.
Shell Structure
Shells belonging to the Myalinidae are among the best preserved pelecypods found in Late Paleozoic rocks. Associated Mytilidae are commonly less well preserved. Reasons for these differences are found in the construction of the shells. Myalinid shells typically are very thick and massive, considering the relatively small volume occupied by the living animal. Some gerontic shells of Myalina copei Whitfield, from Lower Permian rocks, have an interior cavity amounting to less than an estimated one-eighth of the volume of the calcareous matter of the shell. In some instances the maximum thickness of each valve is around one-half inch, which is of the same order of thickness as the visceral cavity between the valves. Some species have comparatively thin shells, as species of Myalinella; but, on the whole, the shells are heavy and therefore relatively strong to mechanical stresses applied before and after burial. Late Paleozoic Promytilus and Volsellina possessed very thin and fragile shells, comparable in every respect with modern representatives of Mytilidae. In part, the rarity of good specimens belonging to these genera may be due to the fragility of their shells.
In pelecypods, as a rule, both calcite and aragonite occur in the same shell, and these two minerals have a separate distribution within the shell. Where both minerals are present, the calcite occurs in an outer layer and the aragonite occurs underneath, in an inner, commonly nacreous, layer. According to Bøggild (1930, p. 239), an exception is in the Pectinidae, where an aragonite layer occurs between two layers of calcite. There are many kinds of shells, however, which are composed (except for a small amount of conchiolin) exclusively of either calcite or aragonite.
According to Bøggild (idem), temperature is not a controlling factor that determines the formation of aragonite or calcite in mollusks. On the other hand
between the salinity of the water and the composition of the shells there seems to be a more pronounced connection. . . . It is very obvious, however, that almost all the forms which consist of both elements (aragonite and calcite) are confined to salt water. Especially among the Mytilidae we find that most members of that family, which are built up of calcite and aragonite, are found in salt water, whereas the comparatively few living in brackish or fresh water, as the Congeria and the Dreissensia, are purely aragonitic. I have only found one example of calcite-bearing shells among all the mollusks living in fresh water, viz. the genus Neritina, all members of which examined by me possess a very thin, upper calcite layer.
Any more direct influence of the salinity of the water upon the composition of the shells is not to be found. If we compare specimens of the same species of Neritina from fresh water with others from brackish water, we shall find that both possess a thin calcite layer which is not more developed in the latter specimens than in the former. And the same is found in Mytilus edulis. Specimens of that animal from water of the least possible salinity (0.5%) are very small and thin as compared with those from salt water, but the relative amounts of calcite and aragonite are almost the same in both forms. (Bøggild, 1930, p. 242).
Owing to the relative instability of aragonite, this mineral tends to be dissolved or to be altered to the more stable calcite, with the common result that fossil pelecypod shells that originally contained some aragonite exhibit unequal preservation of the aragonite structures as compared with the calcite structures. Quite commonly the inner aragonite part of the shell, bearing muscle impressions, hinge, and ligament characters, is lacking in fossil shells; whereas, the outer film of calcite remains, with external ornamentation, perfectly preserved. Circulating ground water undoubtedly will dissolve aragonite from shells under conditions in which the calcite is not affected. Immediate reprecipitation of the carbonate in the form of calcite will cement calcite shells together to form a coquinoid limestone. Probably many organic limestones are formed in just this manner.
The amount of time necessary for the complete alteration of aragonite to calcite varies widely under different conditions. Contrary to popular belief among geologists, aragonite shells are not unknown in Paleozoic rocks, although they certainly are restricted largely to the post-Mississippian part of the Paleozoic sequence. On the other hand, aragonite shells are dissolved, leaving molds in very young rocks ranging up to the Recent. My own observations agree with those of Bøggild, that aragonite has the best chances for survival in calcareous-argillaceous rocks, particularly those in which water permeability is comparatively low. Dense "clay-ironstone" concretions and carbonaceous limestone concretions ranging upward from the Upper Mississippian commonly contain aragonite shells. A less common medium of preservation is found in asphaltic limestones and sandstones in which the bituminous material evidently was introduced early in the history of the rock. Relatively nonargillaceous calcitic limestones and dolomitic limestones, as well as permeable sandstones, practically never contain aragonite shells. Rocks containing very little calcium carbonate, such as siliceous or ferruginous rocks, commonly lose rather quickly both aragonite and calcite shells. Observers seem to be agreed that transformation of aragonite to calcite generally is accompanied by loss in detailed structure. Shells that have undergone this change commonly reveal only coarsely crystalline texture and are true pseudomorphs. As pointed out below, however, many specimens of Myalina show perfectly the nacreous structure of the inner layer, composed now of calcite. There are good reasons to believe that in this instance details of shell structure are retained after the alteration of the original aragonite to calcite.
Structure in Mytilidae—In fossil, as well as Recent representatives of the Mytilidae, the shell consists of an outer thin layer of calcite, ranging between 0.05 mm and 1.0 mm in thickness, and an inner one of aragonite, ranging from 0.5 mm to more than 5 mm in thickness. The structure of the calcite is
typically homogeneous or indistinctly prismatic with the axes horizontal in the radial direction, or nearly so; the aragonitic layer is nacreous and only in a few instances partially prismatic.
The common Mytilus edulis (Recent) possesses a thick calcitic layer of blue colour; the axes are strongly reclined and in the upper part nearly horizontaL The structure is markedly irregular, being sometimes. perfectly homogeneous and sometimes finely prismatic; and the prisms may, in some instances, be very indistinct, in others more marked, and there may be sharp boundaries between the different parts. The directions of the prisms are nearly parallel to those of the optic axes, though generally a little more horizontal so that each prism gets a somewhat oblique extinction. The aragonitic, white layer is normally nacreous. (Bøggild, 1930, p. 272).
Bøggild might have indicated also that the prisms in M. edulis are so oriented that their outer termini are closer to the beak of the shell than the inner ends. In Volsella modiola, according to my observations, the reverse is true, i.e., the inner ends of the calcite fibers are closer to the beak than the outer ends. In the older Mytili studied by Bøggild, the outer calcite layer varies from finely prismatic, with radial, nearly tangential prisms, to a homogeneous calcite, with no trace of prisms. The aragonite layer consists in some instances of prisms arranged normal to the shell surface. Congeria and Dreissena, both of which are composed of aragonite throughout the shell, are known to have had a somewhat different history from other mytiloids, and they are classed by some in a separate family, the Dreissenidae.
Pennsylvanian representatives of the Mytilidae studied by me seem to have had an original shell structure like that of Mytilus edulis. Promytilus swallowi, together with its near relatives, displays an outer prismatic shell layer that, in fundamental respects, is like that of modern Mytilus. The fibers are very fine, less than 2 microns in diameter, and are arranged almost tangent to the outer surface of the shell, extending in a radial direction so that somewhat corroded specimens exhibit radial striations that have been mistaken for ornamentation. Below the surface of the outer ostracum the prisms are rather abruptly declined ventrally at an angle of 45° to 50° with the inner surface of the shell. It now seems possible that it was this kind of shell structure which, in part, I have called radial crossed-lamellar structure (Newell, 1938, pl. 1, figs. 3, 15). The hypostracum, secreted at the surface of attachment of the muscles, is rather obscure in thin-shelled forms such as Mytilus. Imbedded within the nacreous layer, it consists also of aragonite, but the mineral occurs as fine, irregular fibers arranged normal to the growing surface. The nacreous layer does not show the original structure in any of the Paleozoic specimens of Mytilidae that I have seen. However, the coarsely crystalline structure of the inner ostracum indicates the former existence of aragonite.
Structure in Myalinidae—Myalinas showing fine details of original shell structure are not rare. My evidence suggests that the majority of the shells, however, have lost their original aragonite. X-ray analyses were made for me by Bernard Steierman, University of Wisconsin, on the hypostracum, inner, and outer ostracum of excellently preserved specimens of Myalina (Orthomyalina) subquadrata and Septimyalina perattenuata from the Pennsylvanian of Kansas. These specimens show no obvious recrystallization; and, although I had supposed that the shells contain a large amount of aragonite, the X-ray study revealed no trace of that mineral. Good specimens of several of the genera of Myalinidae have not been available for this kind of study. It may be that aragonite will be found in some forms.
A few specimens of Orthomyalina subquadrata at my disposal retain only the outer ostracum, the massive inner part of the shell having been completely dissolved during fossilization. Certainly the inner ostracum in these shells was composed of a less stable mineral-aragonite-than the outer calcite film. Several specimens of Orthomyalina and Septimyalina reveal excellently preserved hypostracum, with long, irregular fibers having a diameter of 2 or 3 microns. These fibers are now composed of calcite. Whether or not they were originally aragonite I am not sure. It is not easy to explain why the microscopic fibers of the hypostracum and the lamellae of the inner ostracum are so well shown if there has been complete transformation from aragonite to calcite. Perhaps such alteration can be accomplished without loss of detailed structures. Microscopic structures of the shell in fossil cephalopods commonly are preserved. As far as we know, the conch of the cephalopod invariably is composed of aragonite.
As in some other pleurothetic pelecypods (Newell, 1938, p. 25), many of the myalinas show marked differences in the outer ostracum of the two valves. Presumably these differences are correlated with the invariable habit of resting on one side in preference to the other. In Pectinacea and seemingly also in the Myalinidae the right valve normally was undermost, with consequent differences in form and structure in the two valves.
The right outer ostracum, 0.1 mm to 0.3 mm in thickness, consists of rather short, robust polygonal prisms of calcite, having irregular form. The prisms range in size up to about 40 microns in diameter but are much smaller when they first are recognizable in the juvenile parts of the shell on the umbones. There seeIllS to be a progressive ontogenetic increase in size of the prisms during shell growth. Neither size nor shape of the prisms characterizes generic types within the family.
The left outer ostracum of different myalinas exhibits one of two types of structure. Shells that are most nearly equivalve, such as Naiadites, Myalinella, and Selenimyalina, seem to have an identical shell microstructure in the two valves. The markedly inequivalve forms, however, such as the species of Myalina, s. s., Orthomyalina, and Septimyalina, are characterized by a left outer ostracum composed of very fine, perpendicular prisms or fibers having a diameter of the order of 1 micron. Only exceptionally well preserved specimens reveal this structure. Other specimens show what seems to be a homogeneous outer ostracum.
The inner ostracum consists of very thin lamellae, probably originally aragonite, built up successively to form a structure that is very massive in the majority of species. This structure seems to be nacreous. The layer ranges in thickness from about 1 mm to 10 mm in thickness in different shells. As far as can be determined, the inner ostracum is identical in both valves.
The hypostracum consists of fibrous calcite, perhaps originally aragonite, oriented normal to the inner surface of the shell. As in other pelecypods, the shell growth causes migration of the muscle "scars" from the beaks in radial directions. Earlier deposits of hypostracum in the muscle areas are covered by later deposits of the inner ostracum, so that the full course of migration of the hypostracum can be studied only in thin sections of the shell, cut in a radial direction through a muscle impression. As in other pelecypods the hypostracum is identical in structure in the two valves.
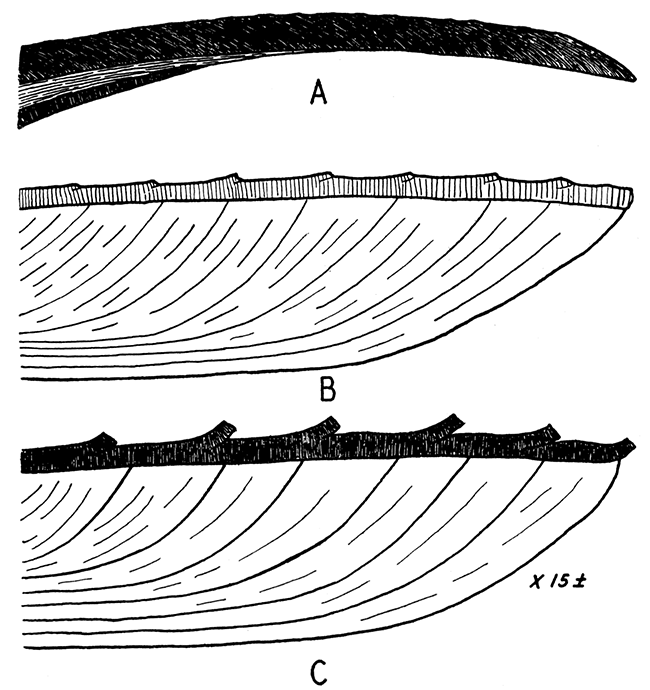
Figure 7—Shell structure of some Mytilacea. Ventral margin at right, beak toward the left; approximately X 15.
A, Mytilus edulis, showing very fine fibrous outer ostracum composed of calcite; the nacreous layer and fibrous hypostracum, both of aragonite are shown below. The Paleozoic Promytilus has a similar shell except that the outer ostracum is relatively thinner.
B, right valve, and C, left valve, of Myalina (Orthomyalina) slocomi. The outer ostracum is calcite; the inner one was probably originally aragonite. In the right valve the prisms are relatively coarse (25 to 40 microns in diameter), but in the left valve the structure is fibrous, individual fibers being in the order of 1 micron in diameter. All of the studied species of Orthomyalina, Myalina, and Septimyalina have the structure described for Orthomyalina slocomi. The two valves in Selenimyalina and Myalinella seem to be identical in shell structure, being comparable to the right valves (B) of other myalinas.
Prev Page--Introduction || Next Page--Systematic Paleontology
Kansas Geological Survey, Geology
Placed on web Dec. 18, 2017; originally published 1942.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/Vol10_2/03_shell.html