The Science behind the Technology
P&T system
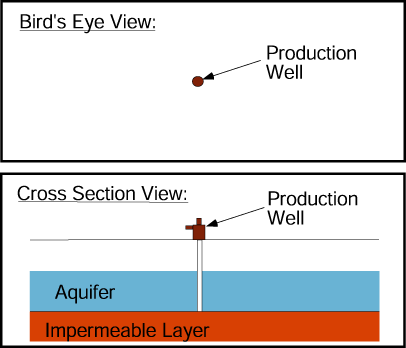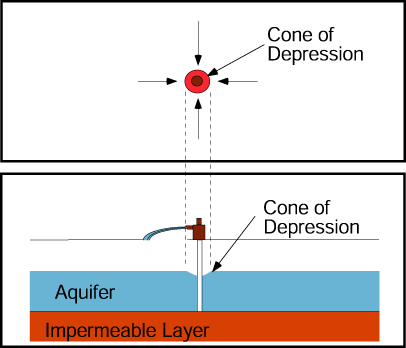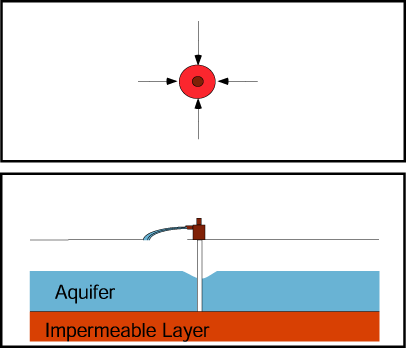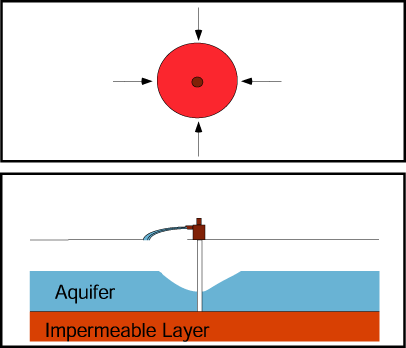
Pumping water from a production well lowers the water table in the aquifer in the well’s vicinity and produces a cone of depression.
.
Adding water to the aquifer through an injection well raises the water table in the well’s vicinity.
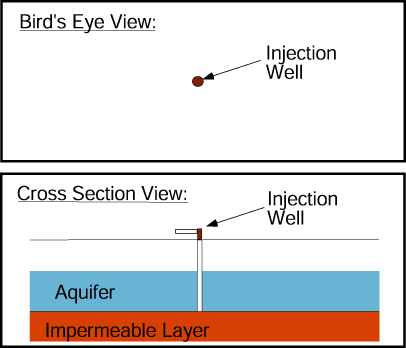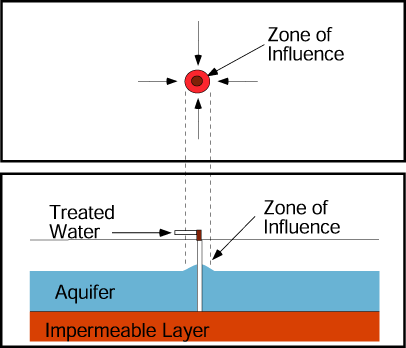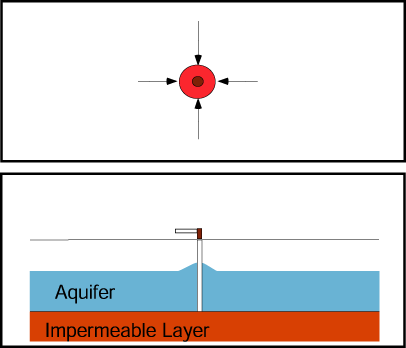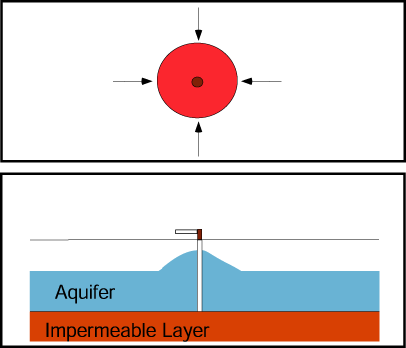
If a production well and nearby injection well operate simultaneously, they create a capture zone that includes the area around each well and is roughly elliptical in shape in a simple aquifer.
The plume of contaminated ground water is contained if the plume boundaries are contained within the capture zone.
Forager™ Sponge
Two types of absorbent sponge are available: one type treated with dithiocarbamate polymer and the other treated polyamine/polyamide group polymer.
Both sorbents have a special affinity for heavy metals. The polymers function by forming coordination complexes preferentially with ions of transition group heavy metals (those metals in Groups IB through VIIB of the Periodic Table of Elements). In comparison, metals, such as calcium, magnesium, or aluminum show poor affinity for the polymers.
The order of affinity of the polymer for metal and non-metal ions in solution is:
(Greatest affinity) Au+3 > Cu+2 > Cd+2 > Hg+2 > Pb+2 > Ni+2 > Mn+2 > Fe+3 > Co+2 > Zn+2 > Au(CN)-2 > SeO4-2 > AsO4-3 > CrO4-2 > UO4-2 > Ag+1 >> Al+3 > Mg+2 > Na+1,K+1,Ca+2, Cl-1 and SO4-2 (Least affinity). The ordering of ions on the list indicates that all to the left of the CrO4-2 ion will be more likely removed from the water to form these coordination complexes than will the CrO4-2 ion. Once these ions have been removed, then the CrO4-2 ion will be the most preferred of those on the list.
At saturation, the polymer will contain between about 20% (dry weight basis) of absorbed ions.