Prev Page--Hydrogeology || Next Page--Conclusions
Chemical quality of ground waters from the lower Paleozoic aquifers
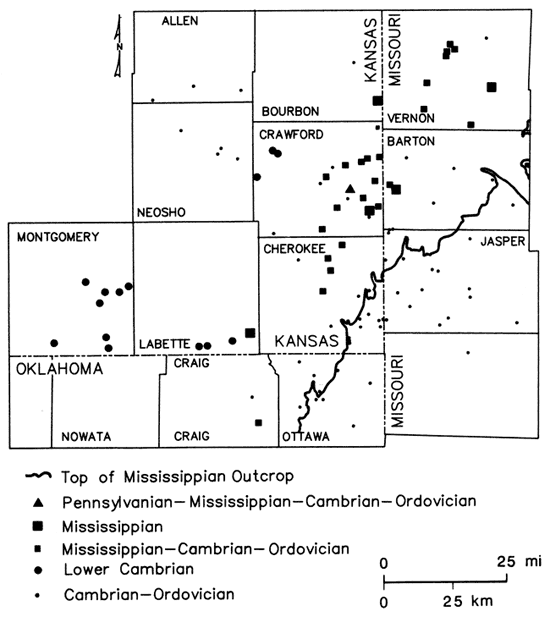
Sampling sites
Water samples from 119 wells were collected during this investigation from domestic, municipal, industrial, oil-field water supply, and oil-producing wells penetrating the lower Paleozoic aquifers in the study area. Of these wells, only two are thought to be open to part of the Pennsylvanian System; seven open only to the Mississippian System; 78 of the wells open to the Cambrian-Ordovician; and 32 to portions of the Mississippian and Cambrian-Ordovician units present. All the wells in the freshwater part of the aquifers were sampled more than once during 1979-1980. The chemical-quality data from the fall 1980 sampling are used as the basis for maps of this report and most of the discussion here. Well locations and aquifer units contributing water are indicated on fig. 7 for all sites used in preparation of the chemical-quality maps. The oil-producing wells and most of the oil-field water-supply wells were sampled only once during 1980-81. Restricted distribution of sampling sites in Kansas and limited information for the Pennsylvanian and Mississippian aquifer systems placed some constraints on the interpretation of water quality in the ground-water systems of the Tri-State region.
Figure 7--Aquifer units contributing ground waters to wells sampled in study area.
Ground-water quality
Feder (1979) noted marked variations in ground-water chemical quality and yields for wells in the Pennsylvanian, Mississippian, and Cambrian-Ordovician aquifer systems of southwestern Missouri, ranging from greater than 1,000 mg/L dissolved solids and 50 gpm (6.4 ft3/min) for Pennsylvanian wells to less than 300 mg/L dissolved solids and 100-500 gpm (13-64 ft3/min) for Cambrian-Ordovician wells. Macfarlane and others (1981) and Hathaway and Macfarlane (1980) found that water quality changes rapidly and deteriorates markedly in a westward direction in the Tri-State region of Kansas, Missouri, and Oklahoma.
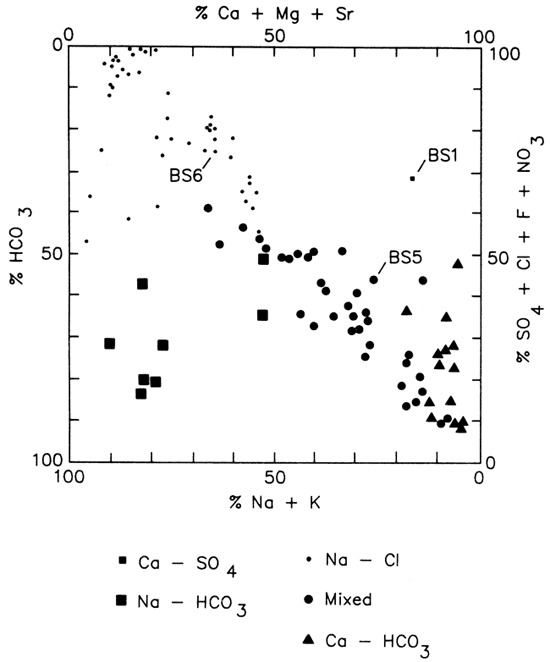
Water-type classifications used in this report are based on percent milliequivalent contributions of the various chemical species to total number of milliequivalents of cations -and anions. Classifications for all sampling sites in the study area are represented by the modified Piper diagram found in fig. 8, which suggests a general transition from Ca-HCO3 or Ca-Mg-HCO3 type waters (southeast portion of the study area) to Na-Cl type waters (west-northwest portions of the study area). This simple picture is complicated by: 1) the occurrence of Na-HCO3 type waters (Crawford County, Kansas, and Barton County, Missouri); 2) the presence of a number of wells open to Mississippian and Cambrian-Ordovician units whose water chemistries fall in the transition region between Ca-HCO3 and Na-Cl type waters; 3) marked variations in water chemistries of wells of similar construction within a given well field; and 4) significant fluctuation in water chemistries noted at some wells open to Mississippian and Cambrian-Ordovician units.
Figure 8--Modified Piper diagram for water samples collected from wells in lower Paleozoic aquifers in Tri-State Region. Percentages represent contributions to the mg/L of cations and anions. BS1, 5, and 6 represent water samples from Baxter Springs wells 1, 5, and 6.
Pennsylvanian aquifers
Water-quality data obtained by Darr (1978) for Pennsylvanian wells in Bates County in southwestern Missouri indicate that Na-Cl type waters are the dominant classification in the region just northeast of the present study area. Total dissolved-solids concentrations in these waters ranged from about 800 to 7,100 mg/L, and sulfide concentrations ranged from less than 0.1 to 30 mg/L. From an application of factor analysis to chemical-quality data for Missouri ground waters, Feder (1979) concluded that Pennsylvanian wells in Cass and Johnson counties of western Missouri exhibit a high loading from a Na-HCO3-Cl type end-member water. Total dissolved solids in these waters ranged from approximately 330 to 1,100 mg/L, with accompanying sulfide levels in the range of less than 0.1 to 8.5 mg/L.
The Pennsylvanian strata in the area covered by the present study, as well as in studies of Darr (1978) and Feder (1979), are generally considered to represent an unconfined aquifer system. The influence of abandoned open-pit and underground coal workings upon ground-water quantity and quality within the study area is largely unknown due to the lack of a premining-era ground-water-quality data base. These features are known to influence locally the quality of surface waters.
Two wells sampled during the present study are open to Pennsylvanian units. One is in NE SW NW sec. 1, T. 27 S., R. 25 E., southeast Bourbon County, Kansas, and is believed to be completed in the lower part of the Pennsylvanian section. The water is a Na-Cl type with a dissolved-solids content of approximately 1,100 mg/L and about 3.5 mg/L sulfide. The second well located in NE SW NE sec. 1, T. 30 S., R. 24 E., is open from the Pennsylvanian to the Cambrian-Ordovician. The relative amounts of ground water contributed by the different stratigraphic units to the discharge of the well are unknown. The water from this second well exhibits some variability in its classification but is predominately a Na-HCO3 type. The dissolved solids level is approximately 650 mg/L. Sulfide values ranged from 8 to 20 mg/L through the duration of the study. The relatively low dissolved-solids level indicates that Pennsylvanian aquifers are not the major contributor of the water produced at this location.
Mississippian aquifers
The Mississippian wells sampled by Feder (1979) are located in the central portion of the Springfield plateau region of southwestern Missouri. This is the outcrop area for limestones of Mississippian age and probably serves as a general recharge area for the Mississippian aquifers. Dissolved solids in waters from these wells ranges from 125 to 404 mg/L and sulfide levels from less than 0.1 to 0.2 mg/L. Factor analysis indicated that the chemistry of waters from Mississippian wells in this part of the unconfined aquifer system reflects the influx of precipitation. Data from Darr's (1978) study indicate a general deterioration in water quality for Mississippian wells northwest of the Springfield plateau area in regions where Pennsylvanian-age units overlay the aquifer. Here the waters fall into a Na-Cl or Na-HCO3 classification. Approximately one-third of the wells investigated exhibited a Na-HCO3 classification. Total dissolved-solids contents range from approximately 500 to 7,300 mg/L and sulfide levels from less than 0.1 to 42 mg/L.
Abandoned lead-zinc mines of northeast Oklahoma and southeast Kansas locally serve as reservoirs for ground and surface water within the Mississippian strata. The mine workings possibly also act as access routes for groundwater movement downward into the Cambrian-Ordovician system where intervening confining units, the Northview and the Chattanooga shales, are missing or are of limited thickness. Playton and Davis (1977) noted that water within the mine shafts was generally stratified with specific conductance, temperature, sulfate, and dissolved-solids and heavy-metal loads increasing with depth. The pH of these waters usually decreases with depth into the mine. Thus, an influx of water from the old mining area would seem to have an undesirable influence upon local ground-water quality.
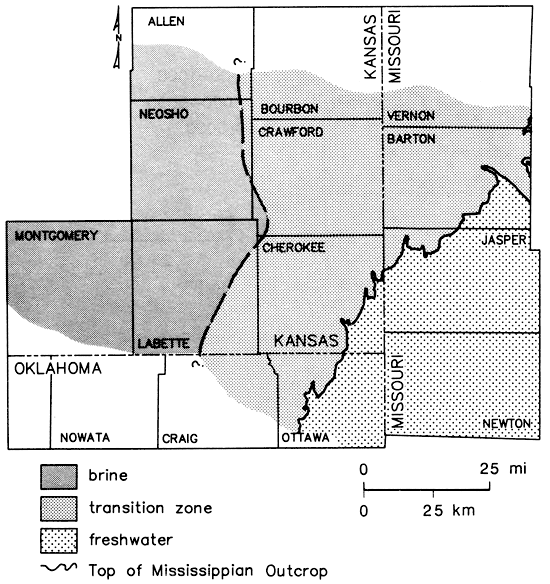
Well logs indicate that a number of the wells sampled in Vernon County (Missouri) and eastern Crawford and north-central Cherokee counties (Kansas) are open to portions of the Lower Mississippian as well as to strata in the Cambrian-Ordovician system (see fig. 7). These wells tend to fall within a general transition zone found in the Cambrian-Ordovician system (fig. 9). The western boundary of this zone was set at a chloride concentration of 2,500 mg/L, approximately 5% of the maximum value determined for oil-field brines collected during this study. The greater depth and aquifer thicknesses intercepted by oil-field water-supply wells in Neosho, Allen, and Crawford counties, Kansas, relative to that of the oil wells of Montgomery and Labette counties, Kansas, lend uncertainty to the placement of this boundary. The eastern limit coincides with the exposed Mississippian-Pennsylvanian boundary.
Figure 9--Generalized representation of transition zone in Cambrian-Ordovician aquifer; solid lines represent Pennsylvanian-Mississippian boundary outcrop and dashed line is the 2,500 mg/L isochlor.
Maps based on a composite of water-quality data for all wells sampled during this study indicate a gradation from fresh, Ca-HCO3 type waters in southwest Missouri to saline, Na-Cl type waters within the transition zone in southeast Kansas. In northeast Oklahoma, low dissolved-solids Na-Cl type waters were found just west of the Mississippian outcrop (figs. 10 and 11).
Figure 10--Classification of ground waters from lower Paleozoic aquifers based on dominant cation and anion present.
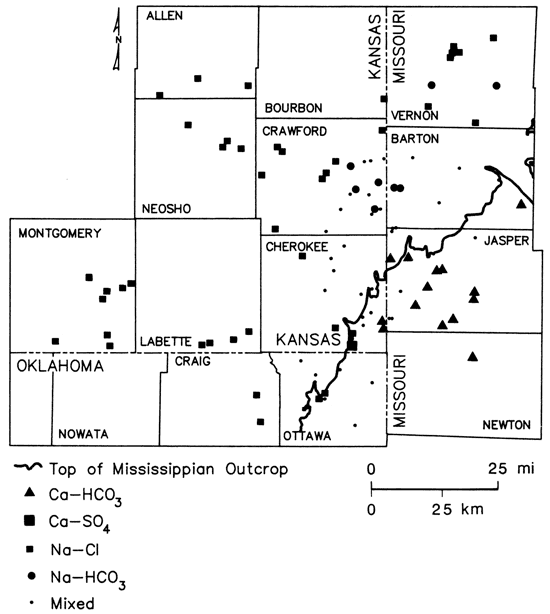
Figure 11--Calculated total dissolved solids (PPM) of ground-water samples from lower Paleozoic aquifers, Tri-State region.
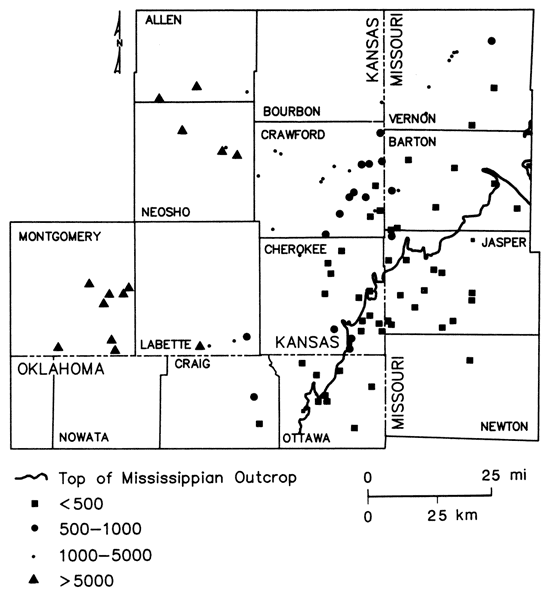
Sulfide concentrations greater than 0.2 mg/L, indicating the presence of reducing conditions, were encountered west of the Mississippian outcrop (fig. 12). The pattern of boron values (fig. 13) corresponded well to the defined brine, transition, and freshwater zones of fig. 9, suggesting its use as a salinity indicator for this region. Other water-quality parameters such as chloride, strontium, and radium-226 (to be discussed later) also exhibited concentration gradations similar to that noted for total dissolved solids.
Figure 12--Sulfide concentrations in ground waters from lower Paleozoic aquifers in Tri-State region.
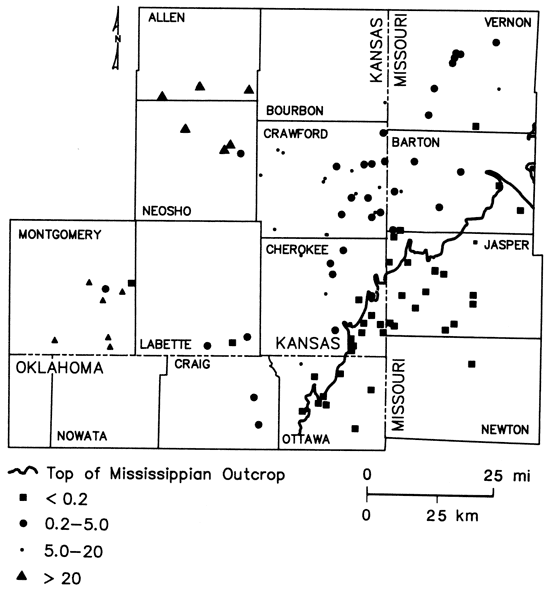
Figure 13--Boron concentrations in ground waters from lower Paleozoic aquifers in Tri-State region.
Na-HCO3 type waters were found in Mississippian and some of the multiaquifer wells in Vernon and Barton counties (Missouri) and Crawford County (Kansas). Excess amounts of HCO3, relative to the Ca and Mg contents of the waters, were noted in a number of other wells open to Mississippian and Cambrian-Ordovician aquifers in the region, corresponding to the quality transition zone extending from Craig County (Oklahoma) through Labette, Cherokee, Crawford, and Bourbon counties (Kansas). However, not all wells which were thought to be open to Mississippian units exhibited excess HCO3. Multiaquifer municipal wells at Mindenmines and Sheldon (fig. 14) in southwest Missouri showed considerable variation in water chemistry over the 2-yr period of this study (table 2). This suggested variable contributions to the output of these wells from the different aquifer sources over a period of time. Data presented by Darr (1978) for a 10-yr timespan also showed similar variations in water quality at those two locations. The origin of the Na-HCO3 type waters is not understood but may lie in complex ion-exchange dissolution reactions between invading freshwaters from the Springfield plateau area and interbedded marine shales and limestones. The occurrence of these waters may reflect the products of biological activity on carbonaceous materials within the strata.
Figure 14--Geographic location of well sites and Picher mining field in Tri-State region: Crawford Rural Water District #7, well #1 (1); Mindenmines, Missouri, city well (2); Sheldon, Missouri, city well #2 (3); Gulf Oil Chemical Corporation well (4); Baxter Springs, Kansas, city wells (5); Quapaw, Oklahoma, city wells (6); Commerce, Oklahoma, city well #3 (7); Clemons Coal, well #1 (8); and Picher mining field (9).
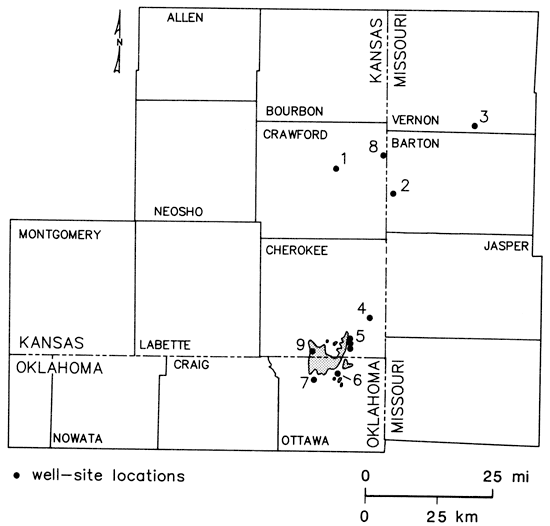
Table 2--Short-term chemical-quality changes associated with two multiaquifer wells from study area.
| Date | HCO3 (mg/L) |
Cl (mg/L) |
Specific conductance (µmho at 25°) |
Water type |
|---|---|---|---|---|
| Sheldon, Missouri | ||||
| 4/17/1979 | 215 | 133 | 780 | Na-mix |
| 9/25/1979 | 314 | 102 | 870 | Na-HCO3 |
| 5/14/1980 | 210 | 136 | 810 | Na-Cl |
| 10/8/1980 | 509 | 36 | 920 | Na-HCO3 |
| Mindenmines, Missouri | ||||
| 4/18/1979 | 327 | 53 | 700 | Na-HCO3 |
| 9/25/1979 | 744 | 39 | 1,530 | Na-HCO3 |
| 5/12/1980 | 522 | 54 | 945 | Na-HCO3 |
| 10/7/1980 | 327 | 700 | ||
The reason for wells in the transition zone being open to Lower Mississippian units is unclear at present. A number of these wells were formerly used in coal-processing operations, and lack of good stratigraphic control or a simple desire for water quantity may have been factors in the placement of well casings in this area.
Sulfide levels in the five Mississippian wells located in the confined portion of the aquifer sampled during the present study had a range of 4.0-13 mg/L. This indicates a reducing environment within that part of the aquifer system. Sulfate levels in the same five wells vary from 16 to 201 mg/L, suggesting that waters from some of these wells may not be in a state of chemical equilibrium but rather represent mixtures of waters of varying chemistries which have been produced by pumpage of the wells. Sulfide levels of less than 0.1 mg/L were found at the two Mississippian well sites located in unconfined portions of the system.
Intrinsic features of the aquifer system such as faults, folding, solution channels, fractures, and areas of silicification may serve to isolate and interconnect water-bearing zones having varied ground-water chemistries. Wells open to Mississippian and Cambrian-Ordovician units exhibit sulfide levels of less than 0.2-8.0 mg/L and sulfate levels of 8-128 mg/L. Here also the higher sulfate and sulfide levels may be found together suggesting a mixing of waters from different sources, but generally this coexistence indicates the presence of a reducing environment.
Ordovician and Cambrian aquifers
In southwest Missouri, northeast Oklahoma, and southeast Kansas, the dolomites and sandstones of Ordovician and Cambrian age serve as important sources of freshwater. Westward into Kansas, these units become saline and supply waters for oil-field water-flooding operations and then become sources for oil and oil-brine production.
Harvey (1980) noted that at West Plains in south-central Missouri, wells into exposed Cambrian-Ordovician units are 1,300-1,500 ft deep and have 950-1,000 ft of casing that is pressure-grouted in place. Yet water from the wells often is turbid after rainstorms. Solution channels in the overlying limestone are clearly making possible the rapid movement of water from the surface down to the Cambrian-Ordovician system at this location. Westward, the dominant constituents in ground water from the Cambrian-Ordovician aquifer system in the region of the Springfield plateau of southwest Missouri are Ca, Mg, and HCO3. In areas with dolomitic source rocks, the Ca/Mg ratio, based on milliequivalents per liter, is nearly 1.0. Ratios of 2.0-3.0 for some wells cemented through Mississippian-limestone sequences suggest that slow leakage through exposed limestone units to the underlying Cambrian-Ordovician system is taking place.
Feder's (1979) study of Missouri waters suggests that two end-member water types may be contributing to water quality of Cambrian-Ordovician units in Dade, Barton, Jasper, Newton, and Lawrence counties. One end member is enriched in Ca, Mg, and HCO3, and the other represents recharging precipitation. Dissolved-solids levels in waters from this aquifer system ranged from 160 to 459 mg/L and sulfide levels from less than 0.1 to 1.4 mg/L. Northward into Vernon County, Missouri, data from Darr (1978) showed a Na-Cl type classification for a Cambrian-Ordovician well indicating a transition in water chemistry northward from the Ca-Mg-HCO3 type waters. Dissolved solids and sulfide levels at this well site were 1,022 mg/L and 3.1 mg/L, respectively.
Cambrian-Ordovician wells in Missouri sampled during the present study exhibited a general transition northward from a Ca-HCO3 classification in Newton and Jasper counties, to a Ca-Mg-HCO3 to Ca-Mg-Na-HCO3 classification (shown as mixed in fig. 10) in Barton County, and a Na-Cl classification in Vernon County. Dissolved-solids levels in the HCO3 type waters ranged from approximately 150 to 525 mg/L. The dissolved-solids level was 824 mg/L in the Na-Cl type water. Westward into Kansas and Oklahoma, the water chemistry of the Cambrian-Ordovician system exhibited a gradation to Na-Cl type waters in Crawford and Cherokee counties, Kansas, and Ottawa County, Oklahoma (fig. 10). This transition was accompanied by a marked increase westward in the dissolved-solids contents of the waters (fig. 11). Macfarlane and others (1981) suggested that chemistries of water samples from Cambrian-Ordovician wells within the Tri-State area can be approximated by mixing different amounts of two general end-member waters, a Ca-Mg-HCO3 freshwater and a Na-Cl brine.
Milliequivalent per liter (meq/L) Ca/Mg ratios for ground-water samples from the Cambrian-Ordovician aquifer collected during this study typically have values in the range of 1.0-2.0. Three wells in central Jasper County, Missouri, are exceptions with Ca/Mg values near 3, suggesting a greater influence of waters in contact with exposed Mississippian limestones in the area. Ground waters from Cambrian-Ordovician wells generally do not contain HCO3 in excess of that necessary to satisfy their corresponding Ca and Mg contents. Brines from two oil wells in southern Labette County, Kansas, are exceptions with HCO3 excesses greater than 6 meq/L. This suggests the possibility of Na-HCO3 type water influx from the Mississippian aquifer at these sites. In this area the Northview and Chattanooga shales only have a combined thickness of approximately 20 ft (6 m).
Sulfide levels did not exceed 0.2 mg/L for wells east of the Mississippian outcrop. Westward from the outcrop zone, sulfide levels increased to approximately 100 mg/L in oil-field brines of Montgomery County, Kansas. The reducing environment in the Cambrian-Ordovician aquifer, as designated by the presence of sulfide levels greater than 0.2 mg/L, seems to coincide very well with the similar conditions found in the overlying confined Mississippian-aquifer system. Nitrate levels in excess of 2.0 mg/L were not observed for any well where the sulfide level was greater than 0.2 mg/L, again substantiating the presence of a reducing environment in parts of the Mississippian and Cambrian-Ordovician systems within the study area. Sulfate concentrations in waters from Cambrian-Ordovician wells were observed to be low (less than or equal to 20 mg/L), generally in areas east of the Mississippian outcrop, to increase to levels of approximately 20-125 mg/L in the freshwater-brine transition zone, and to decrease again to levels of less than or equal to 20 mg/L in brines from the western portion of the study area. The simultaneous occurrence of sulfide levels of 3-100 mg/L and sulfate levels of 75-1,100 mg/L in many waters from Cambrian-Ordovician wells located west of the Mississippian outcrop suggested that a state of chemical equilibrium may not exist in these samples. Sulfate originally present may be incompletely reduced, or a source within the stratigraphic column may be leaking sulfate to the aquifer system faster than it can be reduced. Otherwise pumpage of the wells may simply produce a disequilibrium blend from waters with different chemistries. Within the brine portion of the aquifer, barium levels tended to vary directly with changes in the sulfide content of the waters. Similarly, Gilkeson and others (1983) found that high barium levels occurred in ground water from confined portions of the Cambrian-Ordovician system in northeastern Illinois where dissolved sulfate was depleted through anaerobic microbial reactions.
Areal displays of ground-water chemical-quality data such as those shown in figs. 10-12 may give the impression that quality only varies laterally. However, the complex nature of the carbonate and sandstone units which make up the Cambrian- Ordovician aquifer system and the regional structural fabric almost insure that chemical-quality variations will exist in the vertical profile of the system as well. General information on vertical changes with depth in chemical quality in the Cambrian-Ordovician aquifer system is available for few sites within the study area. The Jayhawk Ordnance well, now located on the Gulf Oil plant site (fig. 14) in southeastern Cherokee County, Kansas, was completed January 1942. Abernathy (1943) presents chemical-quality data for water samples taken from different formation levels during the drilling of the well. These samples were obtained using a bailer and thus may only represent composites from the various units penetrated. The data do suggest a general but erratic increase in chloride and total dissolved-solids levels with depth in the Cambrian-Ordovician system. Chloride data printed on a log dated 1938 for the Clemens Coal Co. well #1 in northeastern Crawford County, Kansas (fig. 14), suggest a decrease in chloride levels with depth in the Cambrian-Ordovician system down into the Roubidoux Formation. Thus, no simple picture of vertical variation in water quality seems to exist within the Tri-State area for the Cambrian-Ordovician aquifer system. Deterioration of quality with depth in the more saline portions of the aquifer in the western half of the study area might be expected.
On a local scale, lateral variations in water quality may be very complex within the Cambrian-Ordovician system. This is best exemplified by the city well field at Baxter Springs, Kansas (fig. 14). Well #5 is about 0.3 mi (0.5 km) north of well #1, and well #6 is about 0.3 mi (0.5 km) north of well #5. All three wells were completed to approximately the same depth and have similar well construction. Samples collected between April 1979 and October 1980 showed well #1 produced a Ca-SO4 type water with dissolved solids of 480-705 mg/L, well #5 produced a mixed cation-HCO3 type water with dissolved solids of 240-305 mg/L, and well #6 produced a Na-Cl type water with dissolved solids of 420-605 mg/L. Changes in the dissolved-solids levels at these three wells were reflected in fluctuations of Cl and SO4 contents of the waters, suggesting that prolonged pumpage of any of the three wells may have induced noticeable changes in water quality. During a 24-hr pump test at well #1 in May 1979, the specific conductance was observed to drop from 1,065 µmho/cm to 930 µmho/cm while water temperature and pH remained constant.
Pumpage at Crawford County Rural Water District #7, well #1 (fig. 14), has induced an increase in the sodium and chloride content of the water produced at that site. Between 1972 and 1980, Na levels increased from approximately 270 to 315 mg/L and Cl levels rose 460-525 mg/L. Water from this well remained a Na-Cl type throughout that period of time. Based on the response of water quality to pumpage in the Baxter Springs well field and at Crawford County RWD #7, well #1, an eastward movement of saline waters toward pumping centers is occurring or seems to be a possibility for Cambrian-Ordovician wells located within the region of the eastern edge of the water-quality transition zone.
Local ground-water chemistry, regional transitions in water quality, and changes induced by pumpage may be controlled to a large extent by the complex subsurface geologic structure associated with the Tri-State area. Fig. 5 provides a general picture of water movement for the Cambrian-Ordovician system within the study area. Seemingly, structural features such as the Pittsburg anticline in Kansas and Chesapeake fault in Missouri (fig. 3) should influence water movement, and thus water quality, in the study area. The presence of the Bourbon arch in Kansas and Missouri may serve as a general barrier in the northern portion of the study area to separate Na-Cl type saline waters to the north of the structure from HCO3 type freshwaters moving to the north and west from the Ozark uplift.
The low dissolved-solids Na-Cl type waters encountered in northeast Oklahoma indicate dilution of the Cambrian-Ordovician brines by vertical leakage with little flushing or displacement of Na-Cl type waters westward from the Mississippian outcrop. However, lead-zinc mining operations in the Picher field have exposed mineralized zones in the Mississippian to oxidizing conditions which favor conversion of sulfide to sulfate. Sulfate concentrations in the Cambrian-Ordovician aquifer at Quapaw wells #2 and #3 and Commerce well #3 (fig. 14) are elevated relative to the areal background value of about 15 mg/L. Also, the sulfate concentration at these sites increased during the time of this study (table 3). This suggests a local vertical recharge to the Cambrian-Ordovician system, which is enhanced by past mining activities and substantial hydraulic-head differences between the Mississippian and Cambrian-Ordovician aquifer systems.
Table 3--Sulfate concentrations for selected wells at Quapaw and Commerce, Oklahoma, April 1979-May 1980.
| Well | Date | SO4 (mg/L) |
|---|---|---|
| Quapaw #3 | 5/1980 | 212 |
| Quapaw #2 | 4/1979 | 44 |
| 9/1979 | 57 | |
| 5/1980 | 70 | |
| Commerce #3 | 9/1979 | 34 |
| 5/1980 | 42 |
Distribution of selected members of uranium-238 and thorium-232 decay chains in waters of lower Paleozoic aquifers of Tri-State area
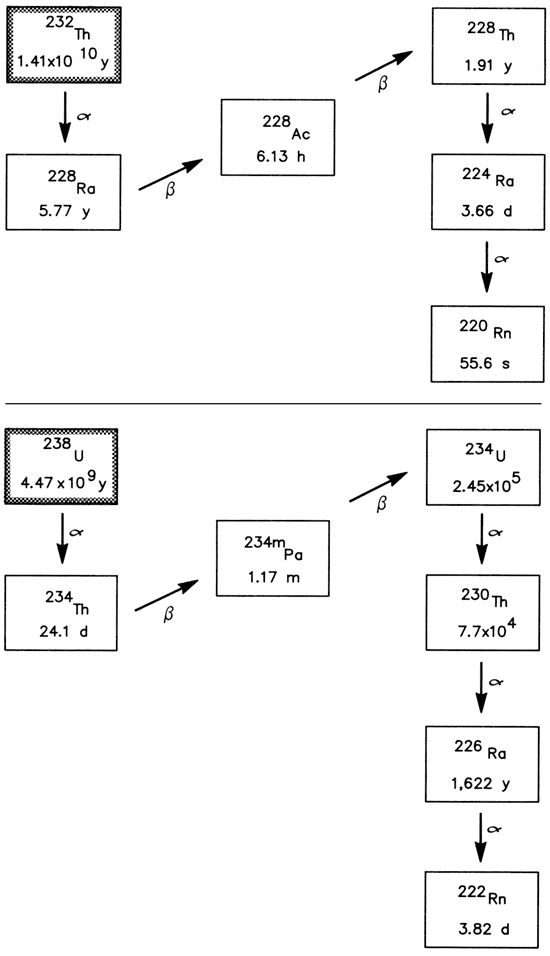
The radioactive isotopes thorium-232, uranium-235, and uranium-238, with half-lives of 1.41 x 1010 yr, 7.04 x 108 yr, and 4.47 x 109 yr, respectively, are long-lived parent isotopes of naturally occurring radioactivedecay chains which are of importance in the areas of geochemistry and environmental health. All three chains undergo a series of alpha and beta decay processes that terminate with the formation of stable lead isotopes. The natural uranium-238/uranium-235 atomic abundance ratio is 137.5/1 and the thorium/uranium mass ratios for whole igneous rocks are approximately 3.5-4.0 (Rogers and Adams, 1978).
Certain members of the thorium-232 and uranium-238 decay chains have received interest in the areas of hydrology (Osmond and Cowart, 1976; Osmond, 1980; King and others, 1982; Krishnaswami and others, 1982; Gilkeson and others, 1983) and human health (Radioactivity Subcommittee-NAS, 1977; Hess and others, 1979). Fig. 15 depicts partial decay chains for these two parent isotopes.
Figure 15--Partial radioactive-decay chains for thorium-232 and uranium-238 showing half-lives in years (y), days (d), or hours (h) and modes, a α- or β-decay.
The radioactivity of an unstable isotope is defined as
A = Nλ = 0.693 (N/t1/2)
where A is the activity in disintegrations per unit time, N is the number of atoms of the isotope in the system, λ is the decay constant for the isotope, and t1/2 is the isotopic half-life. The units used in this report to express the content of many of the radioactive elements are picocuries per liter (pCi/L). A curie is defined as that quantity of a radionuclide in which the number of disintegrations/ sec is 3.7 x 1010. A picocurie (pCi) is equal to 1 X 10-12 curies or 2.22 disintegrations/min. In a closed system the activity of a radioactive daughter (Ad) equals that of the radioactive parent (Ap) if a state of secular equilibrium exists. This state is possible if
λp << λd (t1/2(p) >> t1/2(d))
and the system has reached sufficient maturity. However, at or near the earth's surface the activity ratio Ad/Ap often has a value other than 1.00, indicating that disequilibrium exists within the decay series.
Uranium, thorium, radium, and radon tend to fractionate in the hydrogeological environment because of differences in chemical behavior. Uranium is mobilized in an oxidizing aqueous environment through interactions of carbonate, sulfate, or phosphate ions with hexavalent uranium which produce soluble complex species. In reducing environments, represented in this study by ground waters containing sulfide ions, uranium is reduced to the relatively immobile tetravalent state and precipitated within the aquifer system. Mobility of tetravalent thorium is very limited under almost all near-surface hydrogeochemical environments. Under oxidizing ground-water conditions the solubility of divalent radium seems to be controlled largely by adsorption-desorption processes with the aquifer materials. Radium levels of these waters are generally well below those expected from solubility considerations based on the relatively insoluble RaSO4. Gilkeson and others (1983) have suggested that co-precipitation of radium in the deposition of BaSO4 may be an important control upon radium concentrations in ground waters. Under reducing conditions where sulfate ions are converted to sulfide ions, and with increasing salinity of the ground-water, radium is desorbed and remobilized. Marikos (1981) noted that in Missouri, elevated radium-226 levels from the uranium-238 decay chain may be associated with high dissolved solids in ground water and the existence of surface or subsurface faults. On the other hand, anomalously high radium-228 and radon-222 activities from the thorium-232 decay chain were found in samples from heavily faulted areas.
Activities of radium-226 in ground waters from the Tri-State area in excess of the present maximum contaminant level of 5 pCi/L for radium, as set by U.S. Environmental Protection Agency (EPA), have been reported. Scott and Barker (1962) in a survey of data for uranium and radium in ground waters of the United States for 1954-57 list a value of 8.9 pCi/L of radium-226 for the old well at Girard, Kansas (Crawford County). Hobart (1976) cites a mean value of 11.2 pCi/L for radium-226, based on monthly samplings in 1975, for the Afton, Oklahoma (Ottawa County), water-supply system. A direct correlation between radium-226 and total dissolved solids was observed for this site. Spiker and others (1979) reported that in 1977 and 1978, seven of the 28 public-water supplies sampled in Cherokee and Crawford counties had radium-226 levels which at times exceeded a 5 pCi/L level. They also found that high radium-226 levels tended to correlate with increased chloride contents of the waters.
From 1979 through 1981, water samples were collected for radionuclide analyses at 80 sites in the study area to obtain more detailed information regarding radium-226 in ground waters. Activities of radium-226 were determined in samples from all sites. Total uranium and uranium-234/uranium-238 activity-ratio measurements were made on samples from 35 sites. In addition, values of radium-228 activity were determined for samples from eight sites in the western half of the study area (Montgomery, Neosho, Allen, and Crawford counties in Kansas) which represent Cambrian-Ordovician oil wells or oil-field water-supply wells.
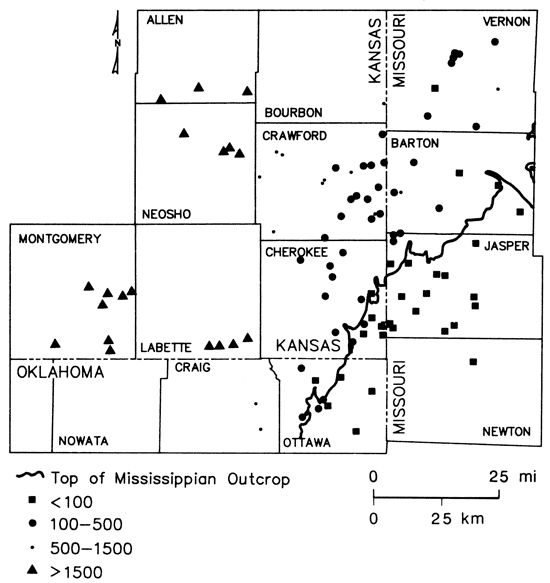
Fig. 16 is an areal display of radium-226 data collected during the course of this investigation. Activities of radium-226 within the freshwater portion of the study area are generally less than 2 pCi/L, levels within the chemical-quality transition zone range from less than 2-9 pCi/L, and radium-226 levels in wells from the brine portion of the study area range upward to 1,600 pCi/L. Overall, radium-226 values greater than 2 pCi/L tend to be associated with the presence of reducing conditions and increased concentrations of dissolved solids. Spiker and others (1979) have suggested that Kansas public-supply wells located within the region of the transition zone in Cherokee and Crawford counties may exhibit elevated radium levels as a result of downward leakage of water from Mississippian units. However, radium-226 values in samples from the three wells in the Mississippian aquifer range only from 0.8 to 3.6 pCi/L. These waters would not be capable of producing the elevated levels noted.
Figure 16--Radium-226 distribution in ground waters from lower Paleozoic aquifers in Tri-State region.
Fluctuations in radium-226 activity with time in samples of water from two multiaquifer wells located within the transition zone were evaluated by analysis of ten samples collected on approximately a monthly basis from April 1979 to April 1980 at two sites. Average values of 2.2 ± 0.2 pCi/L for Mindenmines, Missouri (Mississippian-Cambrian-Ordovician well), and 7.2 ± 0.5 pCi/L for Crawford County Rural Water District #7, well #1 (uppermost Lower Ordovician well), Kansas, indicated limited variation in the radium-226 levels for these types of wells. This is in contrast to wide fluctuations in basic water chemistry noted at the Mindenmines well (see table 2) and only small fluctuations at the rural-water-district well during the same period of time.
The three Cambrian-Ordovician wells of the Baxter Springs, Kansas, municipal well field seem to be located just inside the freshwater, non-reducing side of the freshwater transition-zone boundary (figs. 14 and 9). Radium-226 values of 1.8, 2.3, and 12 pCi/L were observed for samples collected in 1980 from wells #1, #5, and #6, respectively. This trend in the radium level does not correlate with total dissolved-solids contents of the waters (701, 303, and 605 mg/L, respectively) but does vary inversely with sulfate (315, 58, and 22 mg/L, respectively). Additionally, the highest radium level found in well #6 is associated with a Na-Cl type water and a chloride value which is about five times greater than the levels found at wells #1 and #5. This suggests a pumpage-induced eastward movement of saline water toward well #6; local hydrologic conditions producing a back flushing through sediments containing precipitated uranium at sites near or within the transition zone may be very important in determining radium-226 values encountered in waters produced by individual wells.
The direct relationship between radium-226 values greater than 2 pCi/L and sulfide levels greater than 0.2 mg/ L depicted in fig. 16 suggests that the increase in radium-226 activities from the freshwater side into the transition zone may reflect long-term cumulative effects of the reduction and deposition of uranium carried by invading freshwaters from the southeastern portion of the study area. Variations in the concentrations of sulfide, total dissolved solids, and uranium-238 decay-chain radionuclide data for selected Cambrian-Ordovician wells are summarized in figs. 17 and 18.
Figure 17--Location of wells along two traverse lines sampled for selected radionuclides.
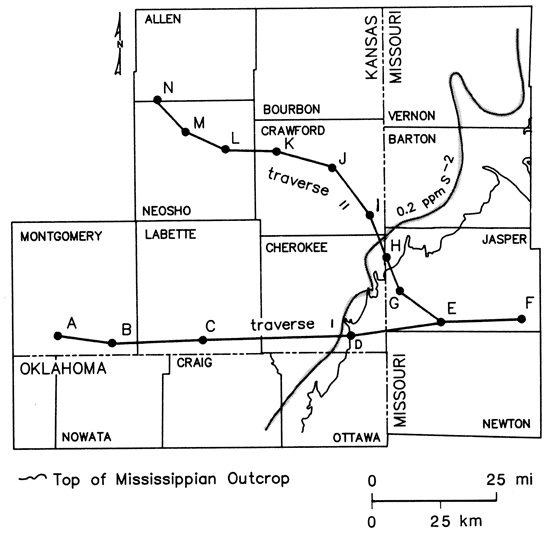
Figure 18-Radium-226 activity, uranium concentration, uranium-234/uranium-238 activity ratio, total dissolved solids, and sulfide concentration variations along two traverses across Tri-State region shown in fig. 16.
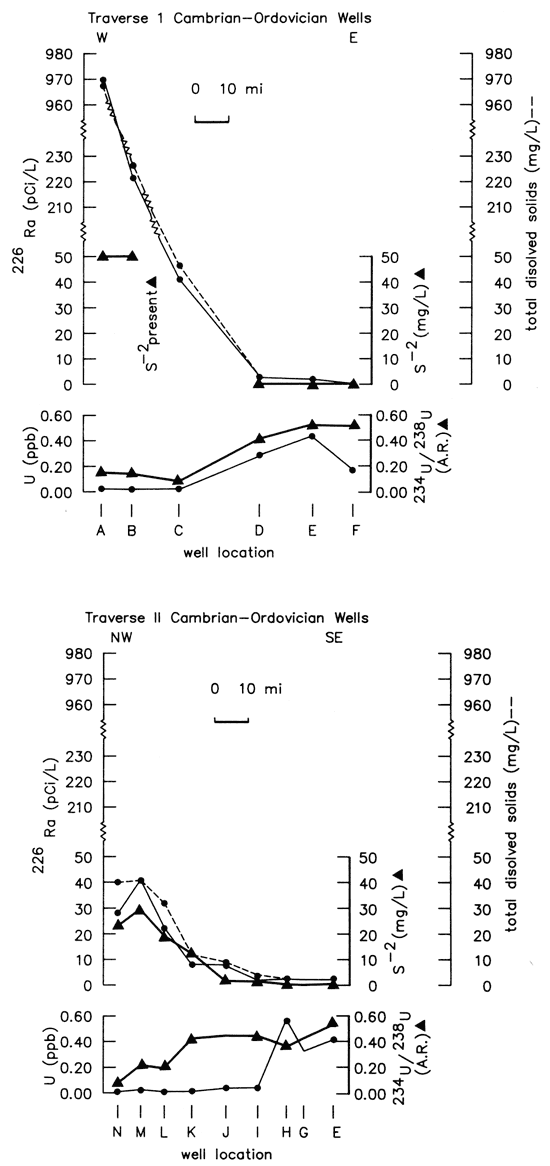
These two figures (17 and 18) show that the concentration of uranium declines and radium-226 activities begin to increase near the eastern boundary of the transition zone as freshwaters encounter sulfide-bearing waters of the transition zone. Radium-226 activities in the two traverses vary directly with concentrations of sulfide and total dissolved solids. The sulfide concentration reported at site A may be too low due to sampling difficulties and subsequent loss of H2S. The uranium-234/ uranium-238 activity ratios exhibit relatively little variation and are anomalously high (7-11) from the freshwater portion of the study area westward and northwestward far into the transition zone. Szabo (1982) found similar uranium-activity ratios in springs of the Pomme de Terre Valley of Southwestern Missouri. In the more saline regions of the study area west of the transition zone, the uranium-234/uranium-238 activity ratios range from 1.5 to 4.0. The differing ranges of uranium-activity ratios suggest two different sources of uranium in ground waters of the study area and the possible eastward migration in recent geologic time of the reducing environment. Deposition of uranium with a high activity ratio along the eastern edge of the transition zone would enhance the potential for radium-226 production. This conclusion is consistent with the observation that radium-226/uranium-234 activity ratios are near equilibrium with values of about 1.0 in the freshwaters but become greater than 1.0 as sulfide levels become greater than 0.2 mg/L (Hathaway and Macfarlane, 1980). However, the high radium-226 levels and low uranium-activity ratios found in ground waters west of the transition zone indicate that a different source for radium-226 probably exists in the western portion of the study area.
Radium-228, unlike radium-226, is not expected to migrate very far within a slow-moving, confined-aquifer system because of its relatively short half-life. Radium-226/radium-228 activity ratios of 0.7-0.9 are to be expected in hydrogeologic systems at equilibrium with materials containing crustal thorium/uranium mass ratios of 3.5-4.5. Radium-228 activities in southeastern Allen and northwestern Crawford counties in Kansas are in the range of 2.0-3.0 pCi/L, and radium-226/radium-228 activity ratios at the corresponding well locations are in the 2.4-2.6 range. To the southwest in Montgomery County, Kansas, the radium-228 levels rise to values of 20-74 pCi/L, and the radium-226/radium-228 activity ratio increases to values of 11-13. Gilkeson and others (1983) found in Illinois that confined sandstone aquifers of the Cambrian-Ordovician system contribute waters with the highest radium concentrations. They also noted that high radium-226 activities were related to the presence of highly mineralized ground water, but that radium-228 activities seem to be unrelated to the ionic strength of the solution. Feldspar grains within the matrix of the aquifer are suggested as the source for radium-228. The limited data, poor spatial distribution, and lack of information regarding uranium/ thorium ratios in materials from the Precambrian basement or Cambrian-Ordovician system make it difficult at present to evaluate the significance of the radium-228 data to an understanding of the hydrogeochemistry of the Tri-State area. Another major problem in the interpretation of radium activity in the lower Paleozoic aquifers of the study area is the lack of specific knowledge of the precise source of the sampled water. The limited availability of well-construction information for the older wells, coupled with long, open bore-hole sections in most of the wells, prohibits precise definition of water sources for most of the wells.
Prev Page--Hydrogeology || Next Page--Conclusions
Kansas Geological Survey, Geohydrology
Placed on web Sept. 1, 2010; originally published 1987.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/GW9/06_qual.html