Prev Page--Ceramic Properties || Next Page--Conclusions
Interpretation and Correlation of Data
The four samples that are the subject of this study have been designated as clays, although the samples contain from 25 to more than 50 percent quartz. The term "clay material" which Grim (1953) uses to describe any fine-grained, natural, earthy, argillaceous material is preferable from the standpoint of accuracy despite the fact that the clay minerals present in the samples must be considered as the dominant factors in attempting to account for the physical and chemical properties of the clays.
The particle size and shape of the clay minerals, the quartz and other so-called nonplastics, and the adsorbed ions and soluble salts determine the clay-water behavior of the clays in respect to such physical properties as plasticity and shrinkage on drying. The effect of heat on the clays, especially in the higher temperature range, is determined to a limited extent by particle size and shape, but largely by the chemical composition of the minerals present. The objectives of this study have been, therefore, to determine the kind and relative percentage of the clay minerals, quartz, and other constituents, by means of x-ray, differential thermal, and chemical analyses, and by examination with the optical and electron microscope; particle size by sedimentation methods; and particle shape by means of the optical and electron microscopes. A further objective of the investigation was the determination of the clay-water characteristics of the clays such as water of plasticity, plasticity, and drying shrinkage; and determination of the effect of heating to relatively high temperatures as measured by shrinkage, water absorption, and (correlatively) porosity. In the following paragraphs we shall attempt to show the interrelation of the fundamental properties, and the relation of the fundamental to the ceramic properties.
Interrelated Methods of Determining Mineral Composition
The kind and percentage of clay minerals and of quartz, feldspar, and muscovite were determined by a combination of methods. In fact, reliable estimates can be achieved only by correlation and interpretation of a variety of data, which have been reported under separate headings.
X-ray diffraction furnished an accurate qualitative basis for estimates of mineral content of the clays (Figs. 2-7). Differential thermal analyses, electron micrographs, and optical examination supplemented the x-ray data. These data also furnished the basis for semi-quantitative estimates which were correlated with the chemical composition in order to obtain greater accuracy. In calculating from the chemical composition, chemical analyses of relatively pure illite, montmorillonite, and mixed-layer (illite-montmorillonite) clay having intermediate compositions were used. The theoretical composition of kaolinite was used because it does not have the range of composition characteristic of the other clay-mineral groups. The rough quantitative estimates for the original or whole clay, the 2- to 8-micron fraction, and the minus 2-micron fraction for each of the four clays, reported to the nearest 5 percent, are given in Table 15.
Table 15--Estimated percentages of the minerals found in the whole clay and in two fractions
| Sample no. | Minerals present, percentage | ||||||
|---|---|---|---|---|---|---|---|
| Kaolinite | Illite | Mixed-layer | Montmorillonite | Muscovite | Feldspar | Quartz | |
| EL-60-6 | |||||||
| Whole clay | 45 | 50 | |||||
| 2-8-micron | 55 | 40 | |||||
| <2-micron | 95 | 1 | |||||
| EL-69-2 | |||||||
| Whole clay | 35 | 20 | 5 | 5 | trace | 35 | |
| 2-8-micron | 20 | 20 | 5 | <5 | 50 | ||
| <2-micron | 60 | 25 | 11 | <5 | |||
| O-5-6 | |||||||
| Whole clay | 40 | 5 | 15 | 10 | trace | 30 | |
| 2-8-micron | 15 | <10 | < 5 | < 5 | 70 | ||
| <2-micron | 55 | 10 | 15 | 10 | <5 | ||
| O-33-4 | |||||||
| Whole clay | 40 | 20 | < 5 | trace | trace | 35 | |
| 2-8-micron | 15 | <10 | < 5 | trace | 70 | ||
| <2-micron | 65 | <25 | < 5 | 5 | |||
The quantitative estimates of mineral content of the clay obtained from the x-ray diffraction data alone agreed within 5 percent with those calculated from the chemical composition, and some of the percentages obtained by the two methods were in much closer agreement.
Although the differential thermal analysis curves indicate accurately the various clay minerals present in the samples, their usefulness is limited so far as quantitative estimates are concerned, chiefly because the intensity of the endothermic and exothermic reactions produced by the different clay minerals differ greatly. Furthermore, both kaolinite and illite, the major clay-mineral constituents of the clays studied, produce endothermic reactions within the range of 400° to 700° C.; therefore, accurate quantitative distinctions can be made only with the aid of an elaborate series of standards. Clay EL-60-6, composed almost entirely of kaolinite and quartz, was in fair quantitative agreement with the Macon, Georgia, kaolin, and the other three clays showed a quantitative relation with each other and with the Champion and Challenger ball clay.
It was possible to determine, however, that the areas obtained under the endothermic peaks ranging from 400° to 650° C. (Figs. 8-10) rather accurately represent the percentage clay-mineral composition. Assuming that the mineral composition of the clays determined from x-ray diffraction data and chemical analyses was accurate, and using the percentages obtained before "rounding off" to the nearest 5 percent (Table 10), the expected relative size of the endothermic peaks was calculated by the following method. Speil and others (1945) tabulated peak areas for various clay minerals as determined with the same apparatus under identical conditions. The area under the 620° C. endothermic peak for kaolinite was found to be 61 square cm under the conditions of their tests, whereas under the same conditions the 605° C. peak area for illite was 16.25 square cm. From data obtained by us a comparable area for mixed-layer clay (illite-montmorillonite) was assumed to be 10 square cm. Using the above described estimated percentages of the various clay minerals in the samples, the theoretical areas were computed by multiplying the percentage of kaolinite by 61, the percentage of illite by 16, and the percentage of mixed-layer clay minerals by 10. Montmorillonite was not included because it does not produce an endothermic peak within the temperature range considered. The computed areas were somewhat larger than those obtained by us due to differences in amplification. The areas of the endothermic peaks determined experimentally are compared to the calculated ones in Table 16. Values for the standard clays previously mentioned were also included, although fewer data were available for calculating the percentage, of clay minerals present in the standard clays. The percentage alumina in the various clays is also compared to the areas of the endothermic peaks in Table 16.
Table 16--Areas of endothermic peaks compared to calculated areas and to percentages of alumina.
| Sample no. | Measured area of endothermic peak, sq. cm. |
Calculated area of endothermic peak, sq. cm. |
Alumina, percentage |
Ratio, calculated to measured areas |
Ratio, measured area to alumina |
|---|---|---|---|---|---|
| EL-60-6 | 21.80 | 27.45 | 17.80 | 1.259 | 0.817 |
| EL-69-2 | 19.19 | 25.05 | 21.18 | 1.305 | 1.104 |
| O-5-6 | 21.65 | 26.70 | 24.29 | 1.233 | 1.122 |
| O-38-4 | 21.13 | 28.10 | 23.09 | 1.330 | 1.093 |
| C. & C.1 | 24.13 | 37.23 | 27.86 | 1.543 | 1.155 |
| Tenn. No. 52 | 26.88 | 39.80 | 29.96 | 1.481 | 1.115 |
| H-4 kaolin3 | 39.14 | 56.73 | 37.20 | 1.449 | 0.950 |
| Bell kaolin4 | 52.84 | 59.78 | 38.40 | 1.131 | 0.727 |
| Average of H-4 & Bell kaolins | 45.99 | 58.25 | 37.80 | 1.290 | 0.823 |
| Average of 4 Kansas clays | 1.282 | 1.034 | |||
| 1Ball clay furnished by H. C. Spinks Clay Co., Paris, Tenn. 2Ball clay furnished by Kentucky-Tennessee Clay Co., Mayfield, Ky. 3Macon, Georgia, Kaolin. A.P.I. standard sample. 4Georgia kaolin furnished by the Bell Foundry, St. Louis, Mo. |
|||||
The ratios of calculated to measured areas of the endothermic peaks for the four Kansas clays show that the differential thermal curves rather accurately reflect the percentage clay-mineral composition of the clays. The maximum deviation from the average is only 3.8 percent. Although there is a marked difference in the two standard kaolins, the average of the two is only 0.6 percent higher than the average for the four Kansas clays. The ratios for the two Tennessee ball clays are similar to each other, but markedly higher than those for the Kansas clays.
The close agreement of the ratios of calculated to measured areas of the endothermic peaks indicates that factors such as particle size and perfection of crystallization have little influence on the size of the peaks. Clays EL-60-6 and O-5-6, for example, range from large, very well-formed crystals to very small, imperfect ones, yet the ratios are in close agreement.
The ratios of measured areas of endothermic peaks to the alumina content of the clays are similar only for clays having similar clay-mineral compositions. This is to be expected, of course, because complete correlation could be obtained only if the percentage of alumina found in each clay mineral were directly proportional to the areas of endothermic peaks produced by them. It will be noted that for clays in which kaolinite is practically the only clay mineral (H-4 kaolin, Bell kaolin, and clay EL-60-6) the ratios compare fairly well. The same similarity can be noted for the other three Kansas clays and the two ball clays, all five of which have similar clay mineral assemblages.
Correlation of Fundamental and Ceramic Properties
The clay minerals under consideration in this study are composed essentially of minute flake-shaped particles. These flakes in the montmorillonites are relatively small and thin; the kaolinite flakes have a wide range in size and shape, but on the average are larger than those of the montmorillonites. Most of the illite flakes are larger than the average kaolinite flakes, and on working with water have a greater tendency to remain in aggregates than flakes of either montmorillonite or kaolinite. On working with water the water film between the clay particles acts as a lubricant and bond, producing the plasticity which is the physical property most characteristic of clays. The clays made up of relatively smaller and thinner flakes also possess a greater total surface, and will therefore retain a greater amount of water and have greater plasticity and bond strength than those made up of larger, thicker flakes. Ion exchange capacity and the presence of soluble salts also affect these properties.
The effects produced by heating or firing clays depend on the mineralogical changes produced by increasing temperatures and the formation of a glassy phase. Kaolinite, consisting only of alumina and silica, above 600° or 700° C. is relatively refractory. Montmorillonite, which usually contains fluxes such as magnesium within the molecule and calcium and sodium as adsorbed ions, is much less refractory than kaolinite. The illites, which contain magnesium, potassium, and iron, are usually the least refractory; quartz acts as a flux to kaolinite at high temperature; and feldspar, muscovite, and iron compounds are also fluxing materials at somewhat lower temperatures. The relation of the individual properties of the various clay minerals to the green or unfired characteristics of the clays is discussed in some detail by Grim (1939, 1939a) and Grim and Bradley (1940).
Those familiar with ceramic tests are well aware that the various properties revealed by the tests are closely interrelated. If the green or unfired properties of the four clays are compared (Table 11) it will be noted that a clay with a low percentage water of plasticity (EL-60-6 for example) has also a low percentage of shrinkage water and low volume and linear shrinkages. A clay such as O-5-6 having a high percentage of water of plasticity has proportionately high percentages shrinkage water and volume and linear shrinkages. The pore water, on the other hand, does not correlate perfectly with the other properties, although the tendency is in the direction of correlation.
The fired properties of clays are also interrelated (Table 11, Figs. 11-15). With increasing temperatures clays normally show increased shrinkage. As the clays shrink the open pores decrease proportionately, thus decreasing absorption at a rate corresponding to the rate of shrinkage. The green or unfired properties of the clays can be considered as one unit, and the fired properties as another but related unit; therefore, the comparison of ceramic data with the more fundamental properties is simplified.
Relation of Unfired Properties to Specific Surface
The plastic and dry, or unfired properties of a clay are directly related to its specific surface. Specific surface, expressed as square meters of surface per gram of sample, should be considered a composite of two properties of a clay: mean diameter of the clay particles and the shape of the clay particles. As stated above, the plasticity of clay is produced by the water film between the clay particles. Drying the plastic clay removes the water film and at a certain point in the drying process the clay particles touch. The amount of water removed up to that point is the shrinkage water. The remainder of the water used to produce plasticity is held in the pores or interstices formed by the imperfect orientation and "fit" of the clay particles. This is given as percentage pore water in Table 11. Volume and linear shrinkage, which are by definition completely correlative, occur only while the shrinkage water is being removed by drying.
The relation of specific surface to the unfired properties of the four clays is shown graphically in Figure 16. The volume shrinkage and shrinkage water are shown to be a linear function of specific surface for the four clays studied, and the water of plasticity shows a close correlation. Harman and Fraulini (1940) have shown that the shrinkage of kaolinite is a linear function of base-exchange capacity. Thus with clays composed dominantly of kaolinite, or kaolinite and quartz, the volume shrinkage on drying or the shrinkage water can be used as an indication of specific surface and base-exchange capacity. Clays such as EL-69-2, O-5-6, and O-38-4 would not show complete correlation between specific surface and base-exchange capacity because of the presence of the illite group of clay minerals and the montmorillonites and because of the considerable variation in these clay minerals, especially those produced by differences in exchangeable cations present. For these three clays the correlation should be fairly good, however, because of general similarity and the dominance of kaolinite.
Fig. 16-Graph showing correlation of specific surface to plastic and drying properties of the four clays.
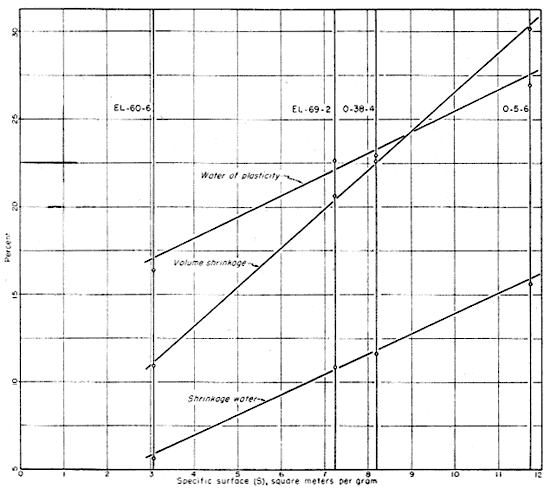
Whitaker (1939) has shown in his work with kaolinite that plasticity is directly related to total surface and inversely related to particle size. He expressed the relation of plasticity to specific surface in the formula: log S = AP + log B, where S equals square centimeters of surface area per 100 grams of clay; P equals the product of yield and strain; and A and B are constants. He has also shown that drying shrinkage increases, in general, with decreasing particle size, and that drying strength also increases with decreasing particle size until a maximum is reached. Norton (1942) also commented on the influence of particle size and shape, the types of clay minerals, and the soluble salts and adsorbed ions on the plastic and drying properties of clays.
Effect of Chemical and Mineral Composition on Fired Properties
In the temperature range within which shrinkage occurs in clays, particle size and shape have little influence on the fired properties. Within this range increased shrinkage and decreased porosity with increasing temperature are caused by the formation of new minerals, and the ultimate formation of a glassy phase due to the fluxing action of some of the components of the clay minerals and to accessory minerals such as limonite, hematite, calcite, and dolomite. Minerals of the illite and montmorillonite groups contain ferric iron replacing the aluminum, and alkalies and alkali earths either within the lattice or as adsorbed ions (Grim and Bradley, 1940). Within the lower temperature ranges K2O and Na2O are the most active fluxes. Calcium, magnesium, and iron oxides are active at somewhat higher temperatures, although in combination with the alkalies they are quite active at relatively low temperatures.
In Figure 17 the percentage volume shrinkage between cone 05 and cone 8 (about 1030° to 1225° C.) has been plotted against the percentages of fluxes contained in the clays and the molecular ratios of the fluxes to the alumina (Table 17). The cone 05 to cone 8 range was chosen because shrinkage calculated from dry size is deceptive. Some of the shrinkage in the early stages of heating reflects particle size, shape, and packing rather than chemical fluxing action. Cone 8 was used as the top temperature because abnormal bloating occurred in some of the clays at cone 10. Figure 17 shows a general correlation between the amount of fluxes present and volume shrinkage on firing. The total percentage of alkalies plotted against volume shrinkage results in a curve which is shown in relation to a segment of an ellipse. Whether or not the shape of the curve has any significance is not known, but there is evidence that the rate of increase in volume shrinkage declines with increasing content of the alkalies. This same type of deviation occurs for the total of CaO, MgO, K2O and Na2O, although the relation is less clear. With Fe2O3 included with the fluxes, the deviations are even more pronounced, although the general relation is still clear.
Fig. 17--Graph showing correlation between fired volume shrinkage and fluxes.
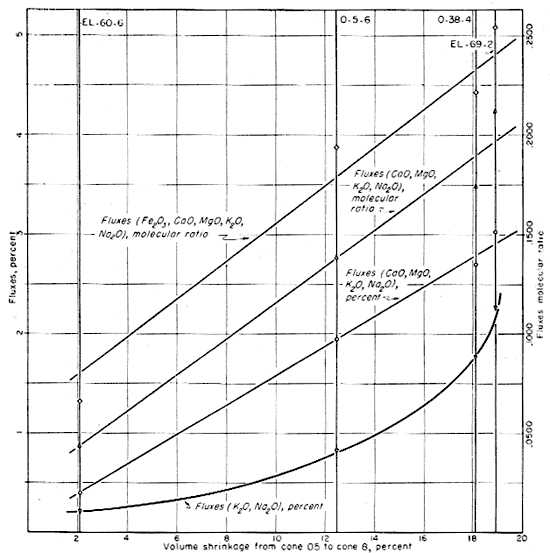
Table 17--Empirical molecular formulas of the four clays, with alumina calculated to unity.
| RO | R2O3 | RO2 |
|---|---|---|
| EL-60-6 | ||
| 0.0034 K2O | ||
| 0.0046 Na2O | 1.0000 Al2O3 | 6.9863 SiO2 |
| 0.0143 CaO | 0.0235 Fe2O3 | 0.0893 TiO2 |
| 0.0212 MgO | 2.0596 H2O | |
| EL-69-2 | ||
| 0.1022 K2O | ||
| 0.0127 Na2O | 1.0000 Al2O3 | 5.5769 SiO2 |
| 0.0209 CaO | 0.0428 Fe2O3 | 0.0613 TiO2 |
| 0.0784 MgO | ||
| 1.8101 H2O | ||
| O-5-6 | ||
| 0.0319 K2O | ||
| 0.0101 Na2O | 1.0000 Al2O3 | 4.3452 SiO2 |
| 0.0403 CaO | 0.0567 Fe2O3 | 0.0571 TiO2 |
| 0.0563 MgO | ||
| 1.9601 H2O | ||
| O-38-4 | ||
| 0.0723 K2O | ||
| 0.0137 Na2O | 1.0000 Al2O3 | 4.5720 SiO2 |
| 0.0214 CaO | 0.0406 Fe2O3 | 0.0688 TiO2 |
| 0.0667 MgO | ||
| 1.7392 H2O | ||
Prev Page--Ceramic Properties || Next Page--Conclusions
Kansas Geological Survey, Geology
Placed on web June 22, 2007; originally published Dec. 1954.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/109_10/07_corr.html