Prev Page--Petrography || Next Page--Correlation
Ceramic Properties
Methods of Testing
Standard or Routine Tests
The procedure and methods used in this investigation followed as closely as was considered advisable the standards recommended by the Committee on Standards of the American Ceramic Society (Watts and others, 1928) and American Society for Testing Materials (1952) standard methods of testing for apparent porosity, water absorption, apparent specific gravity, and bulk density of burned refractory brick (A.S.T.M. designation C 20-46); water absorptions and saturation coefficient (A.S.T.M. designation C 67-50); and pyrometric cone equivalent (A.S.T.M. designation C 24-46).
The amount of water, determined by previous tests, required for optimum workability was mixed with the minus 20-mesh dry clay and the mixture stored for several hours in closed containers. After this the clay was thoroughly mixed and wedged.
Test bars approximately 1 1/8 inches square in cross section and 7 inches long were hand-molded from the prepared clay. The bars were marked immediately with a 14 cm tram (for linear shrinkage measurements) and weighed both in air and suspended in kerosene to determine plastic weights and volumes. After the test bars were completely dried they were again measured and weighed in air and suspended in kerosene to determine dry weights and volume. From these plastic and dry data, water of plasticity, shrinkage water, pore water, volume shrinkage, and linear shrinkage were calculated.
Because test bars from all four clays were fired together, the effects of heat treatment are completely comparable. The bars were fired at a slow rate in a Globar-heated electric furnace. At the end of the firings the kiln temperature was held at near the maximum for at least 1 hour so that the pyrometric cones were "soaked down" and uniform penetration of heat was assured.
The test bars were fired to five temperatures, cone 05 (approximately 1030° C.), cone 02 (approximately 1095° C.), cone 4 (approximately 1165° C.), cone 8 (approximately 1225° C.), and cone 10 (approximately 1260° C). After each firing the test bars were weighed, then saturated in cold water for 24 hours and again weighed to determine the cold-water absorption. The same procedure was repeated after immersion in boiling water for 5 hours, then cooling, to determine the boiling-water absorption. Volumes were determined from the difference between the weight of the saturated test bar in air and suspended in water. Measurements of linear shrinkage were also made. From the above data linear and volume shrinkages, absorptions, saturation coefficient, apparent porosity, apparent specific gravity, bulk specific gravity, and ignition loss were determined. The saturation coefficient is the ratio of absorption determined by 24-hour submersion in cold water to that after 5 hours submersion in boiling water. The saturation coefficient is a more reliable index to the resistance of burned clay products to freezing and thawing and other weathering agents than any other method that has been devised.
A separate and independent test was run on all four clays as a check on the accuracy of the first series. The test bars were fired to cone 4 only, however,
Refractories Tests
Standard 9 by 4 1/2 by 2 1/2-inch fire-clay bricks were prepared from clays EL-60-6 and O-5-6 by the dry press method using 2,000 psi pressure in forming the bricks. Carefully graded grog (calcined and crushed clay) was mixed with the plastic clay. In the preparation of the EL-60-6 batch 70 percent grog was used, and in the O-5-6 batch 80 percent grog was used. The bricks were fired to cone 13 and cone 14 in a down-draft, gas-fired kiln.
pH and Viscosity
Both the pH and the viscosity of the four clays and of Champion and Challenger ball clay from Tennessee were determined on suspensions containing 100 grams of clay and 250 grams of distilled water. The clay suspensions were thoroughly agitated with an electric stirrer. The pH values were determined with a Beckman Model H2 glass electrode pH meter. Apparent viscosities were determined with a Brookfield Model LVF Synco-Lectric viscosimeter (Oliver, 1948). The same suspensions were used for both determinations.
Ceramic Data
Standard or Routine Tests
The data on ceramic tests of these clays (Table 11) show an obvious relationship to the properties of the clays observed in the field and during the process of preparing the test bars. Clay EL-60-6 is a coarse-grained, "lean," relatively nonplastic clay, whereas clay O-5-6 is a fine-grained, "fat" highly plastic clay. Clays EL-69-2 and O-38-4 are also fine-grained, plastic clays, but less so than clay O-5-6. These two clays are very much alike in this respect, but O-38-4 is slightly more plastic than EL-69-2 and EL-69-2 is the coarser of the two. The water of plasticity, shrinkage water, and volume shrinkage on drying accurately reflect these differences. The "fatter," finer grained, and more plastic the clay the higher the water of plasticity, shrinkage water, and volume shrinkage. The order from lean to fat, and from low to high percentages for the above properties is EL-60-6, EL-69-2, O-38-4, and O-5-6. These are empirical characteristics universally observed by workers in clay, but which can be verified by more scientific means. "Plasticity" is a property of clays that is not only difficult to measure accurately, but is in fact incompletely defined. Particle size, however, is a measurable quantity. Specific surface is also measurable and is definitely related to particle size and shape and to the plasticity, or perhaps more accurately the workability of the clays. This relation will be discussed in a separate section.
Table 11--Data on standard ceramic tests of the four clays investigated
| EL-60-6 | EL-69-2 | O-5-6 | O-38-4 | ||
|---|---|---|---|---|---|
| Plastic and Dry Properties | |||||
| Water of plasticity, percent | 16.31 | 22.65 | 26.94 | 22.89 | |
| Shrinkage water, percent | 5.63 | 10.85 | 15.55 | 11.62 | |
| Pore water, percent | 10.68 | 11.80 | 11.39 | 11.27 | |
| Volume shrinkage, percent | 10.91 | 20.61 | 30.19 | 22.55 | |
| Linear shrinkage (measured), percent | 3.51 | 5.90 | 8.08 | 5.33 | |
| Linear shrinkage (calculated from vol. shrink.), percent | 3.51 | 6.44 | 9.20 | 7.02 | |
| Fired Properties | |||||
| Pyrometric cone equivalent | 28-29 | 28-29 | 30 | 27 | |
| Fired color at | |||||
| Cone 05 (1030° C.) | Ivory | Light buff | Light buff | Cream | |
| Cone 02 (1095° C.) | Nearly white | Cream | Dark cream | Dark cream | |
| Cone 4 (1165° C.) | Off white | Cream | Light buff | Dark cream | |
| Cone 8 (1225° C.) | Off white | Dark cream | Dark cream | Yellow cream | |
| Cone 10 (1260° C.) | Off white | Light gray | Yellow tan | Yellow gray | |
| Cone 16 (1450° C.) | Light cream | ||||
| Volume shrinkage, percent at | |||||
| Cone 05 | 1.07 | 2.46 | 6.90 | 5.78 | |
| Cone 02 | 1.15 | 8.67 | 11.54 | 12.54 | |
| Cone 4 | 1.46 | 15.79 | 18.28 | 19.19 | |
| Cone 8 | 3.17 | 21.35 | 19.37 | 23.92 | |
| Cone 10 | 4.39 | 19.59 | 21.20 | 23.60 | |
| Cone 16 | 2.22 | ||||
| Linear shrinkage (measured), percent at | |||||
| Cone 05 | 0.09 | 0.57 | 1.73 | 1.50 | |
| Cone 02 | 0.00 | 2.64 | 3.33 | 3.70 | |
| Cone 4 | 0.37 | 4.84 | 5.58 | 5.83 | |
| Cone 8 | 0.93 | 6.16 | 5.70 | 6.90 | |
| Cone 10 | 1.11 | 5.59 | 6.41 | 6.77 | |
| Cone 16 | 0.37 | ||||
| Linear shrinkage (calculated from vol. shrink.), percent at | |||||
| Cone 05 | 0.36 | 0.83 | 2.36 | 1.97 | |
| Cone 02 | 0.39 | 2.98 | 4.00 | 4.37 | |
| Cone 4 | 0.49 | 5.57 | 6.51 | 6.86 | |
| Cone 8 | 1.07 | 7.69 | 6.93 | 8.71 | |
| Cone 10 | 1.49 | 7.01 | 7.64 | 8.58 | |
| Cone 16 | 0.75 | ||||
| Absorption (24 hours cold water), percent at | |||||
| Cone 05 | 14.85 | 13.74 | 13.27 | 12.95 | |
| Cone 02 | 14.96 | 10.30 | 10.59 | 9.28 | |
| Cone 4 | 14.49 | 5.95 | 5.75 | 4.56 | |
| Cone 8 | 13.43 | 0.83 | 2.62 | 0.02 | |
| Cone 10 | 13.04 | 0.91 | 0.52 | 0.02 | |
| Cone 16 | 11.79 | ||||
| Absorption (5 hours boiling water), percent at | |||||
| Cone 05 | 16.92 | 14.41 | 14.06 | 13.66 | |
| Cone 02 | 17.08 | 10.93 | 11.10 | 9.78 | |
| Cone 4 | 16.74 | 6.17 | 5.97 | 4.85 | |
| Cone 8 | 15.87 | 1.20 | 3.20 | 0.15 | |
| Cone 10 | 15.35 | 2.17 | 1.02 | 0.14 | |
| Cone 16 | 14.97 | ||||
| Saturation coefficient at | |||||
| Cone 05 | 0.88 | 0.95 | 0.94 | 0.95 | |
| Cone 02 | O.,Q8 | 0.94 | 0.95 | 0.95 | |
| Cone 4 | 0.87 | 0.96 | 0.96 | 0.94 | |
| Cone 8 | 0.85 | 0.69 | 0.82 | 0.13 | |
| Cone 10 | 0.85 | 0.42 | 0.51 | 0.20 | |
| Cone 16 | 0.79 | ||||
| Apparent porosity, percent at | |||||
| Cone 05 | 30.97 | 26.51 | 26.71 | 26.23 | |
| Cone 02 | 21.26 | 21.42 | 22.20 | 20.15 | |
| Cone 4 | 30.80 | 13.08 | 12.90 | 10.82 | |
| Cone 8 | 29.84 | 2.72 | 7.01 | 0.35 | |
| Cone 10 | 29.01 | 4.71 | 2.30 | 0.03 | |
| Cone 16 | 27.70 | ||||
| A. Apparent specific gravity; B. Bulk specific gravity at | |||||
| Cone 05 A. | 2.65 | 2.50 | 2.59 | 2.60 | |
| Cone 05 B. | 1.83 | 1.84 | 1.90 | 1.92 | |
| Cone 02 A. | 2.66 | 2.49 | 2.57 | 2.58 | |
| Cone 02 B. | 1.83 | 1.96 | 2.00 | 2.06 | |
| Cone 4 A. | 2.65 | 2.44 | 2.48 | 2.50 | |
| Cone 4 B. | 1.84 | 2.12 | 2.16 | 2.23 | |
| Cone 8 A. | 2.68 | 2.33 | 2.36 | 2.37 | |
| Cone 8 B. | 1.88 | 2.27 | 2.19 | 2.36 | |
| Cone 10 A. | 2.66 | 2.28 | 2.30 | 2.36 | |
| Cone 10 B. | 1.89 | 2.17 | 2.25 | 2.35 | |
| Cone 16 A. | 2.56 | ||||
| Cone 16 B. | 1.85 | ||||
| Hardness as to steel at | |||||
| Cone 05 | Softer | Softer | Softer | Softer | |
| Cone 02 | Softer | Softer | As hard | As hard | |
| Cone 4 | Softer | Harder | Harder | Harder | |
| Cone 8 | Softer | Harder | Harder | Harder | |
| Cone 10 | Softer | Harder | Harder | Harder | |
| Cone 16 | Softer | ||||
The differences noted above are also reflected in the shrinkages and absorptions of the clays when fired to cone 05, but above this temperature other factors such as the fusibility of the clay become increasingly effective. The higher temperature characteristics of the clays will be discussed in relation to the chemical composition in the section on interpretation and correlation. Although the pyrometric cone equivalent of clay EL-60-6 (cone 28-29) is slightly lower than that for clay O-5-6 (cone 30), within the range of the ceramic tests the fine-grained O-5-6 clay is affected more by increasing temperatures than the coarse-grained EL-60-6 clay (Table 11). The same relation can be observed in the fired data on the other two clays, but it is less obvious. The causes for these differences are complex and will be discussed in the last section, but it should be noted in connection with the presentation of the ceramic data that coarse-grained clays like EL-60-6, made up almost entirely of pure kaolinite and pure quartz and containing a very small percentage of fluxes, shrink in firing and show a decreasing porosity largely because the quartz-kaolin proportion approaches a eutectic. Clay O-5-6, on the other hand, contains a much larger percentage of the fluxes such as calcium, magnesium, and the alkalies, but is more refractory because it contains a higher percentage of kaolinite. At the comparatively low temperatures used in these tests the fluxes were active in clay O-5-6 and produced incipient fusion, shrinkage, and decreased porosity. The reaction between the relatively nonrefractory mixture of quartz and kaolinite in clay EL-60-6 produces decided fluxing action only at much higher temperatures.
The fired properties of the four clays are shown graphically in Figures 11 to 15. The relation of the various properties is much more obvious in the graphic representation than it is in Table 11. The curves for the shrinkage characteristics are plotted in reverse in order to facilitate comparison with absorption and porosity. It will be noted that the general trends of all fired properties of each clay are comparable, showing that they are in fact interrelated properties. In Figure 15 the apparent porosity percentages of all four clays are shown graphically. The apparent porosity was chosen from the various fired characteristics because it is calculated from both the absorption and the volume shrinkage and therefore is representative of all the fired properties of each clay. It will be noted that clays EL-69-2, O-5-6, and O-38-4 are very similar, and that EL-60-6 is in a class by itself. This difference is apparent in all the work that was done on these four clays.
Fig. 11--Graph showing changes in fired properties with increasing temperature for clay EL-60-6.
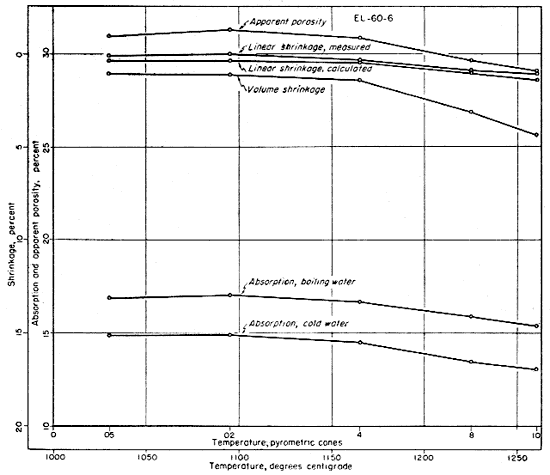
Fig. 12--Graph showing changes in fired properties with increasing temperature for clay EL-69-2.
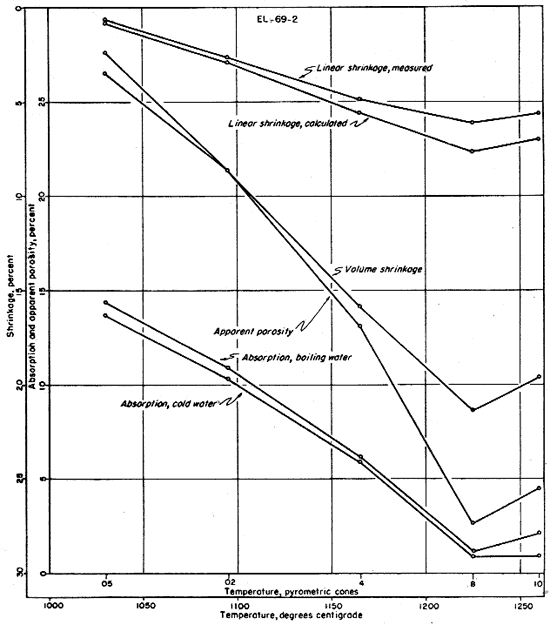
Fig. 13--Graph showing changes in fired properties with increasing temperature for clay O-5-6.
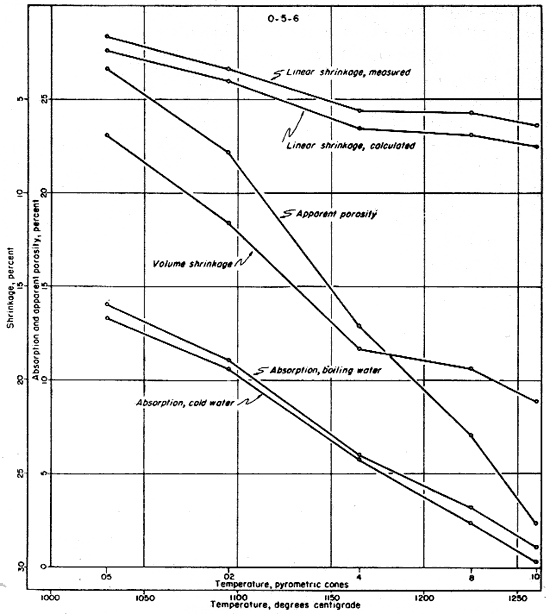
Fig. 14--Graph showing changes in fired properties with increasing temperature for clay O-38-4.
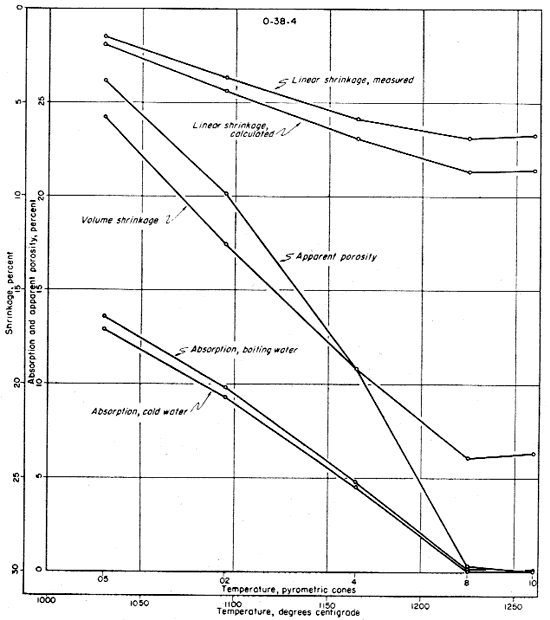
Fig. 15--Graph showing changes in porosity with increasing temperature for the four clays.
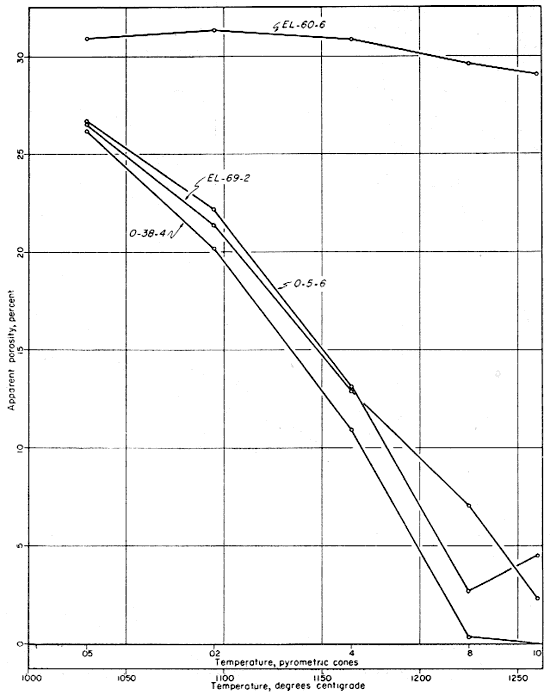
The ceramic test data on clays EL-69-2 and O-38-4 indicate a fairly uniform change in fired properties with increasing temperature up to cone 8. At this temperature clay O-38-4 is almost completely vitrified and clay EL-69-2 is nearly vitrified. At cone 10 the data on both of these clays indicate slight overfiring whereas clay O-5-6 is approaching vitrification at that temperature but is not overfired. The data for clay EL-60-6 produce a quite different pattern (Fig. 11) with only slight differences in fired properties at the temperatures shown. Clay EL-60-6 was fired to cone 16 (about 1450° C.) although the others were fired no higher than cone 10. At cone 16 the EL-60-6 test bars expanded slightly, but were slightly less porous than they were at cone 10, indicating that a decided decrease in porosity would be attained at a considerably higher temperature.
In addition to a separate run on all four clays including a cone 4 firing only, another separate run was fired to cone 10 only. Data on this test indicate that the evidence of overfiring shown in Table 11 and in Figures 12 and 15 was the result of the conditions of firing. The check run was fired very slowly, with the result that shrinkages increased and absorptions and porosity decreased from the cone 8 to the cone 10 temperature. In the graphic representation of the data for clay EL-69-2 (Fig. 12) the upturn from cone 8 to cone 10 would have been represented by a decrease in the rate of change if the check data had been used.
Partial ceramic tests were also run on the minus 2-micron fractions of all four clays. This material is not only fine-grained, but consists almost entirely of clay minerals. As should be expected the percent water of plasticity was much higher on these tests. The linear drying shrinkage, however, is decidedly higher for the finer fraction only for clay EL-60-6. The shrinkage on the fine fraction of clay EL-69-2 is slightly higher, but on the fine fractions of clays O-5-6 and O-38-4 the shrinkage is lower. Firing shrinkages at cone 4-5 were much higher, and the absorptions indicated that the test bricks were near vitrification at that temperature. Test data on the minus 2-micron fractions are shown in Table 12.
Table 12--Ceramic data on the minus 2-micron fractions of the four clays.
| EL-60-6 | EL-69-2 | O-5-6 | O-38-4 | |
|---|---|---|---|---|
| Water of plasticity, percent | 36.67 | 45.95 | 31.97 | 33.75 |
| Linear drying shrinkage, percent | 5.82 | 6.95 | 6.38 | 6.38 |
| Firing shrinkage (cone 4-5), percent | 11.91 | 12.03 | 8.51 | 9.58 |
| Cold water absorption (cone 4-5), percent | 3.85 | 1.00 | 1.00 | 1.00 |
Refractories Tests
Standard 9 by 4 1/2 by 2 1/2 inch fire-brick shapes were made from clays EL-60-6 and O-5-6 containing 70 percent and 80 percent, respectively, of hard-fired grog. The bricks were fired in an A.S.T.M. standard reheat furnace to cone 13 and cone 14. Data on these are given in Table 13.
Table 13--Data on grogged refractory shapes.
| Sample no. | Boiling water absorption, percent | |
|---|---|---|
| Cone 13 | Cone 14 | |
| EL-60-6 | 22.80 | 22.71 |
| O-5-6 | 13.26 | 11.16 |
A characteristic of grogged clays is that they can be fired to much higher temperatures without bloating or other indications of overfiring than is possible with the ungrogged clay. These two clays show the same characteristics; however, the decrease in percentage absorption with increased temperature is much higher for clay O-5-6 than for clay EL-60-6.
pH and Apparent Viscosity Data
The pH and viscosity determinations are discussed as a unit because the tests were run on the same suspensions containing 100 grams of clay to 250 grams of water. The results of the tests are given in Table 14.
Table 14--pH and apparent viscosity of clay slips at various time intervals. C. & C. is Tennessee ball clay.
| Sample no. | pH | Apparent viscosity (centipoises) | |||
|---|---|---|---|---|---|
| Time elapsed after mixing | Time elapsed after mixing | ||||
| 15 minutes | 24 hours | 3 days | 4 hours | 3 days | |
| FL-60-6 | 3.98 | 3.S4 | 3.85 | 18 | 19 |
| EL-69-2 | 3.80 | 3.92 | 3.88 | 25 | 40 |
| O-5-6 | 4.20 | 4.30 | 4.40 | 36 | 40 |
| O-38-4 | 7.20 | 7.20 | 7.25 | 6 | 7 |
| C. & C. | 4.00 | 4.33 | 4.43 | 338 | |
The pH and apparent viscosity of the first three clays in Table 14 are rather uniform and more or less normal. Usually the pH of Dakota formation clays is somewhat higher. The much higher pH of clay O-38-4 is typical for freshly sampled Dakota formation clays, whereas the lower pH obtained on the preceding three is typical for samples that have been in storage for a long time. This explains the low values obtained on three of the clays, but O-38-4 has been in storage for an equal length of time so its pH is anomalous. The viscosities of the first three clays can be readily understood. Samples EL-69-2 and O-5-6 contain a relatively high percentage of fine-grained clay, whereas EL-60-6 has a higher quartz content, and the particle size of both the quartz and the clay mineral is coarse.
The low values obtained for apparent viscosity on clay O-38-4 can be explained by the fact that the pH is on the alkaline side. The extremely high apparent viscosity shown for the Champion and Challenger ball clay is difficult to explain in view of the normal pH obtained. This high viscosity is particularly puzzling in view of the fact that casting bodies containing this Tennessee ball clay are easily deflocculated by the addition of electrolytes. Under similar conditions clay O-38-4 is also easily deflocculated but tends to be thixotropic. Clay EL-60-6 deflocculates very easily, but the other two are not only difficult to deflocculate, but tend to be thixotropic as well.
Prev Page--Petrography || Next Page--Correlation
Kansas Geological Survey, Geology
Placed on web June 22, 2007; originally published Dec. 1954.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/109_10/06_ceram.html