Prev Page--Metals || Next Page--Water
Nonmetallic Mineral Resources
Asphalt Rock
by J. M. Jewett
Summary—One plant in Kansas has been producing asphalt rock for several years. The known reserves in Kansas exceed 25 million tons.
Natural asphalt consists of solid or semisolid hydrocarbons that have been formed by natural processes from petroleum by the evaporation of the lighter hydrocarbons and the partial oxidation of the residue. Artificial asphalt is obtained as a residue from distillation of some crude oils; a large part, 80 per cent or more, of the asphalt produced in the United States is obtained in this way. Natural asphalt may occur in "lakes" at the surface, as in the Burmudez asphalt lake in Venezuela and on the island of Trinidad. A more widespread mode of occurrence of asphalt is in the voids of porous rocks, where it may serve as a cement binding the rock particles together or may occupy interstices in rocks bound by other mineral cementing material. Rock that contains an appreciable amount of asphalt is known as asphalt rock. The asphaltic content in most cases ranges from about 5 to 15 per cent. Asphalt rock generally is in the form of impregnated porous sandstone or limestone. Some writers prefer to use the term bitumen to designate the hydrocarbon material in such rocks. The term asphalt is used in this paper.
Asphalt rock in Kansas
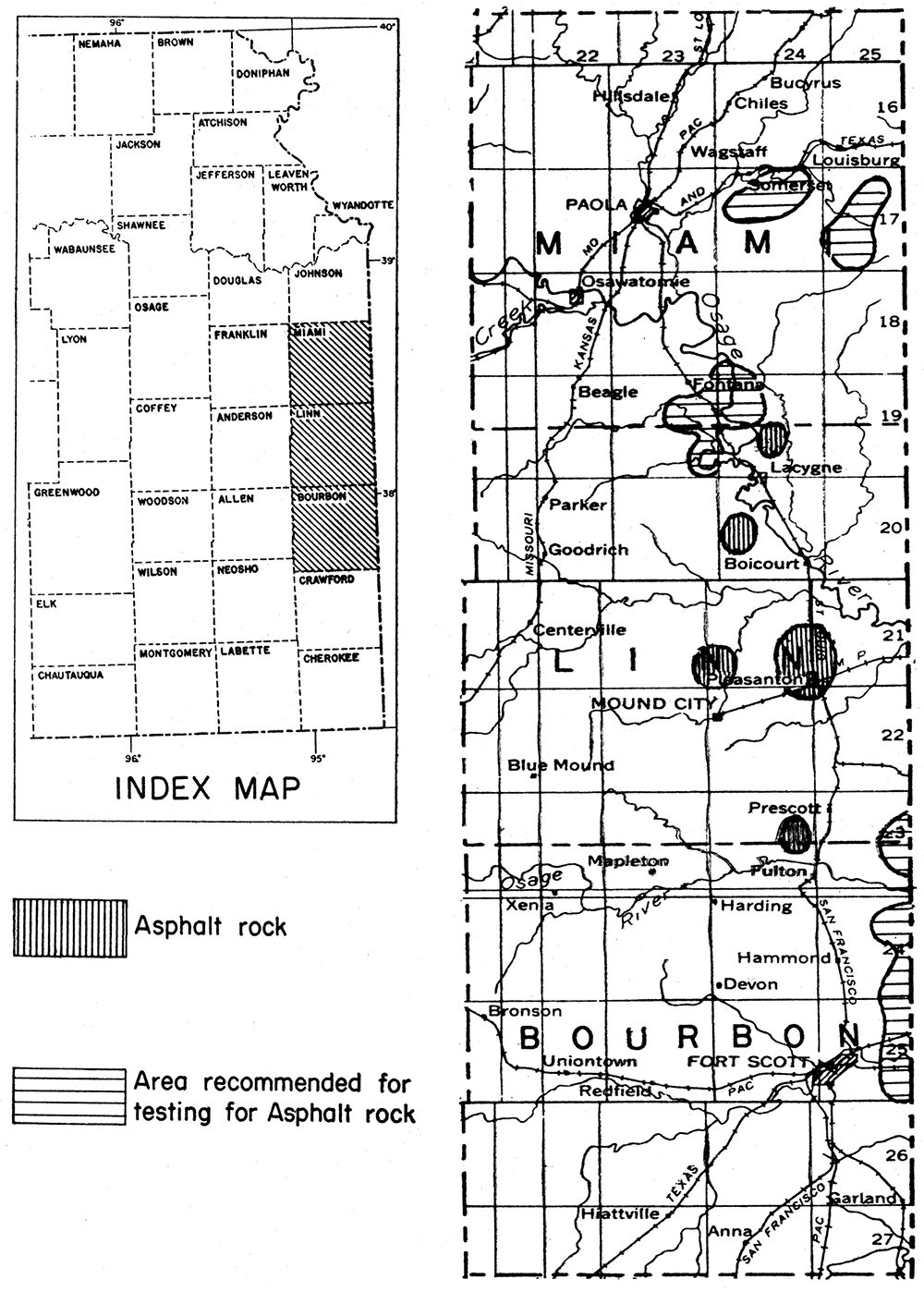
In eastern Kansas there are commercial deposits of asphalt rock in Linn County; in the same county there are several other deposits that are not being worked. Old oil seeps in Miami County would warrant exploration. In Labette County, pockets of very heavy crude oil in porous, cavernous limestone together with partly impregnated porous limestone at the surface indicate that commercial deposits of asphalt rock may be found. Weathered outcrops of impregnated rock have lost most of their asphalt. The commercial deposits have been found in artificial exposures or by shallow drilling. Other counties in eastern Kansas also may be profitably tested by drilling in areas where porous rocks lie close below the surface. It should be noted, however, that the presence of asphalt is unusual; that is, one may expect to examine a great many porous rocks without finding asphalt.
Linn County is the only county in Kansas in which asphalt rock is being produced. A quarry in asphaltic sandstone is located in sec. 25, T. 24 S., R. 24 E. High-grade asphalt rock is being quarried there, and the material is processed in a plant at Pleasanton. The reserves in this part of Linn County are believed to be large. Samples of asphalt rock from Linn County contain approximately 12 per cent hydrocarbons. Asphalt has been seen on the surface or in shallow excavations in Linn County in secs. 19, 24, 25, and 36, T. 21 S., R. 24 K, and in sec. 30, T. 21 S., R. 25 E. All of these 0ccurrences are in the same sandstone stratum.
Limestone asphalt rocks occur in both the northern and southern parts of Linn County. A quarry in sec. 20, T. 20 S., R. 24 K was worked for sometime by the Kansas Rock Asphalt Company, and high-grade material was obtained. A similar occurrence is in sec. 21, T. 19 S., R. 24 K, where a quarry was opened several years ago but has been abandoned. There is good reason to believe that the above-mentioned areas contain a considerable reserve of asphalt rock of limestone type.
It is believed that by systematic exploration additional commercial deposits of asphalt rock could be found in eastern Kansas. Miami, Bourbon, and Crawford counties seem to have favorable indications of the presence of asphalt rock. Areas in Kansas wherein deposits are known or believed to be present are shown in figure 4.
Figure 4—Index map of eastern Kansas showing location of Miami, Linn, and Bourbon counties. Enlarged detail map of the three counties showing asphalt rock deposits and areas recommended for prospecting.
Production in Kansas
For several years high-grade asphalt has been produced at a plant at Pleasanton. This material has found a market in several states, and its fitness as a paving material has been proved.
Uses of asphalt Rock
Where artificial asphalt is used in paving, aggregate must be added; where crushed asphalt rock is employed, both the aggregate and binding material are present. In practice, artificial asphalt' often is added to lean asphalt rocks after crushing. Kansas asphalt rock is of both the sandstone and limestone types; hence two kinds of aggregate are available which can be blended to produce almost any desired mixture. Attention is called to the present demand for asphalt for use in the construction of paved runways at airports.
Kansas reserves
The deposits in Linn and Miami counties lie in an area of asphalt rock extending into Vernon and Barton counties, Missouri; It has been estimated that there are approximately 90,000,000 to 100;000,000 tons of asphalt rock in Linn County, Kansas, and in Barton and Vernon counties, Missouri. Between 20,000,000 and 25,000,000 tons are estimated to be present in Linn County alone. Additional discoveries, through prospecting at the sites of former "tar springs" in Miami County, probably would increase greatly the known reserve.
Age and origin
The asphalt in Kansas asphalt rock deposits is believed to have been formed by evaporation of more volatile parts of crude oil that formerly was held in porous sandstone and limestone. The asphalt is the partly oxidized residue of petroleum. All asphalt rocks in eastern Kansas are Pennsylvanian in age.
Work of the State Geological Survey of Kansas
The State Geological Survey of Kansas (Bull. 29, 1940) has located and described areas in Kansas of known asphalt rock deposits and areas recommended for prospecting. Additional work, especially the investigation of favorable areas, is planned.
References
Heinz, C. E., and Netzebrand, W. F., 1932, The Missouri-Kansas rock-asphalt deposits: Roads and Streets, vol. 65, no. 10, pp. 419-422.
Jewett, J. M., 1940, Asphalt rock in eastern Kansas: Kansas Geol. Survey, Bull. 29, pp. 1-23, pls. 1-2, figs. 1, 2 (including map). [available online]
Redfield, A. H., 1937, Industrial minerals and rocks (chapter on Native Bitumens): Am. Inst. Min. and Met. Eng., New York, pp. 527-532.
Bentonite
by W. H. Schoewe
Summary—Kansas bentonite deposits are unexploited and undeveloped. Experiments have shown that they are well adapted for use in drilling muds, bleaching oils, and bonding material for foundry sands.
Description
Bentonite is a clay or claylike material derived from the alteration of volcanic ash. The chief constituent of bentonite is the clay mineral montmorillonite, a hydrous aluminum silicate. In general, bentonites are of three types: (1) those that swell greatly in water, (2) those that do not swell in water any more than ordinary plastic clays or fuller's earth, and (3) those that show a degree of swelling intermediate between types 1 and 2. The high-swelling type of bentonite absorbs large quantities of water and remains in thin water dispersions, whereas the nonswelling type absorbs no more water than ordinary plastic clays and settles rapidly in thin water dispersions. Mineralogically, bentonites may be classified as alkaline bentonite, alkaline sub-bentonite, alkaline-earth bentonite, and alkaline-earth sub-bentonite.
The Kansas bentonites thus far discovered and investigated belong to the intermediate type (3), as far as swelling is concerned. Mineralogically, they include all four of the above-mentioned classes. They are fine, plastic and most commonly of some shade of gray or green. Colors reported are: very light gray, greenish gray, light-yellow gray, bluish gray, brownish gray, olive green, pale olive-green, gray-green, greenish white, maroon, brown, and chocolate-brown. Some of the bentonites, such as those in Wallace County, are interbedded with soft, dusty grit and sand, whereas others, such as some in Phillips County, are interbedded with shale. Thickness of bentonite beds range from 6 to 30 feet. In Wallace County some of the deposits occur in more or less isolated, long narrow mounds, as a result of erosion. The overburden varies from 3 to 40 feet. Experiments conducted by the State Geological Survey of Kansas show that the Kansas bentonites when immersed in distilled water have a percentage increase of from 200 to 300 per cent.
Chemically, bentonites should be high in silica and alumina in order to be classed as economically important. Analyses by Kinney (1942) of the State Geological Survey of Kansas show that the Kansas bentonites have a high silica and alumina content. The average chemical composition of ten bentonites from Phillips and Wallace counties is giver: in table 13 below.
Table 13—Average chemical composition of ten bentonites from Phillips and Wallace counties. (Analyses by E. D. Kinney).
| Constituents | Percent |
|---|---|
| SiO2 | 54.72 |
| Al2O3 | 18.58 |
| Fe2O3 | 2.77 |
| CaO | 3.50 |
| MgO | 0.38 |
| Na2O and K2O | 0.82 |
| TiO2 | 0.01 |
| H2O lost at 105° C | 0.89 |
| Ignition loss (900° C) | 17.10 |
| SO3 | 1.00 |
| Total | 98.96 |
The chemical analyses also show that Kansas bentonites contain only normal amounts of impurities consisting of lime, iron oxide, and sulphates. It should be noted that impure bentonite deposits often are situated very near purer material, a condition calling for selective mining to assure a uniformly good product.
Distribution
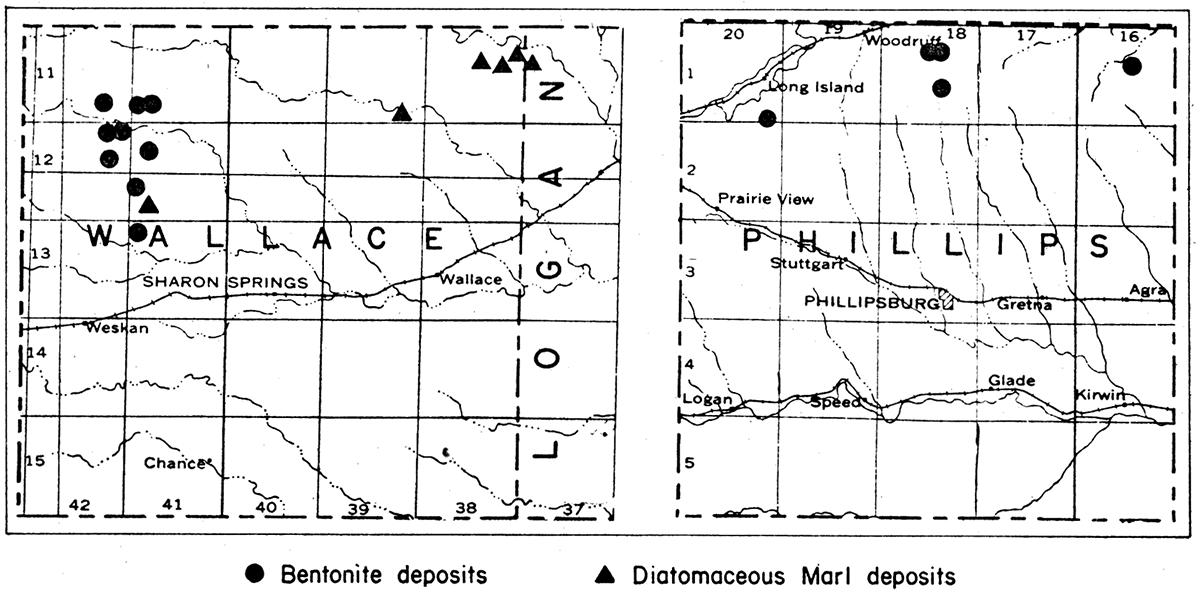
The known deposits of bentonite in Kansas are in Phillips and Wallace counties (fig. 5). The Phillips County deposits occur in an area that extends in a northeasterly direction from sec. 35, 3 miles due south of Long Island, to sec. 11, T. 1 S., R. 18 W. This area is approximately 12 miles long and from one-half to three-quarters of a mile wide. Samples have been collected from the SE sec. 10, T. 1 S., R. 18 W., and NE sec. 35, T. 1 S., R. 20 W. The Wallace County bentonite deposits also are extensive. Bentonite is known to be present in Wallace County at the following locations:
NW NE sec. 29, T. 11 S., R. 41 W.
NW NE sec. 30, T. 11 S., R. 41 W.
NE sec. 26, T. 11 S., R. 42 W.
NW sec. 8, T.12 S., R. 41 W.
NW SW sec. 8; T. 12 S., R. 41 W.
SW sec. 19, T.12 S., R. 41 W.
sec. 1, T. 12 S., R. 42 W.
sec. 2 T. 12 S., R. 42 W.
SE sec. 14, T. 12 S., R. 42 W.
SW NW sec. 6, T. 12 S., R. 41 W.
Figure 5—Maps of Wallace and Phillips counties, and part of Logan County, Kansas, showing location of known bentonite and diatomaceous marl deposits.
Bentonite undoubtedly is present at places other than those mentioned above, not only in Phillips and Wallace counties but also in counties from which bentonite thus far has not been reported.
Uses
Experiments thus far conducted on Kansas bentonites (Kinney, 1942) indicate that they are suitable for the purpose of filtering oils, as an ingredient in moulding sands, and for purposes of sealing to prevent the percolation of water in the construction of dams. Kansas bentonites swell only a little over three times their original volume, compared with 15 times in the case of the Wyoming bentonites. Furthermore, Kansas bentonites tend to form thick colloidal pastes with moderate amounts of water. These facts, together with their cheapness and accessibility, should render them useful as oil-drilling muds, especially for use in local drilling. Experiments have shown that Kansas bentonites are unusually good as bonding cement in foundry sands.
Bentonite is used as a thickening agent in drilling muds, for the purpose of sealing walls against water filtration, and as a bonding medium for heat and sound insulation blocks, plasters and cements. Bentonite is a standard suspending, spreading and adhesive agent in horticultural sprays and insecticides. It is used for the purpose of clarifying turbid waters and purifying sewage, for emulsifying asphalts and other water immiscibles; and as an admixture in concrete to improve workability and flow and to prevent segregation. Bentonite also is employed to inhibit the gumming of screens in dewatering paper pulp, to gelatinize mash poultry foods, and in the preparation of cosmetics and pharmaceuticals.
Production and reserves
Up to the present time (April, 1942), Kansas bentonite deposits have not been exploited or developed, and the extent and volume of reserves is not known. In 1938, a considerable number of auger holes were drilled in sec. 35, T. 1 S., R. 20 W. and in sec. 10, T. 1 S., R. 18 W., in Phillips County. Bentonite was found in most of the auger holes, and thicknesses of 25 feet were reported in some. As indicated previously in this report, further intensified search and test drilling undoubtedly will reveal bentonite deposits other than those already known, not only in Phillips and Wallace counties but also in other Kansas counties.
The importance of searching for and developing Kansas bentonite deposits is suggested by the table below, which shows the great increase in the volume of bentonite sold in the United States from 1931 to 1940 inclusive. During this period production and value increased almost five times.
Table 14—Bentonite sold by producers in the United States, 1931-1940. [Minerals Yearbook, U.S. Bureau of Mines, 1940.]
| Year | Short Tons | Value in Dollars |
|---|---|---|
| 1931 | 52,293 | 429,842 |
| 1932 | 57,743 | 489,803 |
| 1933 | 84,993 | 719,345 |
| 1934 | 146,187 | 977,208 |
| 1935 | 157,445 | 1,047,600 |
| 1936 | 177,807 | 1,367,420 |
| 1937 | 194,768 | 1,500,758 |
| 1938 | 192,183 | 1,373,182 |
| 1939 | 219,720 | 1,702,393 |
| 1940 | 251,032 | 1,919,461 |
Wyoming and South Dakota are the largest producers of bentonite in the United States. Other states in which bentonite is sold or used by producers include: Alabama, Arizona, California, Colorado, Mississippi, Nevada, New Mexico, Oklahoma and Utah.
Origin and age
Bentonite is derived from the alteration or weathering of volcanic ash. Kansas bentonites are of at least two ages. In Wallace County, bentonite is found in the Tertiary Ogallala formation. Some of. the Tertiary bentonite may be Miocene and some early Pliocene in age. The Phillips County bentonite is found in the lower part of the Weskan shale member of the Pierre shale formation of Cretaceous age.
Work of the State Geological Survey
The State Geological Survey has carried on a preliminary exploratory survey in Phillips and Wallace counties. Recently, the Survey has completed a series of chemical analyses of Kansas bentonites and has performed various tests to determine their physical properties and uses, especially as oil drilling muds, bleaching materials for oils, bonding material in ceramic mixtures and as bonding cement in foundry sands. A report on Kansas bentonites and their uses is now in preparation and will be published in the near future as a bulletin of the State Geological Survey of Kansas.
References
Davis, C. W., and Vacher, H. D., 1928, Bentonite, its properties, mining, preparation, and utilization: U.S. Bur. Mines, Tech. Paper 438, pp. 1-51, fig. 1, tables 1-5.
Industrial Minerals and Rocks, 1937, Bentonite (chapter by Paul Bechtner), pp.129-134.
Kinney, E. D., 1942, Kansas bentonite: Kansas Geol. Survey, Bull. (in preparation). [available online]
Ross, C. S., and Shannon, E. V., 1926, Minerals of bentonite and related clays and their physical properties: Am. Ceram. Soc., Jour., vol. 9, pp. 77-96.
Chalk
by Norman Plummer
Summary—The production of Kansas chalk up to the present time has been on a small-scale basis. It is estimated that at least 50 billion tons could be mined in Kansas by stripping methods.
Deposits of chalk are of sparse geographic distribution. The only important deposits of chalk in North America are in a few of the midwestern states. A large proportion of the chalk in this area is within the boundaries of Kansas. In the past, most of the chalk used in the United States has been imported from England and France. These chalks are of late Cretaceous age and, according to Wilson (1937, p. 14), are composed dominantly of the fossil remains of minute organisms, coccalithophores, and foraminifers. The term whiting originally was applied to the water-ground chalks from these countries. Since about 1918, any material composed dominantly of calcium carbonate and having the requisite degree of fineness, plasticity and purity is called whiting.
Chalk industry in Kansas
Very little chalk has been produced commercially in Kansas. This is due in part to the fact that until recent years European chalks could be shipped to this country very cheaply by water. The principal reason for the fact that Kansas chalks have not been developed is that their suitability as a source of whiting was not known until very recently. Subsequent to a preliminary investigation of Kansas chalks in 1937 and 1938, several carloads were shipped from a locality north of Gaylord in Smith County.
UsesChalk, after grinding for use, is given the trade name whiting. At the time whiting was imported in considerable amounts, about 50 per cent of the material was used as a paint filler, 35 per cent as a rubber filler, and about 10 per cent was used in making putty (Wilson, 1937). Putty is manufactured by grinding the chalk or whiting with linseed oil. For these uses, the physical characteristics of the chalk, such as particle size, plasticity and color, are more important than the chemical composition.
The proportion of chalk used for ceramic whiting evidently was not included in the percentages given above. Chalk used for ceramic whiting must meet rather exacting requirements as to chemical composition. Three samples of Kansas chalk that have been analyzed meet these requirements with the exception of the iron oxide content. The standard specifications (American Ceramic Society, pp. 928, 378) designate a maximum Fe2O3 content of 0.25 per cent. In the three Kansas samples analyzed, the Fe2O3 content ranged from 0.33 to 0.60 per cent. It is probable that the Kansas chalks would meet the requirements if carefully quarried so as to eliminate material from iron-stained joints and bedding planes.
English chalk is the standard of purity in the whiting industry. In table 15, analyses of three samples of English chalk and of a sample of Kansas chalk from Smith County are listed for purposes of comparison.
Table 15—Chemical analyses of English chalk and Kansas chalk.
| Constituents | English chalk1 Per cent, three samples |
Kansas chalk (Smith County)2 Per cent |
||
|---|---|---|---|---|
| CaO | 54.4 | 55.0 | 54.0 | 54.80 |
| SiO2 | 1.5 | 0.7 | 1.7 | 1.50 |
| Fe2O3 | 0.08 | 0.D7 | 0.18 | 0.55 |
| Al2O3 | 0.4 | 0.4 | 0.4 | 1.35 |
| MgO | 0.4 | trace | 0.4 | 0.55 |
| Loss on ignition | 43.0 | 43.1 | 42.8 | 42.18 |
| Total | 99.78 | 99.27 | 99.88 | 100.93 |
| CaCO3, calculated | 97.2 | 98.3 | 97.2 | 97.8 |
|
1 Analyses from U.S. Bureau of Mines, Bull. 395. 2 Analysis by Raymond Thompson in the laboratories of the Kansas Geological Survey. |
||||
The slight excess of iron oxide in Kansas chalks would not be detrimental in many ceramic uses of whiting. Whiting is used in glazes, enamels, and in porcelain and whiteware bodies. Chalk is more suitable than other naturally occurring forms of calcium carbonate because of its fine particle size and consequent ability to stay in suspension in water.
Kansas reserves
The Fort Hays chalk formation in Kansas is 60 to 80 feet in thickness and crops out in an irregular belt extending in a southwest direction from the Kansas-Nebraska line in Jewell County to a point about 15 miles northwest of Garden City (fig. 12). There is enough chalk in Kansas to supply the entire United States for many centuries at the present rate of use. It is estimated that at least 50 billion tons could be mined by stripping methods.
Age and origin
The most important commercial reserve of chalk in Kansas is the Fort Hays limestone, basal member of the Niobrara chalk of late Cretaceous age. Chalk is a marine sedimentary deposit derived largely from the calcareous remains of microscopic organisms.
References
Anonymous, 1928, Standard specifications for materials: Am. Ceram. Soc. Jour., vol. 11, no. 6, pp. 378-386.
Wilson, Hewitt, and Skinner, Kenneth, 1937, Occurrence, properties and preparation of limestone and chalk for whiting: U.S. Bur. of Mines, Bull. 395, pp. 1-155, figs. 1-48, tables 1-32.
Chat
by R. M. Dreyer
Summary—Large amounts of chat are available in Cherokee County for concrete aggregate, railroad ballast, and road metal.
Chat is a by-product of the mining of zinc and lead ore in the Tri-State district. Chat constitutes the rock waste with which the lead and zinc minerals are associated. The minerals comprising chat are essentially chert, limestone, and calcite. Mining operations during a great number of years have resulted in the accumulation of large chat piles in the Tri-State district. Until very recently these chat piles were not extensively utilized. At the present time, however, some of the chat piles containing lead and zinc minerals in sufficiently large amounts are being reconcentrated to remove these minerals, which were not recovered during earlier milling operations. In recent years, attempts have been made to find uses for the chat. Considerable amounts now are being utilized for railroad ballast, concrete aggregate and road metal. A large reserve of chat is available in the Kansas portion of the Tri-State area.
Clay
by Norman Plummer and John Romary
Summary—The value of structural clay products produced in Kansas during 1940 was approximately $1,500,000. The known reserves of Cretaceous clay in north-central Kansas are 125 billion tons, and of this amount 46 billion tons are light-firing clays. The reserves of red-firing clay in eastern and southeastern Kansas are enormous.
One of the world's oldest crafts is that of molding useful or ornamental articles from wet clay, drying and firing them to temperatures sufficiently high to make them permanently resistant to weather. Fired bricks over 10,000 years old have been found below the level of the Nile valley, and excellent types of glazed pottery were made in Egypt as early as 3000 B.C.
Clay industry in Kansas
In the early days of Kansas, the brick and pottery industries were much more widely distributed over the state than they are now, although the total tonnage probably was not much greater. Many small communities made hand-molded bricks and fired them in scove kilns. These bricks were used locally in the construction of buildings, many of which are standing today. Small potteries were established over the state to supply local needs.
Production
The high point of brick production in Kansas came in the period from 1900 to 1925. The highest annual production of common bricks was in 1906, when nearly 315 million bricks were produced. The high in vitrified brick production was attained in 1924, with an annual production valued at $1,237,853. The production of vitrified bricks declined rapidly thereafter, because concrete replaced bricks in paving. The highest annual production of face bricks and drain and building tile was attained in 1929, in which year the output of face bricks was valued at $730,116 and that of drain and building tiles at $106,823.
The annual production in 1940 of all structural clay products was valued at approximately $1,500,000. If roofing tiles were included in the list, the figure for 1941 would be considerably higher. Kansas has the second largest roofing-tile plant in the world. During the latter part of 1940 and most of 1941, this plant was running about 20 percent over capacity because of large government contracts.
Clay production in Kansas has been increasing slowly since the extreme low in 1932 and 1933. It is reasonable to expect that production will continue to increase in the future. In the opinion of experts, the timber supplies of this country are not adequate to permit continuance of the present rate of use. In the future the more durable materials, brick and concrete, may replace, wherever possible, lumber used in construction.
Most of the production in Kansas is that of structural clay materials, such as bricks, hollow tiles, and roofing tiles. These products are made mostly from red-firing shale of Pennsylvanian age that occurs in southeastern Kansas. Buff facing brick and tiles are made from a Cherokee underclay at Weir. The demand for light-colored bricks has increased in the past few years. It is probable that within the next few years larger and larger amounts of light-firing clays will be produced from the enormous reserves in north-central Kansas.
Uses
In general, the use of clay and shale for the manufacture of building materials is well known. Less well known are the various types of refractories, chemical ware, and pottery which can be made from these materials.
Refractory fire clays are made into bricks for lining boilers and for constructing blast furnaces, kilns, and regenerators. These fire clays are used also in the manufacture of a variety of special-shaped articles, such as locomotive firebox crowns, glass tank blocks, crucibles, and pot furnaces. Such products are necessities in many industrial processes, including those employed in wartime industries. Perhaps the most critical use of fire clays, and the one in which the requirements are the most exacting, is in the construction of fireboxes of our navy's ships.
Pottery is a general term including a variety of products ranging from flower pots to fine china, and from art ware to chemical stoneware. The types of clays used are equally varied. Some cheaper types of ware are made from red-firing clays, but the greater proportion is made from clays firing to colors ranging from white to buff. White vitreous china or porcelain is made from kaolin, white-firing ball clay, and feldspar. This class of ware includes "china" table ware, spark plug porcelains, chemical ware, lavatory and toilet bowls, and bathtubs.
Many articles that commonly have been made from metals can be made equally well from clay, thus releasing the metals for wartime uses. An extensive list of ceramic (clay and glass) substitutes for materials needed in wartime was compiled by the United States Department of Commerce, and appeared in Ceramic Industry in the issue for December, 194i. The general classes of articles ordinarily made from metal for which fired-clay ware could be substituted are as follows: cooking utensils, electric and gas range parts, officer's mess outfits, refrigerator parts, washing machine parts, containers and linings for use in chemical and industrial processes, and containers for canning food.
Russia has successfully used hard-burned clay or shale containers in military operations for placing charges of explosives in what are termed land mines. Clay containers have an advantage over those made from iron and steel in that they cannot be detected by magnetic devices. In addition, metal is saved for more critical uses. Some Kansas clay is of a type satisfactory for this use.
The ore from which aluminum commonly is extracted is bauxite. In the United States the supplies of high-grade ore, containing 50 to 60 per cent alumina, will last but a very few years. Kansas clays, containing 20 to 40 per cent alumina, are a good potential source of aluminum. It is possible to extract alumina from clay by the acid method, and it seems not too visionary to expect that the vast deposits in Kansas will be utilized in the not too distant future as a source of metallic aluminum. Methods of extracting aluminum from clay are discussed in the section on Aluminum.
An associated Press news item (Feb. 15, 1942) states that Secretary Ickes has proposed a vast program aimed at the utilization of low-grade ores. This program, which was prepared at the request of the Senate Public Lands Sub-Committee, calls for the construction in the western United States of 17 power projects costing $350,603,000. Clay is first on Secretary Ickes' list of substances.
The State Geological Survey is planning to conduct experiments on the concentration of alumina from fire clays by mechanical methods. The process will include heating, grinding, and flotation. This process, if successful, might lower the cost of producing aluminum from clays.
Kansas reserves
Many of the red-firing shales of Pennsylvanian age in eastern and southeastern Kansas are admirably suited to the manufacture of structural clay products, and have been so used for many years. All the important Kansas production is now in that area. It is impossible to estimate the tonnage available, but it is known to be enormous. Usable shale deposits occur also in the Permian system cropping out in the central third of the state.
The most valuable clays in Kansas have been found by the State Geological Survey in central and north-central Kansas (fig. 6). These clays consist of plastic to siliceous fire clays, pottery clays, ball clays, and some kaolin clays. Those of greatest value fire to colors ranging from white to buff. They are capable of withstanding high temperatures, inasmuch as they have fusion points ranging from 2,900 to 3,000° F. An even larger supply of clays firing to colors ranging from dark-buff to red also is available in this area.
Figure 6—Map of Kansas showing location of refractory clay deposits and of ceramic plants.
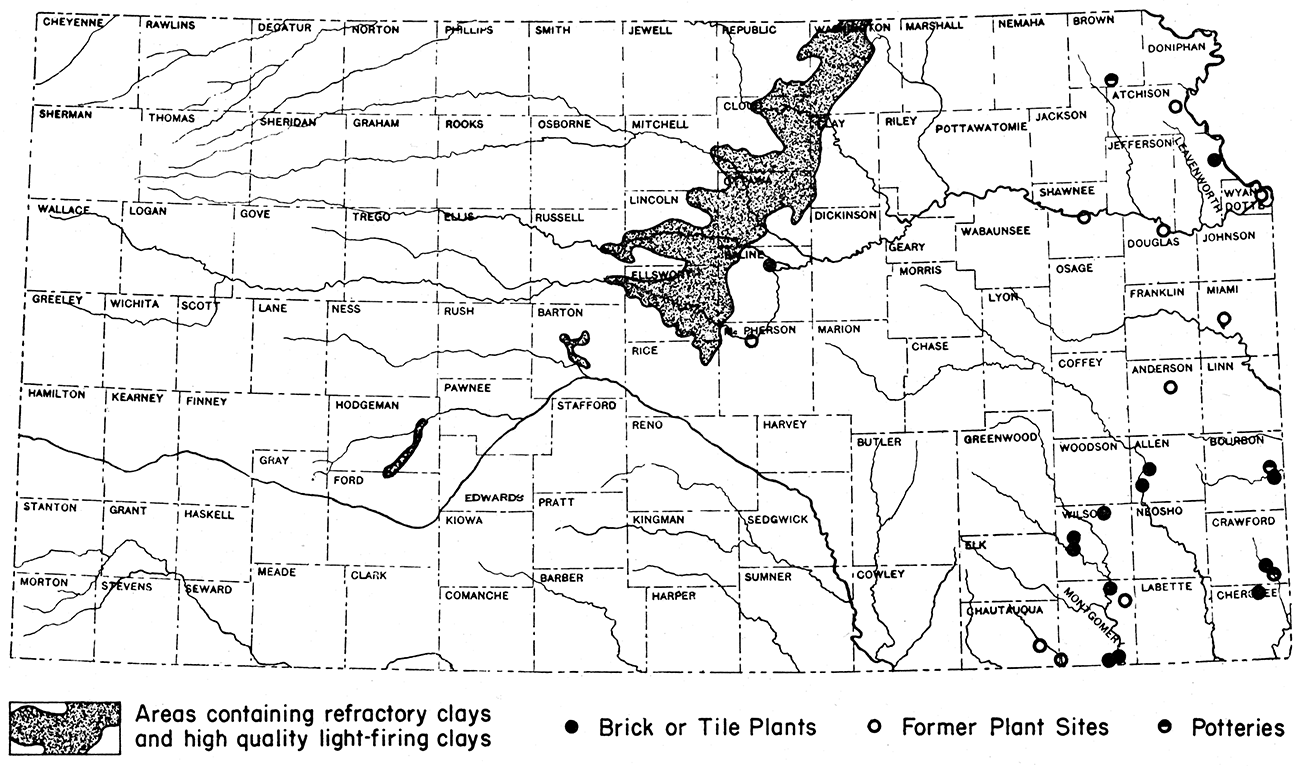
It is estimated that a minimum of 125 billion tons of these clays could be obtained by stripping methods. Of this reserve tonnage, over 40 billion tons consists of high-grade, light-firing clays.
Age and origin of Kansas clays
The clay and shale deposits of Pennsylvanian (late Carboniferous age) that are so extensively used in the manufacture of bricks, hollow tiles, roofing tiles and drainage tiles, are of both marine and nonmarine origin. The formations that have been used most commonly are the upper Cherokee shale, the Galesburg or Coffeyville shale, and Lane-Bonner Springs shale, the Weston shale, and the Lawrence shale.
The large deposits of high-grade light-firing clays-in central and north-central Kansas occur in the Dakota formation of Cretaceous age. They are nonmarine in origin.
References
Anonymous, 1941, Is this the golden age of ceramics?: Ceramic Industry, vol. 37, no. 6, pp. 19-21.
Kruger, Edward V., 1940, The outlook for Kansas clay products industries: Univ, of Kansas, Ind. Res. Ser. no. 1, pp.I-42, tables 1-12.
Diatomaceous Marl
by W. H. Schoewe
Summary—Kansas diatomaceous marl is unexploited and undeveloped. Reserves are estimated to be more than 1,000,000 tons.
Description
Diatomaceous marl is an impure variety of diatomite, the latter being known also as diatomaceous earth or tripolite. Diatomite is a hydrous or opaline form of silica and is composed largely of tiny, skeletal remains of diatoms, which are microscopic, one-celled water plants. Table 16 (Hatmaker, 1931) shows the range in composition of diatomites from various parts of the United States.
Table 16—Range in chemical composition of diatomites from various parts of the United States (Hatmaker, 1931).
| Constituents | Range in Per Cent |
|---|---|
| SiO2 | 65-97 |
| Al2O3 | 0.45-11.71 |
| Fe2O3 | Trace-3.34 |
| CaO | 0.11-2.61 |
| MgO | Trace-1.06 |
| K2O | 0.0-3.58 |
| Na2O | 0.0-1.43 |
| Ignition loss (H2O and organic matter) | 3.40-14.01 |
Diatomite, when pure, is white. When impure, it is gray, brown, pink or green. It is highly porous, bulky, a very poor conductor of heat, and is chemically inert in the presence of ordinary reagents. It appears to be similar to chalk, for which it sometimes is mistaken and from which it can be differentiated by the fact that, unlike chalk, it does not effervesce when acid is applied.
Kansas diatomaceous marl is a snow-white to grayish chalky rock, light and very fragile. Its specific gravity is about 1.53. It is composed of the siliceous tests or hard parts of fresh-water diatoms and of flaky calcium carbonate. Scattered among the diatom tests are long, thin and smooth spicules of sponges, grains of fine- to medium-sized colorless quartz, some pinkish feldspar and occasionally skeletons and scales of small fish. Analyses show that the Kansas marl consists of about 75 per cent of acid soluble material, chiefly calcium carbonate, and the rest of acid insoluble material of which 90 to 95 per cent is made up of the siliceous tests of diatoms and siliceous spicules of sponges. Rough estimates indicate that about one-half of the rock by volume is made up of diatoms. The thickness of the marl varies from 2 to 11 feet. Diatomaceous marl usually is massive and is cut by widely spaced vertical joints into large blocks. It is horizontally bedded and can be broken with comparative ease along the closely spaced bedding planes.
The diatomaceous marl occurs in the Ogallala formation and is overlain by a thin limestone and in some places by grit, gravel and loess as much as 30 feet thick. Inasmuch as the Ogallala formation, which contains the marl, is slightly folded, the marl occurs at various levels. At the Marshall ranch deposit, in Wallace County, the marl is about 60 feet above the level of Smoky Hill river at the west end of the exposure, and from 80 to 120 feet above the river in the middle and eastern parts. The diatomaceous marl, together with the overlying thin limestone, resists weathering and forms low cliffs and benches. In a few places erosion has formed separate cliffs of the marl, which are scattered on the smooth, gently descending slopes of the Smoky Hill river valley.
Distribution
The diatomaceous marl in Kansas has been found by the State Geological Survey to occur at three localities in Wallace County and one in Logan County (fig. 5). The largest known deposits is on the Marshall ranch in secs. 10, 11 and 12, T. 11 S., R. 38 W. and extends into sec. 7, T. 11 S., R. 37 W. in Logan County. It extends for a distance of more than 3 miles. A second deposit is in the SE SE sec. 35, T. 11 S., R. 39 W., at the head of one of the numerous draws on the south side of Lake Creek. The third deposit is in the NE cor. NW sec. 29, T. 12 S., R. 41 W., about one-half mile east of the Collins ranch. Small deposits also occur in Meade and Seward counties, representing extensions of the Beaver County, Oklahoma, diatomaceous marl deposits.
Uses
Kansas diatomaceous marl is especially suitable for use in the manufacture of hydraulic cement or "hydraulic lime," that is, a cement that will "set" under water. Some of the marl possibly may be used as an absorbent for nitroglycerin, as a source of silica for water glass, and as a constituent of sound and heat insulators. It also may be used as an abrasive.
Production and reserves
Up to the present time the Kansas diatomaceous marl deposits have not been developed, and extensive search for them has not been made. It has been estimated that about one million tons of diatomaceous marl are present on the Marshall ranch in secs. 10, 11, and 12, T. 11 S., R. 38 W., in Wallace County, and in the adjoining sec. 7, T. 11 S., R. 37 W., in Logan County. This estimate includes only that marl that could be stripped where the overburden does not exceed 30 feet. The extent of the marl at the other two Kansas localities is unknown.
In 1940, California and Oregon were the chief producing states of diatomite and its varieties. Other producing states include Florida, Idaho, Nevada, New Mexico, New York and Washington. The average annual sales of diatomite for the years 1933 to 1938 amounted to 87,331 short tons valued at $1,335,130 (Minerals Yearbook, 1940, p. 1241).
Origin and age
The diatomaceous marl of Kansas is a fresh-water deposit, as indicated by the tests of fresh-water diatoms and the crushed shells of fresh-water gastropods, pelecypods, and other fossils associated with the overlying limestone cap rock. The diatoms are microscopic water plants that are able to precipitate silica from the waters in which they live; out of this material they manufacture their siliceous skeletons. Diatoms are short-lived but are exceedingly abundant and multiply rapidly, as is suggested by the fact that as many as 50 million individual skeletons may be found in a cubic inch of diatomite (Hatmaker, 1931). On death of the diatom, the organism decomposes but its skeleton sinks to the bottom of the lake, swamp or ocean in which it lived; multitudes of such skeletons gradually build up extensive deposits of diatomite. The Kansas diatomaceous marl occurs in the Ogallala formation of Tertiary age.
Work of the State Geological Survey
The Diatomaceous marl of Wallace County has been studied by Elias (1931) and is described by him in the two State Geological Survey publications cited in the references. Diatomaceous marl has been sent by the Survey to the United States Bureau of Standards for testing in order to determine the suitability of the marl for making hydraulic lime. Preliminary reports indicate that it is satisfactory in quality for such use. A graduate chemical engineer at the University of Kansas has been investigating other possible uses for the marl.
References
Cummins, A. B., and Mulryan, H., 1937, Diatomite: Industrial Minerals and Rocks, Am. Inst. Min. and Met. Eng., New York, pp. 243-260.
Elias, M. K., 1931, The geology of Wallace County, Kansas: Kansas Geol. Survey, Bull. 18, pp. 1-254, figs. 1-7, pls. 1-42. [available online]
Elias, M. K., 1931, Diatomaceous marl from western Kansas, a possible source of hydraulic lime: Kansas Geol. Survey, Circ., 3, pp. 1-5. [available online]
Hatmaker, Paul, 1931, Diatomite: U.S. Bur. Mines, Infor. Circ. 6391, pp. 1-20.
Minerals Yearbook, 1941, Review of 1940: U.S. Bureau of Mines, pp. 1241-1242.
Fillers for Plastics
by Norman Plummer and J. C. Frye
Summary—Kansas contains several types of mineral deposits which may prove to be satisfactory as a filler for plastics.
Plastics is a general term used to designate a wide variety of materials, including hard rubber, phenol-formaldehyde resins, urea-formaldehyde, cellulose compounds, and casein plastics. Such materials are formed into usable articles both by extrusion and by press molding, either at room temperature or at moderate temperatures ranging up to 3000 F. The complex organic compounds are mixed with fillers before being molded into articles. The fillers serve to reduce shrinkage and to increase strength and resistance to abrasion and high temperature.
Organic fillers, such as wood-flour, paper, and cotton flock (a fibrous substance that grows around pods of cotton seeds), commonly are used. Inorganic fillers, such as slate dust, asbestos, mica, graphite, kaolin, pulverized marble and diatomaceous earth, are used for articles in which resistance to abrasion or high temperatures is required. As much as 50 per cent of the plastic substance may consist of filler.
Requirements of a filler for plastics
The specific gravity of the majority of plastic molding compounds ranges from 1.3 to 1.7. In general, it is desirable to use a filler which will not increase the specific gravity. Some plastic compounds have a specific gravity as high as 3.0. The filler should not be of an excessively abrasive character; otherwise it will be injurious to the molds. Furthermore, it should be of a composition such that it will not react chemically with the plastic material used. The maximum particle size should be 300-mesh.
Kansas materials
Kansas could produce enormous quantities of inorganic materials which might prove suitable for use as fillers in plastics. Members of the staff of the State Geological Survey are now undertaking studies of some of these materials to determine possible methods of preparation and grinding. Some of the Kansas materials that seem most promising for this use include diatomaceous marl, volcanic ash, chalk, tripoli, and calcined light-firing clay.
Gypsum
by J. M. Jewett
Summary—Kansas ranks eighth among the states as a gypsum producer. The known reserves, particularly in the southern Kansas area, are very large.
Gypsum has been utilized in a variety of ways since very ancient times. The writings of Pliny show that before the time of Christ the Greeks made plaster of Paris casts from calcined gypsum and that they used transparent gypsum (selenite) in roofs of greenhouses, much as modem greenhouses are made with ordinary glass. Gypsum was used as windows in palaces in ancient Greece and Italy. Rock gypsum was used as a building stone by the Arabs. In Egypt and elsewhere the alabaster variety was used in very remote times in making urns, vases, and sculptured articles.
Gypsum now is used for many purposes, and it is employed both in the calcined and uncalcined form. Large quantities go into the making of cements and plasters and fabricated building materials.
Gypsum is hydrous calcium sulphate. Its formula is CaSO4·2H2O. It is 79.1 per cent calcium sulphate and 20.9 per cent water of crystallization. The mineral calcium sulphate without water is termed anhydrite. When gypsum is completely calcined, the product is termed dead-burned gypsum. This is artificial anhydrite, and, like natural anhydrite, it has no cementing properties. Plaster of Paris is made by calcining gypsum until only a part of the water content is driven off. If about half of the water is removed, the material sets quickly but does not have the strength and hardness of plaster of Paris that has been calcined until only 15 to 18 per cent of the water remains.
Five varieties of gypsum are recognized: (1) tabular crystals and cleavable masses called selenite; (2) a fibrous material with silky luster called satin spar; (3) alabaster, which is a massive, generally fine-grained variety; (4) rock gypsum, the name applied to a compact and granular variety; and (5) gypsite, which is the accepted name for granular gypsum earth of secondary origin.
Gypsum in Kansas
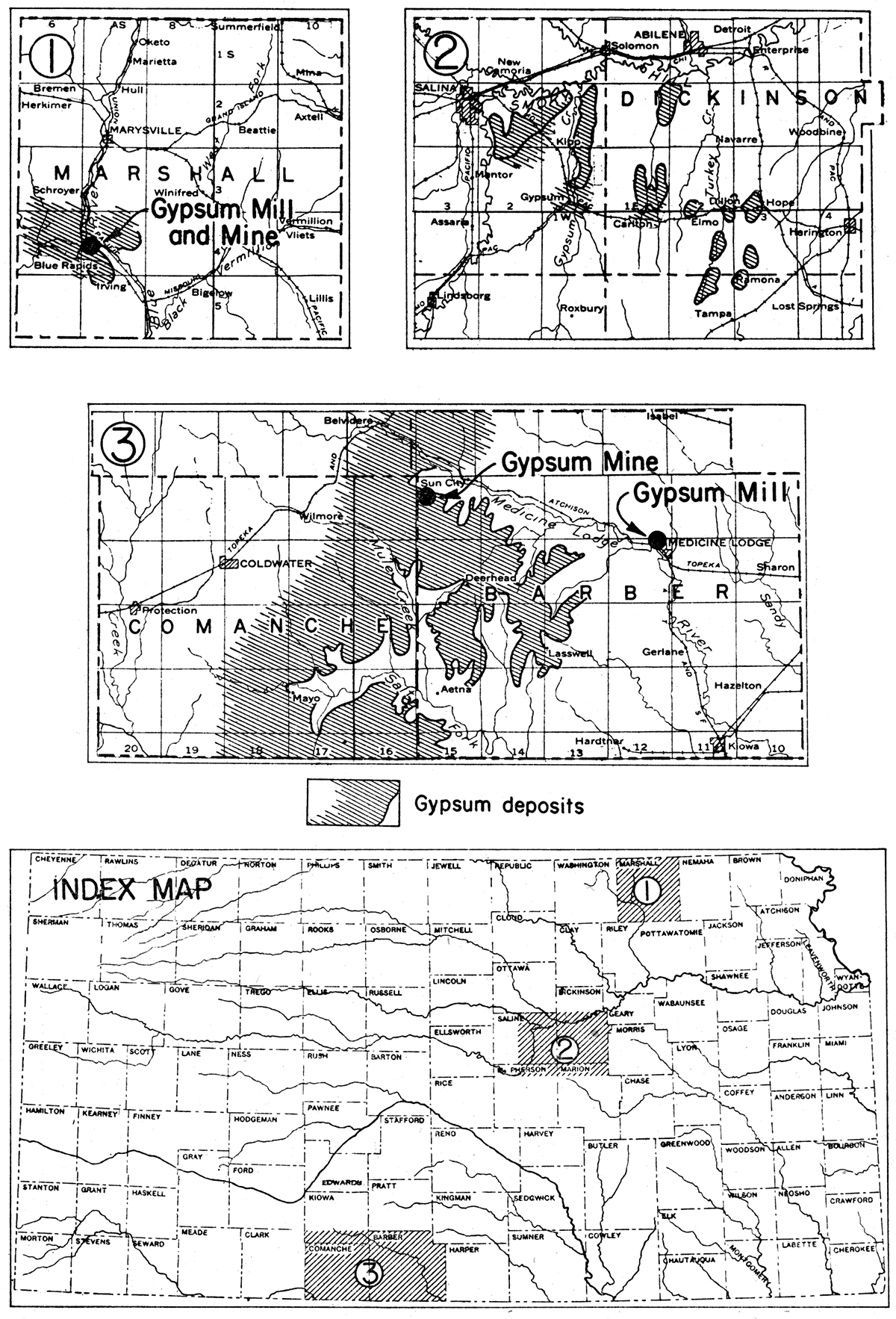
Although gypsum, mostly as disseminated crystals, is of widespread occurrence in Kansas rocks, the workable deposits are chiefly confined to three areas (fig. 7). The areas of workable deposits are (1) the Blue Rapids area in Marshall and eastern Washington counties, (2) the central Kansas gypsum area in Dickinson, Saline, and Marion counties, and (3) the southern Kansas gypsum area in Barber and Comanche counties. Outside of these areas, smaller deposits of both bed-rock gypsum and gypsum earth are known in Clay, Sedgwick, Sumner, and Harvey counties. Gypsum has been mined at several localities from these smaller deposits, and it has been rather extensively produced in the three chief areas. The active plants now are confined to the Blue Rapids and southern Kansas areas.
Figure 7—Index and detail maps showing distribution of three areas of Permian gypsum deposits, and location of gypsum mills and mines.
Plaster of Paris was made experimentally at Blue Rapids in 1871, and the gypsum industry became well established there in the following year. Soon after a gypsum mill was built on the west bank of Big Blue river at Blue Rapids, barges drawn by a small steam tug were used to haul the raw material down the river from the mine to the mill. Water power was used to grind the rock. Blue Rapids has been an important gypsum products center since the first mill was built.
The first gypsum mine in the central Kansas area was a strip mine at Hope, Dickinson County, opened in 1885. In 1894 a shaft mine was sunk at that locality to a depth of 80 feet, and at about the same time a mine and mill were located on Gypsum creek in Saline County, about 6 miles southwest of Solomon. Gypsite or "gypsum dirt" was discovered at Gypsum City, Saline County, in 1873, but development did not start there until 1889. Products from Gypsum City were shipped to many parts of the United States. Plaster made there was used in the World's Fair buildings in Chicago in 1892, and large quantities of plaster made in Gypsum City were used in government buildings at Fort Riley and Fort Leavenworth. These central Kansas gypsum mines and mills operated for several years.
Soon after the discovery of gypsite at Gypsum City, similar deposits were found in Clay, Saline, Dickinson, Marion, Harvey, and Sedgwick counties. Soon seven mills were making plaster of Paris from gypsite. None of these deposits are now being exploited. In 1885, a gypsum mill was built on a farm 5 miles west of Peabody, Marion County. "Rock gypsum" was mined nearby, and the mill operated for about two years.
The first gypsum mill in the southern Kansas area was built at Medicine Lodge in 1889. Keene's cement, which is a very hard and durable cement, and other high-grade products have been produced there for many years. Formerly, a mill was operated at Sun City, about 20 miles northwest of Medicine Lodge. At present, the mining operations of the area are near Sun City. Gypsum is shipped by train to the mill at Medicine Lodge.
Blue Rapids area
As indicated in figure 7, workable gypsum deposits are believed to underlie an area of considerable size in the vicinity of Blue Rapids, Marshall County. As shown by mine workings and outcrops, the average thickness of the bed of gypsum is about 8 1/2 feet. The rock is described as having a sugary texture and is nearly white. A thin layer of satin spar occurs locally in the upper and lower parts of the bed. The gypsum rock crops out along Big Blue river not far above water level. It lies directly on the Middleburg limestone, uppermost member of the Bader formation of the Council-Grove group, Permian system. Information pertaining to whether the rock is anhydrite rather than gypsum at some distance from the outcrops is not at hand.
Central Kansas area
There are several layers of gypsum in the central Kansas gypsum area, as shown in figure 7. Former mine records and outcrops suggest that this area has a large reserve. A bed 14 feet thick and 80 feet below the surface was mined at Hope. At the time of mine workings along Gypsum Creek in eastern Saline County, several gypsum beds were exposed there. A 5-foot bed, a 3-foot bed and several thinner ones were recorded. Natural outcrops occur in the vicinity of Hope and in eastern Saline County. Even though these beds may be represented by anhydrite in areas where they are more deeply covered, there is ample reason to believe that there are large reserves of gypsum in this area. It was in the central Kansas area that several years ago a number of gypsite deposits were developed.
Southern Kansas area
The southern Kansas gypsum area is part of an extensive region underlain by gypsum and anhydrite, extending far southwestward in Oklahoma, New Mexico and Texas. These gypsum beds, associated with red beds of fine sand and silt, crop out in an area extending from near Medicine Lodge westward through Barber and into Comanche County and southward into Oklahoma. They dip westward under younger sediments, and north of their outcrop area they are overlapped by younger beds. The most prominent and thickest gypsum bed of the area caps a range of hills known as the Red Hills. This bed, called the Medicine Lodge gypsum, is about 30 feet thick at its outcrop. Two beds of gypsum that are only slightly thinner occur a few feet higher in the stratigraphic section. These are the Nescatunga and Shimer gypsum members of the Blaine formation. The Medicine Lodge gypsum is the basal member of the same formation.
It is known that at some distance down dip these gypsum beds grade into anhydrite. Great reserves of gypsum, however, are present in the area because the belt of outcrop is a long and winding one, and it is only under an overburden of considerable thickness that anhydrite instead of gypsum is found.
Production in Kansas
Kansas normally holds eighth place among the states as a producer of gypsum; Generally, somewhat less than half of the state's output is marketed as raw or uncalcined gypsum. High-grade gypsum products are being produced at Blue Rapids in Marshall County and at. Medicine Lodge in Barber County.
Uses of gypsum
Uncalcined gypsum is used chiefly as a retarder in Portland cement. In the usual procedure of Portland cement manufacture approximately 2 pounds of raw gypsum are added to every 100 pounds of clinker, either before or after the preliminary grinding. Gypsum ordinarily is shipped in lumps and is ground by the cement producer. Small amounts of raw gypsum are used as fertilizer.
Calcined gypsum leaves the mills in the form of plaster of Paris, cement plasters and fabricated products. Another product is Keene's cement, made by calcining gypsum to red heat and recalcining it after an alum solution is added; it sets slowly into a comparatively hard material that resembles marble. Plaster of Paris is used as molds in pottery and metal plants, relief work on walls and ceilings, making beds for the polishing of plate glass, and in surgical and dental work. Cement plasters are products to which filler and retarders have been added. Fabricated products include wall board, tiles, blocks, and so forth. Gypsum plasters and fabricated forms are excellent heat and sound insulators.
Kansas reserves
As indicated under other headings in this paper, Kansas has great reserves of easily accessible gypsum. Sufficient data are not available to say more than that the reserves in the southern Kansas gypsum area seem to be very large, that large reserves are present also in the two other areas, and that probably the central Kansas area ranks second in this respect.Age and origin of Kansas gypsum
The workable bedrock deposits of gypsum in Kansas are of Permian age. The deposits in the Blue Rapids area are in rocks of the Council Grove group in the Wolfcamp series. The bedrock gypsum deposits in the central Kansas area are in the Wellington shale in the Sumner group, Leonard series. The deposits of the southern Kansas area occur near the top of the Leonard series.
The deposits of gypsite or "gypsum dirt" have been formed recently by the evaporation of ground water that carried gypsum in solution. Some of the deposits were found with a covering of clay 10 feet thick and others with little or no covering. Thus, it seems that the gypsum, which has the appearance of dark granular earth and is commonly found in low, swampy ground, has been deposited during Recent geologic time and chiefly in the present cycle of erosion of the region.
Like salt and anhydrite, gypsum deposits occurring as bed rock have been formed from evaporating sea water in shallow bays or lagoons. The temperature of the water may have been a factor determining whether anhydrite or gypsum was formed. It is believed also that if a deposit of calcium sulphate precipitated from sea water falls through a deeper layer of concentrated brine, it is deposited directly as anhydrite. Gypsum may have been converted into anhydrite by the action of superadjacent salt beds. It is well known that anhydrite changes to gypsum in the presence of water; hence, anhydrite deposits commonly are represented by gypsum at the outcrop and for some distance down the dip of the beds, ground and surface water having brought about the change. This seems to be the case in the deposits of the southern Kansas gypsum area.
It is indicated in the foregoing statements that gypsum and anhydrite may be deposited directly in evaporating sea water in a closed or partly closed basin, and that primary deposits of either mineral may be converted subsequently into the other. It is possible that a given deposit may have undergone more than one conversion.
References
Adams, G. I., and others, 1904, Gypsum deposits of the United States: U.S. Geol. Survey, Bull. 223, pp. 1-129. [available online]
Grimsley, G. P., and Bailey, E. H. S., 1899, Special report on gypsum and gypsum cement plasters: Kansas Univ. Geol. Survey, vol. 5, pp. 1-83, frontispiece, pls. 1-30, figs. 1-20.
Landes, K. K., 1937, Mineral resources of Kansas counties: Kansas Geol. Survey, Mineral Resources Circ. 6, pp. 1-110, maps. [available online]
Landes, K. K., 1938, The Kansas minerals industry: Kansas Yearbook, Kansas State Chamber of Commerce, Topeka, pp. 235-237.
Newland, D. H., and Brown, H. J., 1937, Gypsum: Industrial minerals and rocks, Am. Inst. Min. and Met. Eng., New York, pp. 353-374.
Stone, R. W., 1920, Gypsum deposits of the United States: U.S. Geol. Survey, Bull. 697, pp. 1-326. [available online]
Portland Cement
by J. M. Jewett
Summary—Present production of cement in Kansas is about 4,500,000 barrels annually, valued at $5,200,000. Reserves of cement materials in the state are inexhaustible.
Portland cement consists of compounds, the chemical constituents of which include calcium oxide, silica, alumina, and iron oxides; this material is capable of hardening into a solid mass. In setting, the compounds form chemical combinations with water. This cement is made from calcareous and argillaceous materials. The calcareous material used is some form of calcium carbonate, including limestone, marl, chalk, oyster or other shells, and the precipitates formed in the manufacture of alkalies. Argillaceous materials include clay, shale, cement rock (argillaceous limestone), slate, and blast-furnace slag. Modern cement mills use a combination of one of the calcareous materials and one of the argillaceous materials.
The beginning of Portland cement dates from 1756 when an Englishman, John Smeaton, discovered that by burning and slaking an impure limestone a cement was made that would set under water as well as in air. Forty years later another Englishman, John Parker, obtained a patent for Roman cement, which was made by burning "nodules of clay" in a lime kiln and grinding the clinker. Many plants for making Roman cement were built in England and a little later in America. Portland cement, produced by calcining a mixture of limestone and clay, was patented in England by Joseph Aspdin in 1824. The early Portland cement differed from that of today chiefly in that it was burned at a lower temperature and was pulverized by slaking rather than by grinding. Cement made at Allentown, Pennsylvania, in 1875 was probably the first true Portland cement made in America.
Operations involved in Portland cement manufacture are: (1) mining the raw materials, (2) crushing, (3) drying, in the "dry process", or adding water to produce "slurry", in the "wet process", (4) grinding, (5) mixing the desired proportions, (6) pulverizing, (7) burning to incipient fusion at about 2500 to 3000° F., and (8) grinding the clinker to fine powder. About 2 per cent of raw gypsum is added to the mix before or after grinding the clinker. Gypsum serves as a retarder.
Raw materials in KansasKansas contains an abundance of raw materials suitable for the making of Portland cement. The supply is virtually inexhaustible. Limestones, including chalk of central and western Kansas, are the source of the calcareous materials, and shales and clays provide the argillaceous materials. Limestones of Carboniferous and Permian age crop out in bands crossing the eastern third of the state in a north-south direction. Many of the limestones occur in beds a few tens of feet in thickness, and many of the beds are nearly pure calcium carbonate. Shales occur interbedded with the limestones. At many sites the two materials can be taken from the same quarry. Cement rocks are also abundant in Kansas, and some are reported to be natural Portland cement rocks. At six places in eastern Kansas Portland cement is being made from Pennsylvanian (Upper Carboniferous) limestones and shales.
In central and western Kansas Cretaceous chalk and shales are abundant. Some years ago chalk and shale were used in cement making in Ellis County.
Gypsum is produced in Kansas in Marshall and Barber counties. Figure 7 shows the areas in Kansas underlain by gypsum deposits; they have been discussed elsewhere in this report.
Both natural gas and powdered coal have been used as fuel in cement mills in Kansas. Natural gas now is used exclusively. As indicated in other sections of this report, Kansas has an abundance of both coal and natural gas.
Production of Portland cement in Kansas
Six Portland cement mills are now operating in Kansas. The locations are: (1) Bonner Springs, Wyandotte County; (2) Iola and (3) Humboldt, Allen County; (4) Chanute, Neosho County; (5) Fredonia, Wilson County; and (6) Independence, Montgomery County. All the Kansas plants use Pennsylvanian limestone and shale. Natural gas is used as fuel.
Figure 8—Map of eastern Kansas showing location of Portland cement mills and of region of abundant raw materials for cement manufacture.
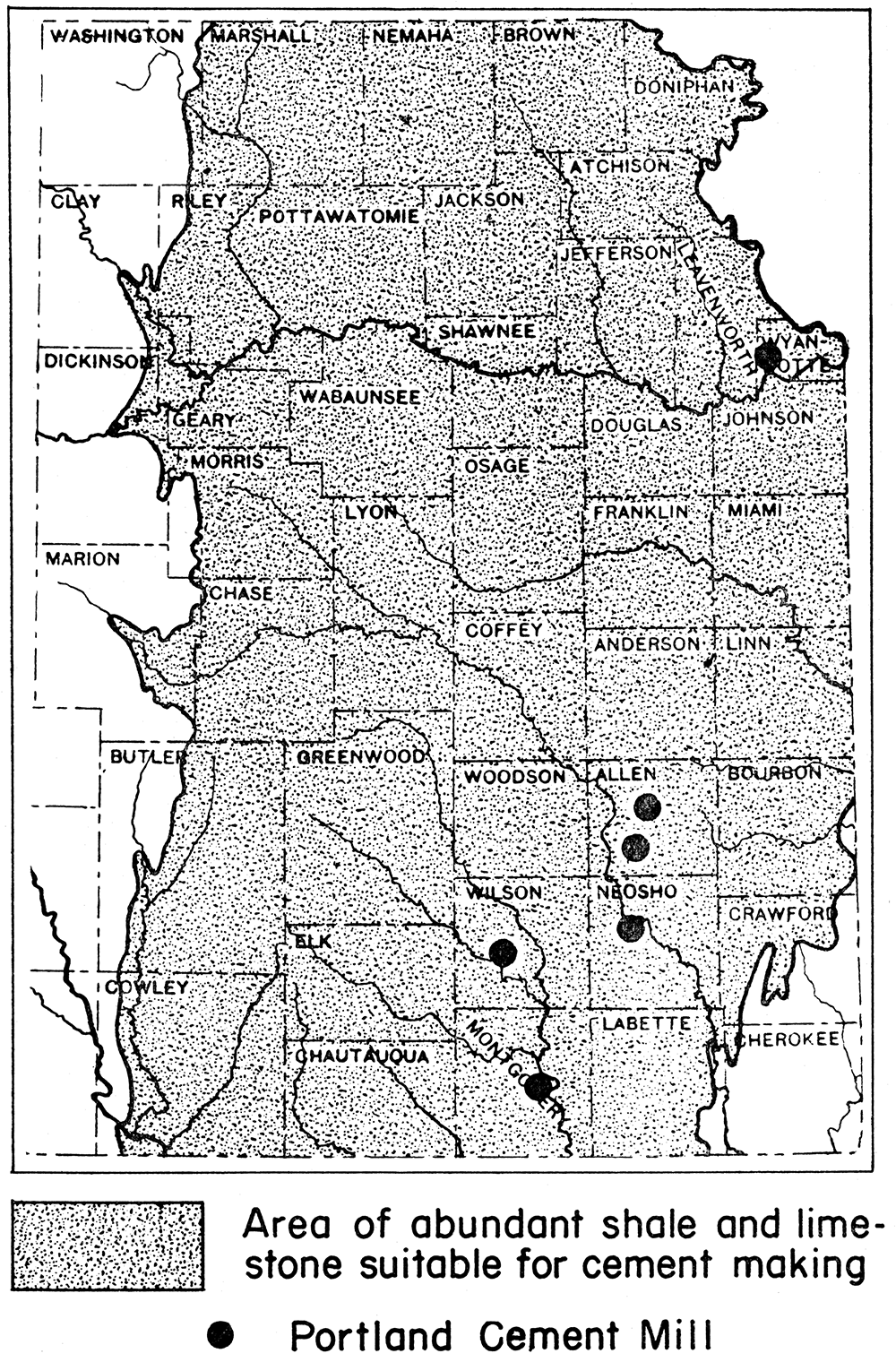
The capacity of the plants now operating in Kansas is said to exceed 8,000,000 barrels per annum. From 1910 to 1928 the total yearly output ranged from a low of 2,586,834 barrels (1918) to a high of 6,574,219 barrels (i928); and the average yearly production for the years 1927 to 1936, inclusive, was 4,254,417 barrels. The production in 1940 was 4,509,742 barrels, having a total value of $5,192,160. The greater part of the cement produced in Kansas has its market outside the state.
Table 17, below, shows annual production figures of Portland cement in Kansas for the ten year period prior to 1941.
Table 17&mash;Portland cement production in Kansas
| Year | Barrels | Year | Barrels | |
|---|---|---|---|---|
| 1931 | 4,478,823 | 1936 | 3,568,090 | |
| 1932 | 2,224,079 | 1937 | 3,500,684 | |
| 1933 | 2,189,137 | 1938 | 3,217,407 | |
| 1934 | 2,425,867 | 1939 | 3,746,370 | |
| 1935 | 2,487,888 | 1940 | 4,509,742 |
References
Bowles, Oliver, 1918, Rock quarrying for cement manufacture: U.S. Bur. Mines, Bull. 160, pp. 1-160.
Giese, Henry, 1939, A practical course in concrete: Pamphlet published by Portland Cement Association, pp. 1-62, illustrated.
Miller, B. L., 1937, Cement materials: Industrial Rocks and Minerals, Am. Inst. Min. and MetalL Eng., New York, pp.163-190, figs. 1-2, tables 1-7.
Rock Wool Materials
by Norman Plummer
Summary—Rock wool has been manufactured in Kansas for so few years that no significant data are available on production in the state. The reserve of raw materials is greatly in excess of any future needs.
Rock wool is a fluffy, usually white material composed of minute fibers of glass. It is manufactured by melting rock in a copola or reverboratory furnace and pouring the molten glass into a blast of steam or air. The glass is drawn out into thin fibers by the current of steam or air. Rock wool is made either from a mixture of approximately 50 per cent limestone and 50 per cent shale, clay, or sand or from naturally occurring rocks, such as calcareous shales, siliceous marls, or siliceous limestones, which have the required chemical composition. The dominant chemical constituents of most rock wools are calcium carbonate and silica, these being present in approximately equal proportions.
Rock wool industry in Kansas
The State Geological Survey of Kansas tested rock samples for their suitability in the manufacture of rock wool in the years 1936 and 1937. These tests demonstrated that rock wool of excellent quality could be made from Kansas rocks. Since that time three plants have been built in the state. The two larger plants at Winfield and at Parsons are still operating. The third operated in conjunction with the brick plant at Neodesha.and is now shut down because the manufacture of bricks has been discontinued.
Uses
The chief use of rock wool is in the insulation of dwellings and other buildings, but it has many other applications. It is effective in preventing freezing in house pipes and underground pipes. Its use prevents loss of heat from pipes carrying steam, molten sulphur, hot oil, and so forth. Rock wool is valuable in the insulation of ovens, furnaces and stoves, including steam boilers, fractionating columns, and towers; in the insulation of annealing, baking and smelting ovens, retorts, and metallurgical and chemical furnaces. It is used also in various refrigeration devices, and for sound control. Rock wool is used as a packing for acid carboys, as a filter medium for corrosive fluids, as an air filter in hot air heating, as. a component of insulating cement, as a fire preventive, and as lining between planking and metal sheathing of ships.
The many uses of rock wool are determined by the fact that it is relatively inexpensive and by the fact that it is essentially a refractory glass, which means that it can withstand relatively high temperature, that it does not transmit heat readily, and that it is non-deteriorating, fireproof, and vermin proof.
Kansas reserves
The Kansas rocks tested for suitability in manufacture of rock wool are of widespread geographic distribution (fig. 11) and range in age and stratigraphic position from the Cherokee shale of lower Pennsylvanian age to Pleistocene "mortar beds". The quantity of material available at anyone of the locations sampled is far in excess of future needs. The majority of the fifty samples tested were mixtures of calcarious and siliceous rocks, but several were natural wool rocks. The latter class includes the Lenapah limestone of southern Kansas, the Florence limestone and the Grant shale of central Kansas, and the "mortar beds" of western Kansas.
References
Lamar, J. E., Willman, H. D., Fryling, C. F., and Voskuil, W. H., 1934, Rock wool from Illinois mineral resources: Illinois Geol. Survey, Bull. 61, pp. 1-262, figs. 1-34, tables 1-39.
Plummer, Norman, 1937, Rock wool resources of Kansas: Kansas Geol. Survey, Mineral Resources Circ. 5, pp. 1-74, figs. 1-28. [available online]
Plummer, Norman, 1937, Rock wool resources of Kansas, Appendix: Kansas Geol. Survey, Mineral Resources Circ. 8, pp. 1-28, figs. 1-13. [available online]
Salt
by J. M. Jewett
Summary—The present production of salt in Kansas is about 700,800 tons annually. The reserves are estimated at 5,000 billion tons.
Common salt, or halite, is composed of sodium chloride (NaCl). In the pure state it contains, by weight, 33.34 per cent sodium and 66.66 per cent chlorine. The greatest reserves are in ocean waters, but vast amounts are stored as layers of rock, and large quantities are present as a part of natural brines in deeply buried porous rocks.
Human existence is dependent on salt. It is an essential part of man's diet and must be fed to his domestic animals. Salt always has been a strategic mineral; large quantities are used in industries that are essential to modern civilization.
Salt in Kansas
The salt industry in Kansas had its beginning in about 1892. The industry grew rapidly, and Kansas is now the most important salt-producing state west of the Mississippi river. Production has centered around Lyons and Little River in Rice County, Hutchinson in Reno County, Kanopolis in Ellsworth County, and Anthony in Harper County. As early as 1898 the state produced yearly 250,000 tons of salt and recently the average yearly production has ranged around 800,000 tons. Plants and mines are now operating at Hutchinson, Lyons and Kanopolis.
As shown in figure 9, salt underlies a large area in central and southwestern Kansas. An area extending along the Kansas-Oklahoma line from Sumner County westward to Seward County and northward to Rooks, Osborne and Mitchell counties is underlain by a thick vertical section of salt beds in the Wellington shale formation. In central Reno County salt beds form the dominant portion of a rock section 500 feet thick; the salt-bearing section is about 200 feet thick in central Osborne County. The deposits are thicker in the central part of the salt area. A well in Pratt County has been drilled through as much as 800 feet of rock, which is chiefly salt. This deposit extends for some distance into Oklahoma.
Figure 9—Map of Kansas showing location of salt plants and of areas underlain by Permian salt deposits.
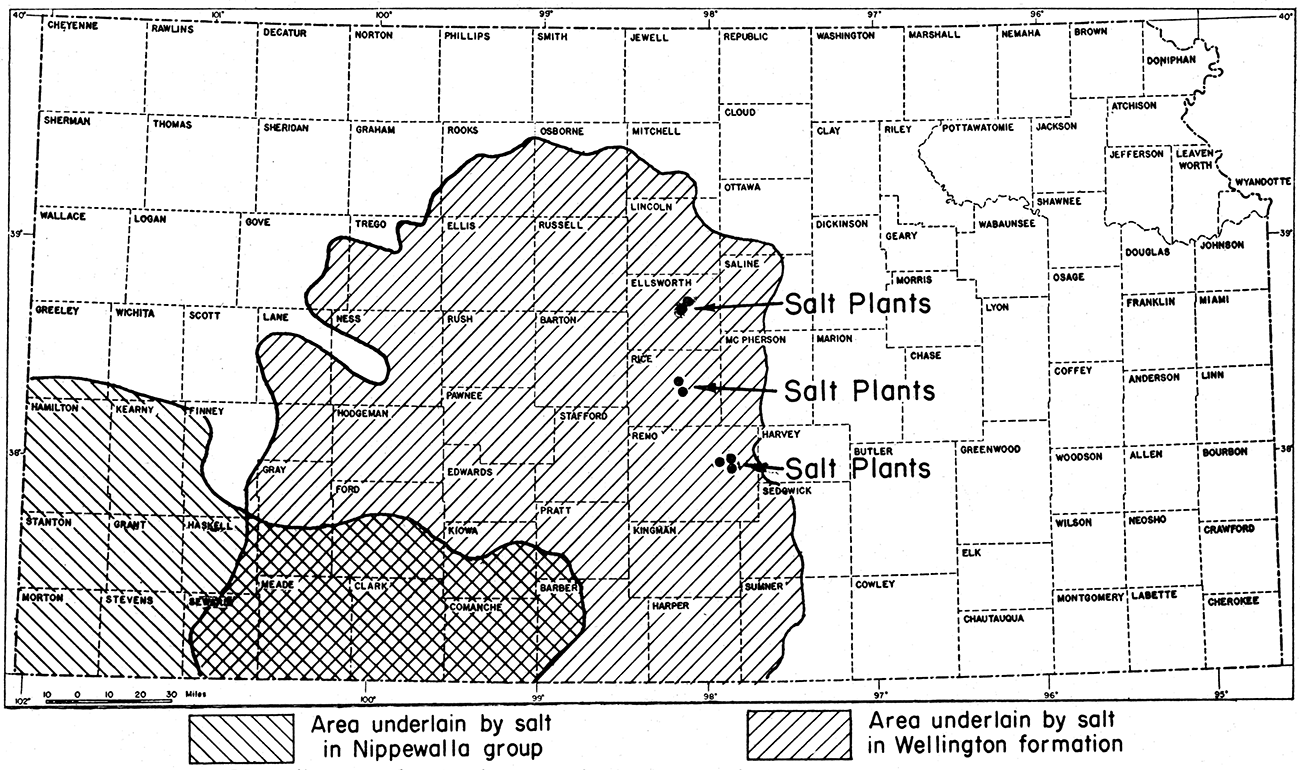
The layers of rock, including the rock salt, dip gently westward, Because of this westward dip and because of the westward increase of land surface elevation, the salt is nearest the surface along the eastern margin of the salt area. Because of its solubility the salt does not crop out at the surface. That part of the salt beds mined by shaft methods is about 600 feet below the surface at Hutchinson, about 900 feet below the surface at Lyons, and about 860 feet at Kanopolis. At Anthony the same portion of the salt section is about 1200 feet below the surface. Logs of oil wells in northwestern Hodgeman County indicate that salt beds are encountered about 1800 feet below the surface.
The southwestern Kansas salt deposits are part of a large assemblage of salt beds extending far to the south and west in Colorado, Oklahoma, New Mexico, and Texas. The southwestern Kansas area (fig. 9) is underlain by one or two thick deposits of rock salt that occur in red beds of the Nippewalla group of Permian rocks. The area underlain by Nippewalla salt is partly coextensive with the area of Wellington salt (fig. 9).
Production in Kansas
Kansas normally produces about 800,000 tons of salt each year. The average value of a year's output is approximately $3,000,000. Table 18, shown below, lists production figures for the twenty year period prior to 1941.
Table 18—Salt production in Kansas.
| Year | Tons | Year | Tons | |
|---|---|---|---|---|
| 1921 | 665,968 | 1931 | 691,160 | |
| 1922 | 749,459 | 1932 | 688,178 | |
| 1923 | 845,163 | 1933 | 732,947 | |
| 1924 | 794,303 | 1934 | 768,133 | |
| .1925 | 812,540 | 1935 | 608,204 | |
| 1926 | 729,880 | 1936 | 704,164 | |
| 1927 | 794,780 | 1937 | 654,089 | |
| 1928 | 821,950 | 1938 | 597,909 | |
| 1929 | 840,370 | 1939 | 641,752 | |
| 1930 | 759,800 | 1940 | 684,053 |
Two methods of extracting salt are employed. One is direct mining, in which shafts are driven to the deposits and the salt is mined by room and pillar method. Modern electrically driven machinery is used. The other method is hydraulic mining in which wells are drilled to the salt deposits and water is pumped down to dissolve the salt. The water, saturated with salt, is returned to the surface and is evaporated by heating. Brine wells are in operation at Hutchinson and at Lyons. Shaft mines are in operation at Hutchinson, Lyons and Kanopolis.
Uses of salt
The uses of salt are numerous. In a large industrial country such as the United States, chemical industries consume more than half of the salt used. A large number of sodium-bearing chemicals are made from it. Of these, the soda alkalies are quantitatively the most important, but many other sodium salts are made from common salt. Metallic sodium, hydrochloric acid, and chlorine are other derivatives.
Salt is of great importance in meat packing, and much Kansas salt is used for this purpose. Kansas City, Kansas, ranks second in the United States as a meat-packing city, and packing plants are in operation in other Kansas towns, including Wichita, Topeka and Salina. Food manufacturing and processing consume great quantities of salt. Its use in the home is on a smaller scale but is for the same general purposes as in the packing and food industries, that it, to give flavor to food and to aid in its preservation.
Salt is used as a fertilizer, as a refrigerant, as an ingredient of stock feeds, and for the purpose of killing weeds. It is used also in making and packing ice cream, as a refrigerating agent in refrigerator cars and trucks, and for the purpose of melting ice on railroads and highways. Salt is used in the manufacture of many articles, including glass and clay products, dyes, pulp and paper, rayon, soap, and textiles. It is used in the iron and steel industry, in tanning, in tobacco manufacture, and in the making of rubber.
Kansas reserves
It is estimated that 5,000 billion tons of salt lie beneath the surface of Kansas. At the present rate of consumption this amount would supply the entire United States for a period of a half million years.
Age and origin of Kansas salt deposits
Salt occurs in Permian rocks in Kansas. Salt beds constitute the major portion of an 800-. foot rock section included in the Wellington shale, a rock unit of the Leonard series of the Permian system. This deposit is of great economic importance. Other salt beds in Kansas, approximately 700 feet higher in the stratigraphic column, occur in the Nippewalla group of the Leonard series. It is not easy to account for the accumulation of such vast deposits of salt, and detailed descriptions of the theories would be out of place here. It is sufficient to say that the salt accumulated through evaporation of sea water in subsiding shallow bays, lagoons, or inland seas or lakes. Arid climatic conditions must have prevailed. The areas of accumulation continued to subside for some time, and the salt was buried beneath layers of mud and sand. The salt, other chemical precipitates, mud, and sand are now the materials of rock beds. Subsequent uplift has brought the rocks into their present position.
References
Landes, K. K., 1937, Mineral resources of Kansas counties: Kansas Geol. Survey, Mineral Resources Circ. 6, pp. 1-110, maps. [available online]
Landes, K. K., 1938, The Kansas mineral industry: Kansas Yearbook, Kansas State Chamber of Commerce, Topeka, pp. 235-237.
Phalen, W. C., 1917, Technology of salt making in the United States: U.S. Bureau of Mines, Bull. 146, pp. 1-149.
Phalen, W. C., 1919, Salt resources of the United States: U. S. Geol, Survey, Bull. 669, pp. 1-284.
Phalen, W. C., 1937, Salt: Industrial minerals and rocks, Am. Inst. Min. and Metall. Eng., New York, pp. 643-670.
Sand and Gravel
by W. H. Schoewe
Summary—Kansas possesses unlimited supplies of sand and gravel. Annual production in 1940 was 2,264,871 short tons valued at $893,962.
Sand and gravel are unconsolidated materials resulting from the disintegration, decomposition or weathering of rocks, and the transportation and sorting of the rock fragments by running water. The terms sand and gravel denote size and shape of particles rather than mineral or chemical composition. At the present time there is no strict conformity in the usage of the terms sand and gravel. According to Thoenen (1937, p. 671), sand generally is considered to be unconsolidated material, the grains of which are coarser than 0.0029 inches and finer than 0.25 inches in diameter; this material is retained on a 200-mesh screen (0.074 sieve openings). Gravel represents similar unconsolidated particulate material, the grains of which are coarser than one-fourth of an inch and finer than 3 1/2 inches in diameter. Another classification scheme employed by certain workers designates sand and gravel according to the Wentworth classification, as given below in Table 19.
Table 19—Wentworth classification of sand and gravel.
| Size Class | Limiting Dimensions, in mm |
|
|---|---|---|
| Gravel | ||
| Boulder | Greater than 256 | |
| Cobble | 64 - 256 | |
| Pebble | 4 - 64 | |
| Granule | 2 - 4 | |
| Sand | ||
| Very coarse | 1 - 2 | |
| Coarse | 1/2 - 1 | |
| Medium | 1/4 - 1/2 | |
| Fine | 1/8 - 1/4 | |
| Very fine | 1/8 | |
Sand and gravel may be well sorted or homogeneous as to grain 'Size or composition, or both. The flint gravels of southeastern Kansas are an example of a deposit well sorted or homogeneous as to composition. On the other hand, sand and gravel may be heterogeneous as to size or composition; an example of this condition of poor sorting is provided by glacial sands and gravels of north-eastern Kansas.
Sands
Dune sand commonly has a larger percentage of quartz than has ordinary river sand and, furthermore, tends to be of more uniform grain size.
Kansas stream sands generally consist of a mixture of quartz, feldspar, mica, hornblende, magnetite, and other mineral grains; quartz commonly predominates. Texturally, stream sands vary from very fine to very coarse, changing both vertically and horizontally in short distances. The grains are angular to well rounded. Cross-bedding and pocket-and-lens types of structure are common. More or less discontinuous gravel layers are associated with the sands. Stream sands are commonly buff, yellowish :gray, or brown in color. Thickness of the sand deposits vary considerably.
North and west of Atchison, in Atchison County, and in the vicinity of Frankfort, Marshall County, very light buff to white lake sand deposits occur. These sands in general are much finer than are river sands, are horizontally bedded, and consist dominantly of quartz grains. They are believed to represent lake deposits; at places they have a thickness of more than 50 feet. Lake sands also are known to be present in the vicinity of St. George, in Pottawatomie County, and southeast of Holton in Jackson County.
Gravels
Gravel deposits vary greatly in composition, grain size, shape, roundness, thickness, color, and structure. Glacial stream and lake gravels occur in northeastern Kansas and stream gravels are present in the High Plains of the western part of the state. In southeastern Kansas there are gravels which consist essentially of only one kind of material; namely, flint or chert. At certain places in Kansas gravels are made up entirely of local limestones. The flint or chert gravels of southeastern Kansas are composed of flint or chert weathered from Carboniferous and Permian rocks, and distributed by streams probably during Tertiary or Quaternary times. These gravels generally cap the uplands. The flint or chert fragments are rounded to angular and commonly are of light-buff color, although shades of yellow, red and dark gray are not uncommon. Limestone gravels are known to be present in Brown, Douglas, Jewell, Lincoln and Smith counties; they are composed of fragments of local limestone beds and vary in color and texture depending upon the source rocks.
Distribution of sands and gravels in Kansas
Kansas has unlimited reserves of sand and gravel, and deposits occur in almost all counties. In some areas the deposits may not be of great commercial value, but may be important for local purposes. The accompanying map (fig. 10) shows the general distribution of the more important sand and gravel deposits of the state. Sand and gravel, especially, sand, may be found almost anywhere along the major stream courses in the state, such as those of the Arkansas, Big Blue, Cimarron, Kansas, Little Blue, Marais de Cygnes, Missouri, Ninnescah, Neosho, Republican, Saline, Smoky Hill, Solomon, and Verdigris.
Figure 10—Map of Kansas showing distribution of sand and gravel pits.
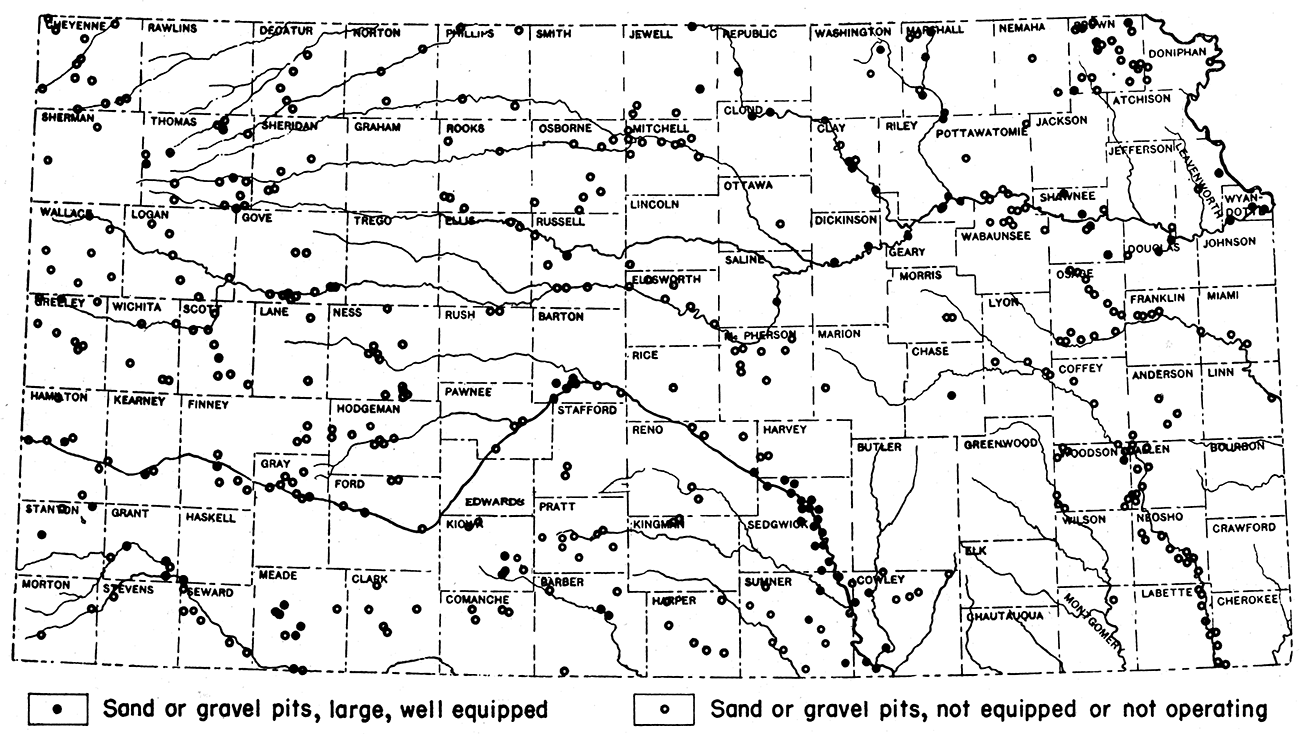
The Tertiary sands and gravels are confined chiefly to the western part of the state in the region known as the High Plains. Quaternary sands and gravels are found chiefly in the same region as the Tertiary deposits. Glacial deposits are confined for the most part to northeastern Kansas.
Production of Kansas sand and gravel
During 1940, Kansas produced 2,264,871 short tons of sand and gravel, valued at $893,962. Of this amount, 1,791,103 tons are classified as commercial and the remaining 473,768 tons as government and contractor types. Wyandotte County leads in the annual production of sand in the state. The relative importance of the sand and gravel industry in Kansas is indicated in table 20, which gives statistics of the tonnage and value of the Kansas production from 1905 to 1940.
Table 20—Production of Kansas sand and gravel, 1905 to 1940.
| Year | Production in Short Tons |
Value in Dollars |
Year | Production in Short Tons |
Value in Dollars |
|
|---|---|---|---|---|---|---|
| 1905 | 70,988 | 21,552 | 1923 | 1,950,411 | 1,039,064 | |
| 1906 | 293,918 | 66,762 | 1924 | 1,882,968 | 1,223,016 | |
| 1907 | 556,625 | 117,313 | 1925 | 2,198,870 | 1,303,060 | |
| 1908 | 320,150 | 64,328 | 1926 | 2,489;343 | 1,491,492 | |
| 1909 | 977,918 | 188,708 | 1927 | 2,254,648 | 1,456,130 | |
| 1910 | 776,638 | 165,659 | 1928 | 2,760,277 | 1,532,399 | |
| 1911 | 734,507 | 164,058 | 1929 | 3,389,783 | 1,879,899 | |
| 1912 | 1,381,586 | 287,352 | 1930 | 3,232,858 | 1,649,476 | |
| 1913 | 1,119,990 | 271,509 | 1931 | 2,893,249 | 1,333,175 | |
| 1914 | 1,347,394 | 381,065 | 1932 | 1,851,211 | 878,733 | |
| 1915 | 1,128,496 | 378,355 | 1933 | 2,015,799 | 734,343 | |
| 1916 | 1,164,995 | 303,630 | 1934 | 1,681,619 | 698,461 | |
| 1917 | 823,403 | 195,578 | 1935 | 1,570,975 | 666,529 | |
| 1918 | 761,110 | 264,073 | 1936 | 2,454,017 | 920,730 | |
| 1919 | 954,121 | 507,642 | 1937 | 2,495,196 | 1,017,053 | |
| 1920 | 1,275,309 | 905,936 | 1938 | 2,962,831 | 1,117,053 | |
| 1921 | 1,082,914 | 647,723 | 1939 | 1,934,759 | 822,305 | |
| 1922 | 1,398,996 | 790,763 | 1940 | 2,264,871 | 893,962 |
Uses
Sand and gravel are used chiefly in Kansas as structural and paving material. The material is used also as traction sand, filter sand, railroad ballast, grinding and polishing sand and molding sand. Studies made by the State Geological Survey during the past two years show that some Kansas sands are suitable for use as foundry sands. There are 41 plants in Kansas and 21 in Kansas City, Missouri, which use foundry sands. Many of these foundries are making castings, especially aluminum castings, for wartime industries. The greatest part of the molding sand used at these foundries is taken from glacial or Pleistocene hight-terrace deposits along the north side of the Kansas river in and near Kansas City, Kansas. A part, however, is taken from deposits formed by glacial outwash streams, and from deposits in the flood plain of the Kansas river.
Age and origin
Sand and gravel deposits of the western part of the state, in the High Plains area, are either Tertiary or Quaternary in age and were deposited either by streams coming from the west or by wind action, the latter being in the form of sand dunes in the valleys of Arkansas and other rivers. The deposits in northeastern Kansas, on the other hand, are mostly of glacio-fluvial origin; that is, they were deposited by streams deriving sediment from a melting continental glacier or glaciers during the Great Ice Age some 750,000 years ago. Some of the sands, such as those near Atchison and Frankfort, were deposited in glacial lakes during the Great Ice Age. Much of the sand and gravel dredged up in our major stream courses represents older material reworked by the streams in modern times.
Work of the State Geological Survey
Sand and gravel resources of Kansas have been studied by members of the State Geological Survey during the past two years. A report on these investigations is now being prepared and will be published in the near future as a bulletin of the Survey. Sand and gravel deposits in Kansas have been described in various publications of the State Geological Survey and in other publications; these are listed below.
References
Bass, N. W., 1926, Geologic investigations in western Kansas: Kansas Geol. Survey, Bull. 11, pp. 1-96, pls. 1-9, figs. 1-27. [available online]
Bass, N. W., 1929, The geology of Cowley County, Kansas: Kansas Geol. Survey, Bull. 12, pp. 1-203, figs. 1-23, pls. 1-12 (including map). [available online]
Charles, Homer, 1927, Oil and gas resources of Kansas, Anderson County: Kansas Geol. Survey, Bull. 6, pt. 7, pp. 1-95, pls. 1-10 (including map), figs. 1-13.
Elias, M. K., 1931, The geology of Wallace County, Kansas: Kansas Geol. Survey, Bull. 18, pp. 1-254, pls. 1-42 (including map), figs. 1-7, tables 1-2. [available online]
Frye, J. C. and Hibbard, C. W., 1941, Pliocene and Pleistocene stratigraphy and paleontology of the Meade basin, Kansas: Kansas Geol. Survey, Bull. 38, pt. 13, pp. 389-424, figs. 1-3, pls. 1-4. [available online]
Haworth, Erasmus, 1896, Surface gravels: Kansas Univ. Geol. Survey, vol. 1, pp. 246-255. [available online]
Haworth, Erasmus, 1897; Physical properties of the Tertiary: Kansas Univ. Geol. Survey, vol. 2, pp. 247-284, pls. 38-44.
Haworth, Erasmus, 1897, The McPherson Equus beds: Kansas Univ. Geol. Survey, vol. 2, pp. 287-296.
Landes, Kenneth K., and Ockerman, J. W., 1930, The geology of Mitchell and Osborne counties, Kansas: Kansas Geol. Survey, Bull. 16, pp. 1-55, pls. 1-15 (including map), fig. 1. [available online]
Landes, Kenneth K., 1937, Mineral resources of Kansas counties: Kansas Geol. Survey, Mineral Resources Circ. 6, pp. 1-110, maps. [available online]
Latta, Bruce F., 1941, Geology and ground-water resources of Stanton County, Kansas Geol. Survey, Bull. 37, pp. 1-119, figs. 1-6, tables 1-5, pls. 1-9 (including maps).
Lohman, S. W., and Frye, J. C., 1940, Geology and ground-water resources of the "Equus beds" area in south-central Kansas: Economic Geology, vol. 35, pp. 839-866, figs. 1-5, tables 1-2.
Moore, R. C., and Landes, Kenneth K., 1927, Underground resources of Kansas: Kansas Geol. Survey, Bull. 13, pp. 1-154, figs. 1-115 (including map), tables 1-2. [available online]
Newell, N. D., and Jewett, J. M., 1935, The geology of Johnson, Miami, and Wyandotte counties, Kansas: Kansas Geol. Survey, Bull. 21, pp. 1-205, pls. "1-23 (including map). [available online]
Rubey, W. W., and Bass, N. W., 1925, The geology of Russell County, Kansas: Kansas Geol. Survey, Bull. 10, pp. 1-104, pls. 1-7 (including map), figs. 1-11. [available online]
Schoewe, W. H., 1923, Glacial geology of Kansas: Pan-Am. Geologist, vol. 40, pp. 102-110, pl. 11.
Seaton, R. A., and Taylor, I. I., 1916, Tests. of Kansas sands for use in mortar and concrete: Kansas State Agricultural College, Bull. 3, Eng. Experiment Station, vol. 42, no. 45, pp. 1-44, figs. 1-8, tables 1-5.
Smith, H. T. U., 1938, Preliminary notes on Pleistocene gravels in southwestern Kansas: Kansas Acad. Sci., Trans., vol. 40, pp. 283-291, fig. 1, table 1.
Smith, H. T. U., 1940, Geologic studies in southwestern Kansas: Kansas Geol. Survey, Bull. 34, pp. 1-212, pls. 1-34, figs. 1-22. [available online]
Thoenen, J. R., 1937, Sand and gravel: Industrial Minerals and Rocks (dept. 38), pp. 671-720.
Todd, J. E., 1918, Kansas during the Ice Age: Kansas Acad. Sci., Trans., vol. 28, pp. 33-47, map.
Todd, J. E., 1918, History of Kaw lake: Kansas Acad. Sci., Trans., vol. 28, pp. 187-199, maps.
Wooster, Lyman C., 1934, The chert gravels of Lyon County, Kansas: Kansas Acad. Sci., Trans., vol. 37, pp. 157-159.
Stone
by J. M. Jewett
Summary—Several million tons of Kansas stone are. used annually. The reserves of stone, including limestone, sandstone and hard shale, are extremely large.
This section of the report describes the economic aspects of firmly consolidated rocks that are used for purposes other than those discussed in other sections of this report. Limestone and sandstone have many uses; hard shale is useful as road metal. Locations of important quarries as shown in the Mineral Resources Map of Kansas (part 4, Bulletin 41); quarries from which various types of stone are taken are distributed throughout a majority of the counties in Kansas.
Figure 11—Map of Kansas showing location of rock wool plants and location of outcrops from which rock has been tested to determine suitability for use as rock wool material.
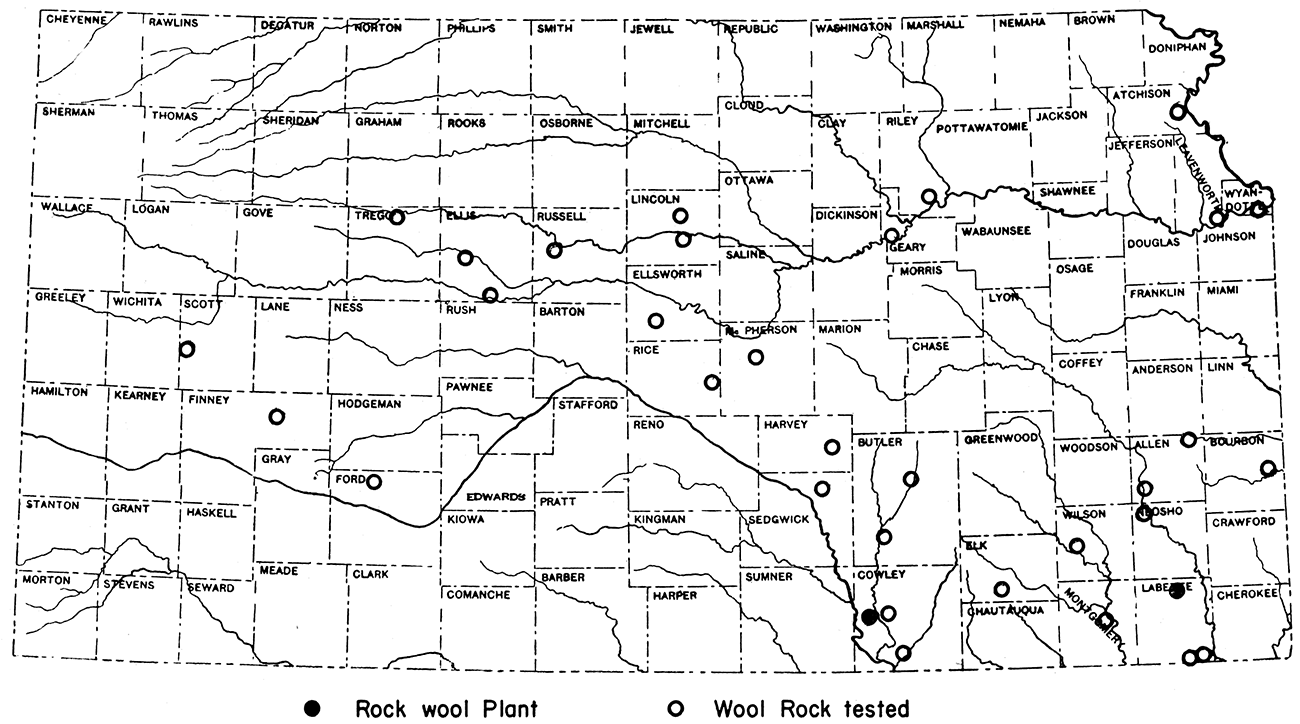
Limestone
Limestone is a sedimentary rock composed primarily of calcium carbonate (CaCO3), occurring in strata or beds. The normal color is white to light gray or buff. Limestones may pass by gradation into shales and sandstones, and it is customary to use adjectives in describing the intermediate varieties. Thus, one may speak of sandy or arenaceous limestone, calcareous sandstone. argillaceous limestone, calcareous shale, and so forth. In addition, there occur ferruginous, carbonaceous, magnesian, and phosphatic limestones, which are, respectively, those with a high percentage of iron, carbonaceous matter, magnesia, and phosphate. There are relatively few pure limestones, but a surprisingly large number consist of 90 per cent or more of calcium carbonate.
Limestones in Kansas
The supply of limestone in Kansas is practically unlimited; areas having abundant reserves are shown in figure 12. All counties in the eastern third of the state and several of the north-central counties have large reserves. Limestone for various purposes has been quarried at one or more places in nearly every township in eastern Kansas.
Kansas limestones range in composition from nearly pure calcium carbonate to those composed of mixtures of calcium carbonate, silica, clay minerals, various iron compounds, and other impurities. Limestones composed of nearly pure calcium carbonate are rather abundant in the state. The rocks occur in beds ranging up to 50 feet thick. As a rule the purer material is found in the thicker beds, those which are 20 feet or more in thickness.
Table 21, below, presents analyses of samples taken from three quarries in eastern Kansas. The rocks from which the samples were taken are believed to be somewhat representative of many of the thicker limestone beds of eastern Kansas.
Table 21—Analyses of calcined samples of eastern Kansas limestones.
| Constituents | Drum limestone Montgomery County (per cent) |
Iola limestone Allen County (per cent) |
Iola limestone Neosho County (per cent) |
|---|---|---|---|
| SiO2 | 11.50 | 4.76 | 1.76 |
| Al2O3 | 3.02 | 0.46 | 2.08 |
| Fe2O3 | 2.14 | 2.14 | 2.75 |
| CaO | 82.59 | 87.50 | 88.80 |
| MgO | 0.84 | 3.40 | 4.60 |
Figure 12—Map of Kansas showing location of areas of abundant limestone, sandstone, and chalk deposits.
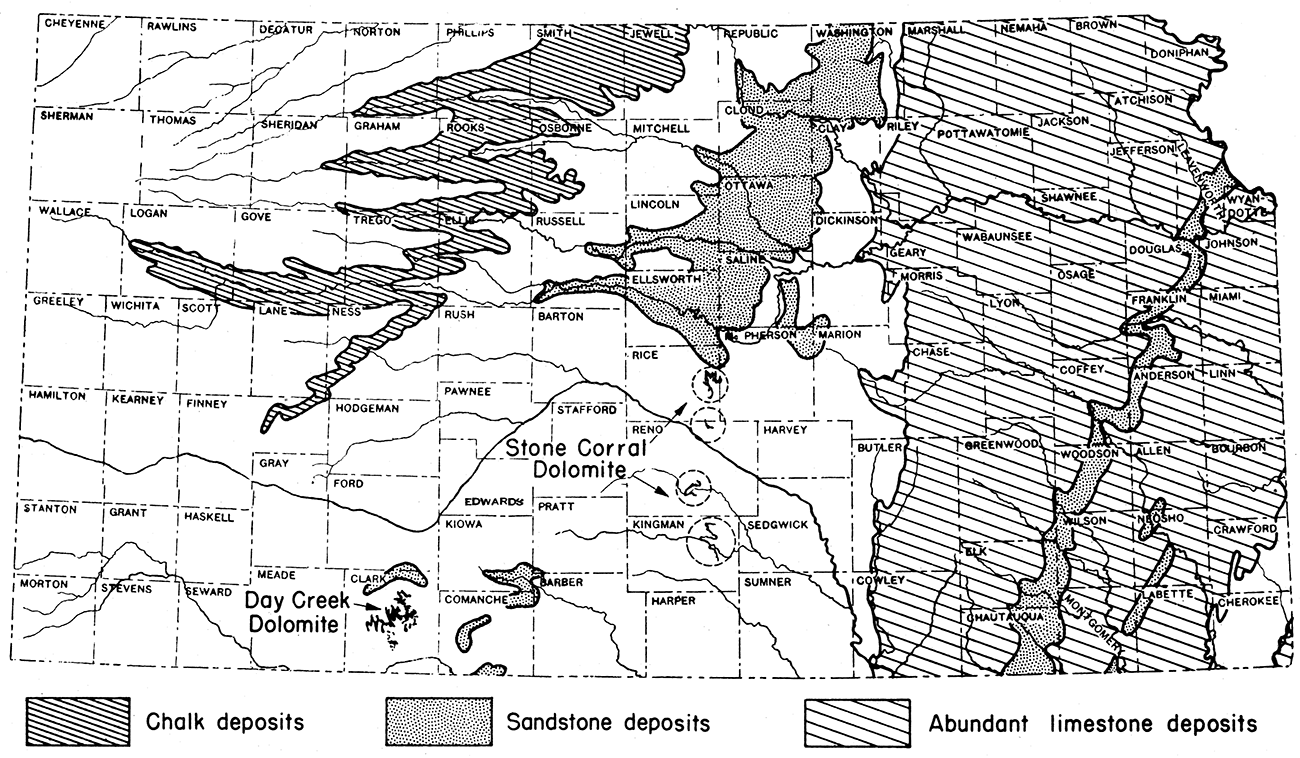
Limestones interbedded with shales and sandstones crop out in the eastern third of Kansas, forming narrow, tortuous bands of outcrop trending in a general north-south direction. The thicker limestones generally cap eastward facing escarpments, west of which they lie with little cover below "dip slopes" inclined gently to the west. The limestones are readily accessible for quarrying operations.
Limestone beds generally are less abundant in central and northern Kansas than in the eastern part of the state. Much of the limestone of central Kansas is in the form of chalk, a soft rock composed of the calcareous remains of minute organisms. Because of its physical properties and high degree of purity, chalk has uses that ordinary limestones do not have; it therefore is described in a separate section of this report.
Limestone production in Kansas
Available quantitative figures on the production of limestone in Kansas are of little value because large quantities are quarried and used without being reported for incorporation in available records. Commercial quarries produce limestone in many places. Near each population center quarries have been established to supply local demands. The demand is variable, depending upon the extent of local concrete paving operations, building programs, and so forth. Railroad companies have quarried immense quantities that have been used largely as track ballast. The cement-manufacturing companies operate their own quarries. In the past few years a large amount of limestone has been quarried by W.P.A. workers, and the stone has been used in public works projects. It follows from the foregoing statements that a considerable portion of the product of limestone quarries does not enter the regular channels of trade; a large tonnage, nevertheless, is sold each year by quarry operators. Table 22 shows production figures as reported for the period from 1937 to 1940.
Table 22—Reported stone production in Kansas (chiefly limestone).
| Years | Tons |
|---|---|
| Yearly average for 10-year period (1927-36) |
1,571,699 |
| 1937 | 3,540,860 |
| 1938 | 3,676,230 |
| 1939 | 3,405,640 |
| 1940 | 2,880,930 |
Uses of limestone
Kansas limestone is used in large quantities as road metal, railroad ballast, rip-rap, concrete aggregate, in cement making, and to a considerable extent as building stone. In addition to these, limestone has many other uses. It is used as a flux in blast furnaces and in metallurgical work; it 'is used as agricultural limestone and in making lime; its products are used for refining sugar, manufacturing refractories, paper, and glass; with coke it is used in making calcium carbide from which acetylene gas is generated. Limestone is used as filter bed material and in making fillers and whiting.
When Kansas was being settled, limestone was used as building stone to a large extent. Some of the early settlers came directly from northern Europe, where wooden buildings are uncommon. They and others recognized the value of Kansas limestones and constructed many stone farm buildings. In certain areas, for example in the Flint Hills region, there are many farm buildings, rural schools and churches, constructed of limestone; some of these were built as early as the 1860's and still are in excellent condition. Some stone buildings built in territorial days still are in use.
From time to time a considerable amount of Kansas limestone has been used in public buildings. The athletic stadium of the Kansas State College at Manhattan, some of the University of Kansas buildings at Lawrence, and most of the permanent buildings at Fort Riley and at Fort Leavenworth have been constructed of limestone. In nearly every town in eastern Kansas there are churches and other buildings that have been built of limestone taken from local quarries. Farther west in the state limestone has been less extensively used.
In recent years limestone has been used extensively in public buildings constructed chiefly by W.P.A. workers. These buildings, of pleasing appearance, indicate that Kansas limestones may be used in masonry construction and that dimension blocks can be prepared without the use of highly skilled labor.
Space does not permit description here of individual limestone formations, but it should be noted that much of the best building stone occurs in thin regular beds and requires but little dressing.
Oolitic limestones represent a type of Kansas limestone that may be fashioned into beautiful building blocks. Such rock, quarried in eastern Kansas, was used recently in building the new Sweeney Gymnasium at the University of Kansas City, Missouri.
It is well to mention here that, although at the present time there are no lime kilns operating in Kansas, there is a great abundance of limestone suitable for use in lime manufacture. In the past, lime has been made at many places in eastern Kansas.
Kansas limestone reserves
As indicated above, Kansas has unlimited reserves of various kinds of limestones adapted to numerous uses. The greatest reserves are in the eastern part of the state, but much limestone also is available in the central and northern parts.
Origin and age of Kansas limestones
Most of the limestones of the world have been formed by the consolidation of calcareous mud that was deposited on the floors of shallow seas; this mud was derived largely from the accumulation of calcareous tests or shells of marine animals and hard parts of certain plants. Some limestone beds were formed largely by direct chemical precipitation of calcium carbonate from sea water, and perhaps nearly all limestone beds contain some precipitated material.
The limestones in eastern Kansas are Carboniferous and Permian in age. Those of central and northern Kansas are Cretaceous in age.
Work of the State Geological Survey
Various members of the State Geological Survey of Kansas have carefully mapped and studied the limestone and other formations of the state. An abundance of data pertaining to thickness, lithologic character, and geographic distribution are at hand. The geologic map of Kansas, published by the Survey in 1937, shows the areal distribution of various formations. Bulletin 22 (Moore, 1936) presents general descriptions of limestone beds and other rock units of Pennsylvanian (Late Carboniferous age in Kansas. Numerous bulletins deScribing the rocks of individual counties have been published. Mineral Resources Circular 6 (Landes, 1937) describes briefly the limestone and other resources of every county in Kansas. The use of limestone and other rock in rock wool manufacture has been investigated and has been discussed in Mineral Resources Circular 5 (Plummer, 1937).
Limestone is one of Kansas' great mineral resources, on which additional research is needed. Studies of polished and thin sections of Kansas limestones have been initiated. These investigations are expected to yield valuable information pertaining to the use of limestone as building stone and for other purposes as well as information of important scientific value. Chemical analyses of Kansas limestones are being made in the Survey laboratories. Data obtained by these study methods will be of great value in determining potential economic uses of various limestones of the state.
Sandstone
Sandstone is a sedimentary rock occurring in strata or beds that is composed of and grains cemented together so as to form a more or less solid mass. Most sandstones are composed largely of quartz grains held together by some natural cement, such as calcium carbonate, iron oxide, or clay. Sandstones may grade vertically or laterally into shales and limestones; intermediate rock types include sand limestones or calcareous sandstones, argillaceous sandstones or sandy shales.
Sandstone in Kansas
The reserves of sandstone in Kansas are inexhaustibly great, but even so the rock is not as abundant as limestone. Sandstone occurs interbedded with shale and limestone in the eastern part of the state, and sandstone is present in the Smoky Hill region in north-central Kansas in a wide belt extending from Rice and McPherson counties to Washington County. Figure 12 shows the locations of principal sandstone outcrop areas in Kansas.
Sandstone production in Kansas
The production (in tons) of sandstone marketed in Kansas are included in the figures given in table 22. There is a large deposit of "quartzite", a firmly cemented sandstone, in Lincoln County; the crushed "quartzite" has been taken from a quarry in sec. 7, T. 12 S., R. 7 E., Lincoln County. Pressed cement bricks are made from fine material derived from this quarry. For many years a unique flagstone type of sandstone has been produced in Bourbon County. This stone has been shipped to various points in Illinois, Missouri, Kansas, Oklahoma, and Texas, where it has been used chiefly as paving slabs in walks; more recently the rock has been used as building stone and as ornamental stone.
Uses of sandstone
Sandstone has a variety of uses. Like other firmly cemented rocks it is used widely as aggregate in concrete. It is.probable that some of the eastern Kansas sandstone could be employed as abrasives. As stated above, some of the Kansas rock is suitable for use as building stone and flagstone. Several years ago sandstone in southeastern Kansas was used as a source of quartz sand in glass manufacture. Cretaceous sandstones are being used as road metal material in central Kansas.
Age and origin of Kansas sandstones
Sandstones in Kansas are chiefly nonmarine in origin and were formed largely as coastal plain deposits. Some are of marine origin and were deposited in deltaic or littoral environments. The deposits in eastern Kansas are Pennsylvanian (late Carboniferous) in age. Those in central Kansas are of Cretaceous age.
Work of the State Geological Survey
Studies pertaining to potential uses of Kansas sandstones have been planned. The Survey has carefully mapped and studied the surface and subsurface distribution of Kansas sandstones and has carried out studies of the character of the various sandstone types.
Shale
Shale is a stratified sedimentary rock made up of thin layers ordinarily composed of silt or clay particles; it tends to be softer than limestone and sandstone and therefore does not have the same uses as have harder rocks. Many shales consist chiefly of clay particles, and when subjected to weathering, the rock generally slakes. Certain shales in Kansas and elsewhere are an important source of clay used for ceramic purposes; such clay shales are discussed in another section of the present report.
Kansas shale used as road metal
In general, shale is a relatively non-resistant rock; there occur, however, in Kansas two or three types of more resistant shale that are used as road metal material. These resistant shales provide road-surfacing material that is very easily obtained.
At many localities in eastern Kansas sandy shale, or very argillaceous sandstone, crops out and has been used for "sanding" roads; Another kind of shale that may be used as a road metal is a black, platy carbonaceous shale that occurs in several parts of the eastern Kansas stratigraphic section. This material has been used on roads for several years; in Labette County, especially, black shale has been used in large quantities, and farm-to-market roads, having a limited amount of traffic, have stood up well when surfaced with it. Black shale suitable for surfacing secondary or temporary roads is readily available in all counties in the eastern part of Kansas.
The Kansas shales discussed above occur interstratified with other shales, sandstones and limestones in the Pennsylvanian (Upper Carboniferous) rock section of eastern Kansas. It is probable that shales of Cretaceous age that are dark and somewhat similar to the black Pennsylvanian shale would be useful. Cretaceous shales of this type crop out in Kiowa, Comanche, Ellsworth and McPherson counties. Calcareous shale from the Greenhorn formation of Cretaceous age has been used as road metal material in Cloud and Republic counties, Kansas.
References
Bowles, Oliver, 1937, Dimension stone: Industrial minerals and rocks: Am. Inst. Min. and Metall. Eng., New York, pp. 763-794.
Eardley, Wilmot, 1937, Abrasives: Industrial minerals and rocks: Am. Inst. Min. and Metall. Eng., New York, pp. 1-58.
Landes, K. K., 1937, Mineral resources of Kansas eounties: Kansas Geol. Survey, Mineral Resources Circ. 6, pp.1-110, maps. [available online]
Moore, R. C., 1936, Stratigraphic classification of Pennsylvanian rocks of Kansas: Kansas Geol. Survey, Bull. 22, pp. 1-256, figs. 1-12. [available online]
Patterson, S. B., 1937, Crushed and broken stone: Industrial minerals and rocks, Am. Inst. Min. and Metall. Eng., New York, pp. 795-836.
Plummer, Norman, 1937, Rock-wool resources of Kansas: Kansas Geol. Survey, Mineral Resources Circ. 5, pp.1-74, figs. 1-20. [available online]
Tripoli
by J. C. Frye
Summary—One tripoli processing plant is situated near Baxter Springs, Kansas. Tripoli occurs in Cherokee County; the reserves are inadequately known.
Tripoli consists essentially of silica (SiO2), in a cryptocrystalline state, that can be ground to a fine abrasive of uniform texture. It occurs in limestones of Mississippian age in southeastern Kansas, northeastern Oklahoma, and southwestern Missouri, and probably is formed by ground water leaching of the calcium carbonate from a secondarily silicified limestone, leaving a siliceous residue. In some parts of the world the terms tripoli or tripolite are used to designate a siliceous deposit consisting almost entirely of the siliceous skeletons of diatoms; such deposits should be termed diatomite rather than tripoli. Diatomaceous marl deposits in Kansas are described in another part of this report.
Tripoli is a uniform-textured abrasive used mainly as a polishing, and filtering material. It is used locally as an ingredient of drilling mud and can be used successfully in scouring powders and mechanics soap. A mill for the processing of tripoli is located near Baxter Springs, Kansas; according to H. W. Nesch this mill can process 6 tons an hour, the material being ground to a size of 300 mesh or smaller.
Tripoli deposits are known to occur in Cherokee County, Kansas, but it is impossible at the present time to make an accurate estimate of the known reserves, and the purity of the material is not adequately known.
Volcanic Ash
by W. H. Schoewe
Summary—Kansas is the leading state in the production of volcanic ash. Production in 1940 was 39,215 short tons valued at $129,959. Reserves, 10,000,000 short tons.
Description
Volcanic ash consists essentially of very small, sharp and angular fragments of natural or rock glass. In addition, volcanic ash usually contains minor amounts of mineral feldspar, a small amount of quartz and occasionally muscovite mica. Impurities likely to be present are calcite, either in the form of a cement or concretions, sand and clay. Texturally, volcanic ash is very fine; mechanical analyses of Kansas deposits show that a very large percentage of the ash particles are finer than 0.147 mm in diameter, or will pass through a 100 mesh sieve. Much of the ash consists of particles smaller than 0.046 mm in diameter, or will pass through a 300 mesh sieve. As a general rule, the finer the ash the greater its commercial value. Kansas volcanic ash is white to light gray or bluish gray in color, the differences in color being due to differences in age. Volcanic ash is known also as pumicite, and erroneously as silica, feldspar, spar and geyserite.
The various deposits of volcanic ash in Kansas are widely separated and completely isolated from one another (fig. 13). Field observations indicate that individual deposits as seen in outcrop cover from a few square feet to more than a quarter of a section of land with an average areal extent of several acres. The ash beds are highly variable in thickness; at places they are but a few inches thick, whereas thicknesses of 20 to 30 feet or more have been reported from some localities. Thicknesses of 6 to 14 feet are common. The ash crops out at the surface at numerous places; at other places, however, it is overlain by soil, a veneer of stream-carried pebbles or by Pleistocene deposits. At no place is the known overburden greater than 20 feet in thickness. In general, the ash deposits are massive in structure; thin-bedded and cross-bedded deposits, however, are relatively common. In Norton County much of the ash shows distinct horizontal bedding, indicating deposition under quiet water conditions in lakes.
Figure 13—Map of Kansas showing distribution of volcanic ash deposits in Kansas.
Chemically, volcanic ash is a solid solution of silicates, the combined constituents being alumina, soda, potash, magnesia, lime, and iron oxide. The feldspars found in Kansas ash thus far examined consist of the potash and soda aluminum silicates, the lime feldspars being absent. The chemical analyses shown in table 23 are typical of the Kansas deposits.
Table 23—Chemical analyses of Kansas volcanic ash.
| Constituents | McPherson Co. (per cent) |
Lincoln Co. (per cent) |
Meade Co. (per cent) |
Satanta, Meade Co. (per cent) |
McPherson Co. (per cent) |
|---|---|---|---|---|---|
| SiO2 | 72.50 | 73.30 | 72.40 | 72.36 | 72.30 |
| Al2O3 | 14.15 | 14.46 | 10.65 | 12.20 | 16.62 |
| Fe2O3 | 1.65 | 1.54 | 2.65 | 1.40 | 2.33 |
| CaO | 0.70 | 1.00 | 1.68 | trace | 0.99 |
| MgO | 0.30 | 0.21 | 0.13 | 0.58 | |
| K2O | 4.70 | 5.64 | 4.82 | 3.00 | 2.00 |
| Na2O | 6.52 | 4.00 | |||
| Ignition loss | 5.00 | 4.60 | 9.60 | ||
| Total | 99.00 | 100.75 | 96.93 | 100.00 | 98.24 |
Distribution
In 1927 volcanic ash was known to occur in 13 counties, and at the present time 36 Kansas counties are known to contain volcanic ash deposits. At least 115 separate deposits are known in these counties. Table 24 shows the distribution of volcanic ash in Kansas by counties together with the number of deposits thus far reported; the geographic distribution of the deposits is shown also in figure 13.
Production
Kansas is the leading volcanic ash producing state in the United States. In 1940, Kansas produced 39,215 tons of ash or 47.58 per cent of all the ash mined in the United States during; that year. The value of the Kansas ash production for 1940 amounted to $129,959. Table 25 indicates that Kansas has led the states in the quantity and value of ash produced since 1916. The table shows also that although Kansas has ranked first in ash production since 1916, the percentage of ash mined in Kansas is gradually becoming less and is considerably less than it was in the early years of the industry. Meade County leads all other counties in the state in ash production.
Table 24—Number of known volcanic ash deposits (by counties) in Kansas on February 1, 1942
| County | Number of deposits |
County | Number of deposits |
|
|---|---|---|---|---|
| Chautauqua | 1 | Meade | 12 | |
| Clark | 8 | Nemaha | 2 | |
| Comanche | 2 | Ness | 1 | |
| Decatur | 2 | Norton | 20 | |
| Doniphan | 1 (?) | Osborne | 2 | |
| Ellsworth | 3 | Phillips | 1 | |
| Gove | 1 | Pratt | 2 | |
| Graham | 1 | Rawlins | 4 | |
| Grant | 4 | Reno | 1 | |
| Hamilton | 7 | Rooks | 2 | |
| Harper | 4 | Russell | 2 | |
| Haskell | 1 | Seward | 5 | |
| Hodgeman | 2 | Sheridan | 4 | |
| Jewell | 4 | Sherman | 1 | |
| Kiowa | 1 | Smith | 1 | |
| Lincoln | 1 | Stafford | 1 | |
| Logan | 2 | Trego | 2 | |
| McPherson | 4 | Wallace | 3 |
Volcanic ash has been mined in Kansas since 1915 or earlier. During 1941, five companies were operating in six counties, namely Comanche, Grant, Meade, Norton, Osborne and Sheridan. At various times volcanic ash was mined by local individuals or owners. The names and home office locations of the most important volcanic ash or pumicite producers in Kansas are listed below.
The Cudahy Packing Co., Chicago, Illinois
Davidson Pumice Co., Norton, Kansas
Dodson Concrete Board Co., Wichita, Kansas
J. B. Ford Co., Wyandotte, Michigan
Mid-Co Products Co., Kansas City, Missouri
The Pumicite Co., St. Louis, Missouri
Table 25—Volcanic ash production in Kansas.
| Year | Production, short tons | Per cent of U.S. production from Kansas |
Value of Kansas production, in dollars |
|
|---|---|---|---|---|
| Kansas | United States | |||
| 1916 | 23,804 | 33,320 | 71.44 | 51,648 |
| 1917 | * | 35,293 | * | * |
| 1918 | * | 28,637 | * | * |
| 1919 | * | 34,051 | * | * |
| 1920 | * | 41,838 | * | * |
| 1921 | 34,172 | 37,108 | 92.09 | 125,642 |
| 1922 | * | 45,262 | * | * |
| 1923 | 51,907 | 56,575 | 91.75 | 170,459 |
| 1924 | 39,489 | 43,651 | 90.47 | 148,177 |
| 1925 | 35,385 | 40,380 | 87.63 | 122,349 |
| 1926 | 48,869 | 53,887 | 90.69 | 152,190 |
| 1927 | 45,439 | 53,298 | 85.26 | 152,748 |
| 1928 | 46,836 | 57,430 | 81.57 | 183,589 |
| 1929 | 49,768 | 67,013 | 74.26 | 195,784 |
| 1930 | 38,024 | 56,843 | 66.89 | 134,896 |
| 1931 | 47,783 | 69,819 | 69.43 | 152,520 |
| 1932 | 39,375 | 53,214 | 73.99 | 117,558 |
| 1933 | 42,355 | 61,220 | 69.18 | 109,454 |
| 1934 | 39,283 | 56,169 | 69.94 | 102,668 |
| 1935 | 41,111 | 57,116 | 71.98 | 108,349 |
| 1936 | 42,057 | 72,915 | 57.68 | 117,757 |
| 1937 | 38,438 | 71,007 | 54.13 | 111,655 |
| 1938 | 38,136 | 65,742 | 58.00 | 112,823 |
| 1939 | 41,643 | 89,159 | 46.70 | 123,163 |
| 1940 | 39,215 | 82,407 | 47.58 | 129,959 |
| * No data available. | ||||
Kansas has produced more than 1,023,129 tons of volcanic ash since 1916, an amount valued at $2,613,399.
Uses
The uses of Kansas volcanic ash are many and varied. It is used as an abrasive, especially as a polishing and cleansing agent. It is used in toothpastes and powders, as a high-grade silver polish, in making mechanics paste soaps, abrasive hand soaps, sweeping compounds, and rubber erasers. Because of its low heat conductivity and high porosity, volcanic ash can be utilized efficiently in heat-and-cold insulations and as a packing for water and steam pipes and lagging boilers. It may be used as a filler in paints and as an agent in filtering oils for purposes of purification and clarification. Experiments now being conducted by the State Geological Survey are designed to test its suitability as a filler in certain plastics. Volcanic ash has been used in the preparation of dynamite. It can be used in the making of puzzolan and trass or tufa cements. Puzzolan cement, because of its hydraulic properties, is especially valuable in sea works; tufa cement has been used in the construction of aqueducts and dams. Recent experiments have shown that Kansas volcanic ash may be used by ceramic industries in the manufacture of glazes, enamels, glass, and vitreous pottery. The use of volcanic ash in a pottery body solved for a Kansas City firm the problem of manufacturing a container in March, 1942. The State Geological Survey recommended a mixture of 25 per cent volcanic ash and 75 per cent Kansas pottery clay, from which the firm is able to manufacture a container which is impervious to water and which can be fired at the relatively low temperature attained by their kiln.
Reserves
The total volcanic ash reserves in Kansas are unknown. This is due to the fact that relatively few of the 115 known localities distributed throughout 36 counties in Kansas have been surveyed and only a few have been exploited. It is reasonably certain, however, that the Kansas reserves of volcanic ash are several times the total amount thus far mined. Table 26 gives estimates of the reserve tonnage of some of the larger known ash deposits.
Table 26—Estimated reserves of volcanic ash in the larger known ash deposits.
| County | Reserves, Short Tons |
|---|---|
| Grant | 75,000 |
| Gove | 60,000 |
| Jewell | 4,000,000 |
| McPherson | 48,000 |
| Meade | 1,600,000 |
| Norton | 50,000 |
| Phillips | 500,000 |
| Total | 6,333,000 |
It is reasonable to assume that an additional 3 to 4 million tons of ash are present in the unsurveyed but known volcanic ash deposits in the remaining 29 counties not included in Table 26. It should be noted that undiscovered volcanic ash deposits undoubtedly exist not only in the counties known to have volcanic ash but also in counties in which volcanic ash has not as yet been reported. Recently (February, 1942), the State Geological Survey of Kansas received and examined volcanic ash from two hitherto unreported deposits in Hodgeman County. The total reserve tonnage of volcanic ash in Kansas may be estimated as approximating 10,000,000 short tons.
Origin and age
Volcanic ash or pumicite is the finest material erupted from volcanoes. Inasmuch as no volcanoes are known to have existed in Kansas, it follows that the volcanic ash represents transported material brought to the state from volcanoes in other regions. The Kansas deposits are not continuous but occur in scattered and isolated patches in the counties cited above. A study of their distribution indicates that the area covered by the ash is fan-shaped with the apex of the fan pointing toward the southwest. In general, the coarser ash lies southwest of the finer ash. The fineness of the material, the absence in some places of horizontal bedding, and locally the characteristic eolian or wind-type of crossbedding strongly indicate that wind was the transporting agent which brought the volcanic ash to Kansas. It has been suggested that the source of the Kansas volcanic ash was a group of now extinct volcanoes in the northeastern corner of New Mexico. These volcanoes, known as the Capulin group of volcanoes, lie to the southwest of the previously mentioned fan-shaped ash area in Kansas. The Capulin volcanoes are post-Tertiary or Pleistocene in age.
Not all of the ash deposits in Kansas are of Pleistocene age. In Norton, Decatur and Rawlins counties that ash is much more compact than elsewhere in the state and is bluish in color, in contrast with the white or light gray ash found in other counties. The compact and bluish ash occurs in the "mortar beds" of the Ogallala formation which is considered to be of Pliocene (late Tertiary) age; this ash, therefore, is older than the white and more prevalent Pleistocene ash.
Work of the State Geological Survey
The State Geological Survey has undertaken the work of examining samples of volcanic ash sent in by individuals for identification. Recent, intensive studies have been carried on by the Survey on the subject of the ceramic uses of Kansas volcanic ash and the control of iron oxide in the ash suitable for making glazes and low-cost glass. Bulletins published by the Survey dealing with volcanic ash are listed below.
References
Frye, J. C., and Hibbard, C. W., Pliocene and Pleistocene stratigraphy and paleontology of the Meade basin, southwestern Kansas: Kansas Geol. Survey, Bull. 38, pt. 13, pp. 389-424, figs. 1-3, pls. 1-4. [available online]
Landes, K. K., 1928, Volcanic ash resources of Kansas: Kansas Geol. Survey, Bull. 14, pp. 1-58, figs. 1-3, pl. 1-4. [available online]
Landes, K. K., 1937, Mineral resources of Kansas counties: Kansas Geol. Survey, Mineral Resources Circ. 6, pp. 1-110, maps: [available online]
Landes, K. K., 1938, Distribution of volcanic ash: Am. Ceramic Soc., Bull., vol. 17, no. 8, pp. 323-325.
Kinney, E. D., 1941, Control of iron oxide in volcanic ash: Am. Ceramic Soc., Bull., vol. 20, no. 4, pp. 118-121, fig. 1, tables. 1-6.
Moore, R. C., and Landes, K. K., 1927, Underground resources of Kansas: Kansas Geol. Survey, Bull. 13, pp. 1-154, figs. 1-115. [available online]
Plummer, Norman, 1939, Ceramic uses of volcanic ash: Am. Ceramic Society., Bull., vol. 18, no. 1, pp. 8-11; also Kansas Geol. Survey, Mineral Resources Circ. 13, pp. 8-11.
Smith, H. T. U., 1940, Geologic studies in southwestern Kansas: Kansas Geol. Survey, Bull. 34, pp. 1-212, figs. 1-22, pls. 1-34. [available online]
Prev Page--Metals || Next Page--Water
Kansas Geological Survey, Geology
Placed on web Nov. 2, 2017; originally published May 9, 1942.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/41_3/05_nonmet.html