Prev Page--Ground-water Occurrence, Recharge, Discharge || Next Page--Formations
Ground Water
Utilization of Water
During this investigation, data on 226 wells in Rawlins County were obtained. All types of wells in all parts of the area were inventoried in order to get a representative tabulation of the wells in the county. Most of them were domestic or stock wells; 7 were municipal wells, 11 were irrigation wells, and 2 were railroad supply wells. Wells from which the pumping equipment had been removed and which did not seem to be in usable condition were classified as unused.
Domestic and Stock Supplies
Nearly all domestic and stock supplies in the county are obtained from wells. The domestic use of water generally includes drinking, cooking, washing, and in some cases, the disposal of sewage. Water supplies for those schools not served by public-supply systems are considered domestic. In general, ground water in Rawlins County, although hard, is suitable for most domestic and stock uses. In several small areas of Rawlins County, ground-water supplies are inadequate, and water for domestic use must be hauled.
Public Supplies
Three municipalities in Rawlins County obtain public water supplies from wells. Each municipal supply is described briefly in the following paragraphs. The geologic characteristics and the water-bearing properties of the aquifers are discussed in the section on geologic formations and their water-bearing properties.
Atwood
The city of Atwood in Beaver Creek valley obtains its water from two drilled wells near the water plant in the south part of town. Both wells extend into sand and gravel deposits underlying the Crete terrace surface upon which they are located. These wells are 65 feet deep and are cased with 12-inch steel casing equipped with a copper screen. They are equipped with turbine pumps powered by 40-horsepower electric motors. The static water level in each well is reported to be 40 feet below the land surface.
Water from the wells is pumped directly into the mains, the excess going into a 300,000-gallon concrete storage tank at the southeast corner of town. In August of 1952, the city of Atwood consumed about 14,900,000 gallons of water, which is the most ever used by the city up to that date. The chemical analysis of a composite sample of water from both wells is given in Table 3.
Herndon
The city of Herndon obtains its water supply from two drilled wells penetrating sand and gravel of the terrace upon which the city is located. Well 2-31-3aa, in the northeast part of town, has a reported depth of 69 feet and a static water level of 45 feet below the land surface. This well is cased with 10-inch steel casing and is equipped with a turbine pump powered by a 10-horsepower electric motor. Well 2-31-3db, at the city water plant, has a reported depth of 61 feet and a static water level of 43 feet below the land surface. This well is eased with 12-inch steel casing and has a bronze screen 18 feet long. It is equipped with a turbine pump and a 15-horsepower electric motor. The bottom of this well is on Pierre shale, and the well when first drilled was pumped at the rate of 500 gallons per minute for a period of 9 hours.
Water is pumped from the wells directly into the mains, the excess going into a 55,000-gallon elevated steel storage tank in the northwest part of town. The chemical analysis of a composite sample of water from both wells is given in Table 3.
McDonald
The city of McDonald obtains its water supply from a drilled well (3-36-21ba) near the water tower in the east part of town. This well is reported to be 302 feet deep and has a static water level of about 198 feet below the land surface. It is cased with 16-inch steel casing and is equipped with a turbine pump and a 40-horsepower electric motor. Two other wells near the city hall are maintained on a stand-by basis for emergency use. All three wells obtain water from the Ogallala formation. The chemical analysis of a sample of water from well 3-36-21ba is given in Table 3.
Water from the well is pumped directly into the mains, the excess going into a 50,000-gallon elevated steel storage tank. The average daily water consumption of McDonald is about 75,000 gallons.
Table 3--Analyses of water from typical wells in Rawlins County, Kansas Analyzed by Howard A. Stoltenberg. Dissolved constituents in parts per million. (One part per million is equivalent to one pound of substance per million pounds of water or 8.33 pounds per million gallons of water.)
| Well number |
Depth, feet |
Geologic source | Date of collection |
Temp. (°F) |
Dissolved solids |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium and potassium (Na+K) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) |
Hardness as CaCO3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Carbonate | Noncarbonate | ||||||||||||||||
| 1-31-7aa | 240.5 | Ogallala | 9-23-1952 | 60 | 281 | 43 | 0.06 | 36 | 14 | 39 | 244 | 14 | 9.0 | 0.7 | 4.9 | 148 | 148 | 0 |
| 1-31-36dd | 22.0 | Terrace deposits | 9-23-1952 | 55 | 495 | 31 | 0.10 | 88 | 23 | 56 | 337 | 69 | 59 | 0.6 | 2.6 | 314 | 276 | 38 |
| 1-33-8ad | 152.3 | Ogallala | 9-23-1952 | 60 | 301 | 43 | 1.5 | 37 | 15 | 42 | 232 | 21 | 13 | 1. 1 | 15 | 154 | 154 | 0 |
| 1-35-13bc | 148.7 | Ogallala | 9-23-1952 | 59 | 335 | 58 | 0.56 | 41 | 17 | 41 | 242 | 28 | 11 | 1.7 | 18 | 172 | 172 | 0 |
| 1-36-5dc | 37.3 | Terrace deposits | 9-23-1952 | 59 | 1,920 | 35 | 0.38 | 322 | 86 | 159 | 415 | 983 | 52 | 0.4 | 80 | 1,160 | 340 | 820 |
| 1-36-27cd | 87.7 | Ogallala | 9-23-1952 | 59 | 384 | 53 | 1.2 | 54 | 22 | 38 | 246 | 64 | 19 | 1.6 | 11 | 225 | 202 | 23 |
| 2-31-3aa and db1 | 61-69 | Terrace deposits | 5-1-1952 | 690 | 29 | 0.82 | 109 | 31 | 81 | 461 | 137 | 37 | 1.0 | 1.5 | 400 | 378 | 22 | |
| 2-32-5bb | 205.7 | Ogallala | 9-23-1952 | 60 | 308 | 46 | 1.0 | 38 | 18 | 40 | 262 | 17 | 10 | 1.1 | 8.4 | 169 | 169 | 0 |
| 2-35-31da | 117.1 | Ogallala | 9-24-1952 | 59 | 312 | 50 | 1.0 | 51 | 17 | 25 | 239 | 26 | 11 | 1.5 | 12 | 197 | 196 | 1 |
| 3-31-23bb | 88.5 | Ogallala | 9-23-1952 | 59 | 317 | 48 | 0.13 | 44 | 18 | 36 | 261 | 18 | 12 | 1.1 | 12 | 184 | 184 | 0 |
| 3-33-8db1 and 2 | 65 | Terrace deposits | 2-12-1953 | 710 | 32 | 0.06 | 111 | 35 | 93 | 498 | 131 | 44 | 1.6 | 14 | 421 | 408 | 13 | |
| 3-33-31bc | 165.5 | Ogallala | 9-25-1952 | 61 | 314 | 50 | 2.6 | 41 | 18 | 37 | 254 | 22 | 10 | 1.3 | 10 | 176 | 176 | 0 |
| 3-34-1bd1 and 2 | 45 | Terrace deposits | 9-20-1952 | 56 | 776 | 36 | 0.14 | 96 | 40 | 123 | 484 | 189 | 51 | 1.6 | 1.0 | 404 | 397 | 7 |
| 3-35-24cb | 50 | Terrace deposits | 9-20-1952 | 57 | 423 | 40 | 0.06 | 68 | 22 | 50 | 350 | 46 | 16 | 2.0 | 7.1 | 260 | 260 | 0 |
| 3-36-21ba | 302 | Ogallala | 5-28-1952 | 332 | 51 | 0.08 | 37 | 16 | 46 | 220 | 28 | 15 | 1.7 | 28 | 158 | 158 | 0 | |
| 4-31-15bb | 30 | Terrace deposits | 9-24-1952 | 54 | 501 | 35 | 1.5 | 78 | 22 | 75 | 454 | 49 | 16 | 1.0 | 0.7 | 285 | 285 | 0 |
| 4-32-5ca | 167.5 | Ogallala | 9-26-1952 | 59 | 297 | 49 | 0.82 | 47 | 18 | 26 | 264 | 12 | 8.0 | 1.2 | 6.2 | 191 | 191 | 0 |
| 5-31-23dd | 136.1 | Ogallala | 9-26-1952 | 59 | 313 | 46 | 0.10 | 46 | 19 | 30 | 238 | 29 | 16 | 1.3 | 8.8 | 193 | 193 | 0 |
| 5-33-12ad | 35 | Terrace deposits | 9-26-1952 | 539 | 41 | 0.14 | 84 | 31 | 67 | 470 | 60 | 22 | 1.1 | 1.0 | 337 | 337 | 0 | |
| 5-34-1bb | 128.0 | Ogallala | 9-25-1952 | 336 | 56 | 0.20 | 40 | 19 | 40 | 237 | 31 | 13 | 1.9 | 18 | 178 | 178 | 0 | |
| 5-36-32aa | 187.5 | Ogallala | 9-25-1952 | 61 | 286 | 44 | 2.9 | 38 | 15 | 35 | 220 | 25 | 9.0 | 2.0 | 10 | 156 | 156 | 0 |
Industrial Supplies
The Chicago, Burlington, and Quincy Railroad has two wells in Rawlins County that are used principally to provide water for filling locomotive boilers. Well 3-36-20ad, at McDonald, is a dug well 208 feet deep and about 16 feet in diameter. The well is walled with rock and equipped with a plunger pump powered by a kerosene engine. The average monthly pumpage from this well, which obtains water from the Ogallala formation, is about 90,000 gallons. At Herndon, the C. B. and Q. has a drilled well 40 feet deep, cased with 12-inch steel casing. It is equipped with a turbine pump and 5-horsepower electric motor. This well obtains water from terrace sand and gravel, but no information on yield or pumpage is available.
Irrigation Supplies
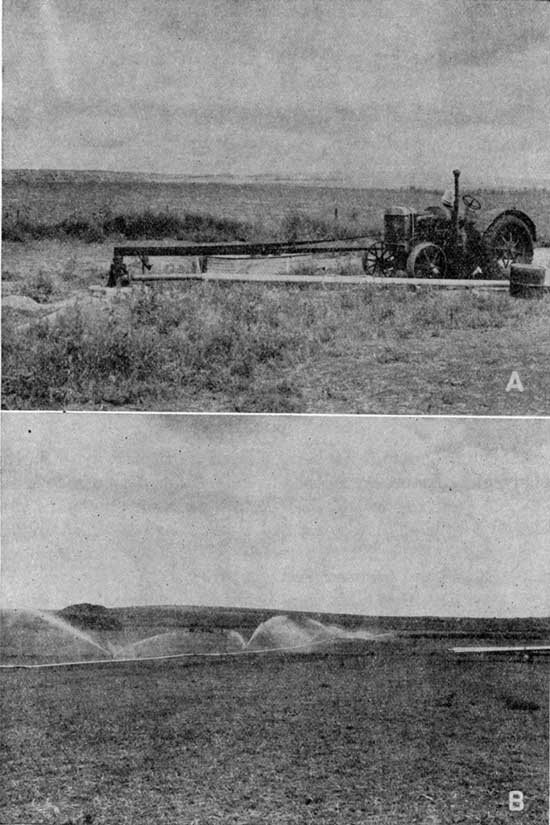
Although irrigation is not being carried on extensively in Rawlins County, eleven of the wells listed in Table 7 are classed as irrigation wells. Of these, one well (2-32-15cc) did not have a pump installed, and well 2-32-13ad, although it had a pump, had not been used for several years. Wells 3-33-8cd and 3-33-17ba were being drilled in April 1953 and had not been used when they were inventoried. The other seven irrigation wells were being used to irrigate an average of about 40 acres per well. Alfalfa, corn, and grass are the principal crops irrigated in Rawlins County. A typical irrigation well and sprinkler system are shown in Plate 3. All irrigation wells inventoried in Rawlins County were in valley areas, and their depths ranged from 29 to 84 feet. Static water levels ranged from about 10 to 42 feet below land surface. The chemical analysis of water from well 3-35-24cb and an analysis of a composite sample from wells 3-34-1bd1 and 3-34-1bd2 are given in Table 3.
Plate 3--A, Irrigation well 3-35-24cb in Little Beaver Creek valley; B, Irrigation sprinkling system in operation, NW SW sec. 24, T. 3 S., R. 35 W., applying 450 gallons of water per minute to grass.
Possibilities of Future Development of Irrigation Supplies
The economic feasibility of future development of irrigation supplies in Rawlins County depends upon the value of agricultural products compared to the cost of drilling and operating an irrigation well. The cost of drilling and operating an irrigation well depends to a large extent on the depth to water and the thickness of saturated material.
At the time of the field work for this report, all irrigation wells in the county were in the valleys of the major streams. Wells can be drilled and operated economically in the valleys because the depth to water generally is not more than 20 feet. Also wells in the valleys generally have large yields because of the high permeability of the water-bearing material.
A satisfactory irrigation supply probably can be developed anywhere in the valleys of Beaver Creek, Little Beaver Creek, South Sappa Creek, and Middle Sappa Creek where there is 30 feet or more of saturated material. The water-bearing material, in general, is most permeable in the valleys of Beaver Creek and Little Beaver Creek, and as a result, wells have larger yields from the same thickness of saturated material than wells in South and Middle Sappa Creek valleys. Wells in the valleys of Rawlins County obtaining water from alluvium and terrace deposits yield 400 to 750 gallons per minute.
The saturated-thickness map (Fig. 9) shows that about 5 townships in the south and west parts of Rawlins County have 100 feet or more of saturated thickness. If test drilling is done to locate the most permeable areas in the Ogallala formation, satisfactory irrigation wells probably can be developed in much of the upland areas of these townships. Yields from the Ogallala formation would probably be less than from the alluvium and terrace deposits, and the cost of drilling and operation would be greater because of the greater depth to water.
Chemical Character of Ground Water
The chemical character of ground water in Rawlins County is indicated by the analyses given in Tables 3 and 5, and shown graphically in Figure 10. The analyses were made by Howard Stoltenberg in the Water and Sewage Laboratory of the Kansas State Board of Health. Samples of water were collected for chemical analysis from 18 wells distributed as uniformly as possible within the county and representing the principal water-bearing formations of the area. Analyses of the water from three municipal supplies are also included. The effect of the geologic character of the aquifer upon the quality of water is discussed in the section on geologic formations and their water-bearing properties. The results of the analyses of the water samples shown in Table 3 are given in parts per million. Factors for converting parts per million of mineral constituents to equivalents per million (Fig. 10) are given in Table 4.
Figure 10--Graphic representation of analyses of water from the principal water-bearing formations in Rawlins County.
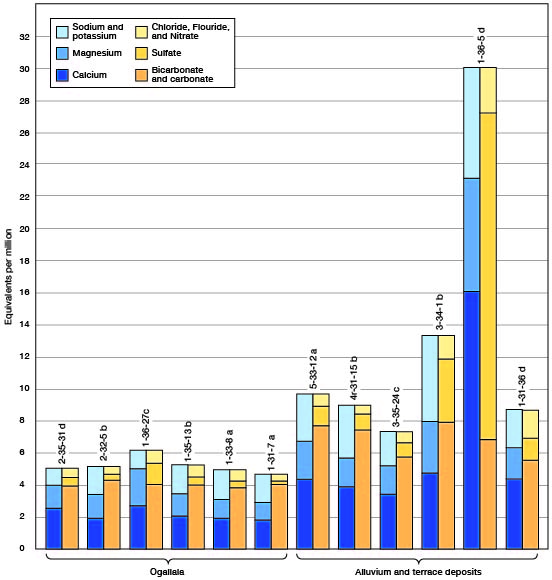
Table 4--Factors for converting parts per million of mineral constituents to equivalents per million
| Cation | Conversion Factor | Anion | Conversion Factor |
|---|---|---|---|
| Ca++ | 0.0499 | HCO3- | 0.0164 |
| Mg++ | 0.0822 | SO4-- | 0.0208 |
| Na+ | 0.0435 | Cl- | 0.0282 |
| NO3- | 0.0161 | ||
| F- | 0.0526 |
Table 5--Range in dissolved solids, hardness, fluoride, and nitrate in samples of water from typical wells in Rawlins County.
| Range in parts per million | Number of samples | |
|---|---|---|
| Ogallala formation |
Terrace deposits |
|
| Dissolved solids | ||
| 250-300 | 3 | 0 |
| 301-400 | 10 | 0 |
| 401-500 | 0 | 2 |
| 501-600 | 0 | 2 |
| 601-1,000 | 0 | 3 |
| More than 1,000 | 0 | 1 |
| Hardness | ||
| 100-150 | 1 | 0 |
| 151-200 | 11 | 0 |
| 201-250 | 1 | 0 |
| 251-300 | 0 | 2 |
| 301-400 | 0 | 3 |
| 401-600 | 0 | 2 |
| More than 600 | 0 | 1 |
| Fluoride | ||
| 0 0.5 | 0 | 1 |
| 0.6-1.0 | 1 | 3 |
| 1.1-1.5 | 7 | 1 |
| 1.6-2.0 | 5 | 3 |
| Nitrate (NO3) | ||
| 0-10 | 6 | 6 |
| 11-20 | 6 | 1 |
| 21-30 | 1 | 0 |
| 31-80 | 0 | 1 |
Chemical Constituents in Relation to Use
The following discussion of the chemical constituents of ground water has been adapted in part from publications of the Federal Geological Survey and the State Geological Survey of Kansas.
Dissolved Solids
When water is evaporated, the residue consists mainly of the mineral constituents given in the tables of analyses and generally includes a small quantity of organic material and some water of crystallization. Waters containing less than 500 parts per million of dissolved solids generally are satisfactory for domestic use except for difficulties resulting from their hardness or excessive iron content. Waters containing more than 1,000 parts per million are likely to include enough of certain constituents to cause a noticeable taste or to make the water unsuitable in some other respect.
The dissolved solids in 21 samples of water from Rawlins County ranged from 281 to 1,920 parts per million. Fifteen samples contained less than 500 parts per million, 5 samples contained between 500 and 1,000 parts per million, and only one sample contained more than 1,000 parts per million (Table 5).
Hardness
The hardness of water, which is the property that generally receives the most attention, is recognized most commonly by its effects when soap is used with the water. Calcium and magnesium cause almost all the hardness of ordinary waters. These constituents are also the active agents in the formation of the greatest part of the scale formed in steam boilers and other vessels used to heat or evaporate water.
In addition to the total hardness, the table of analyses shows the carbonate hardness and the noncarbonate hardness. The carbonate hardness is due to the presence of calcium and magnesium bicarbonates and can be removed almost completely by boiling. This type of hardness is sometimes called "temporary" hardness as opposed to "permanent" or noncarbonate hardness due to the presence of sulfates or chlorides of calcium and magnesium, which cannot be removed by boiling. With reference to use with soap, the carbonate hardness and noncarbonate hardness do not differ. In general, water with noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness of less than 50 parts per million is generally rated as soft, and treatment for the removal of hardness is not necessary under ordinary circumstances. Hardness between 50 and 150 parts per million does not interfere seriously with the use of water for most purposes, but the hardness does increase the amount of soap used, and its removal by a softening process is profitable for laundries and certain other industries. Water having a hardness in the upper part of this range will cause considerable scale in steam boilers. Hardness exceeding 150 parts per million is very noticeable, and if the hardness is 200 or 300 parts per million, water for household use is commonly softened. Where municipal water supplies are softened, an attempt generally is made to reduce the hardness to about 80 parts per million. The additional improvement from further softening of a public supply generally is not deemed worth the increase in cost.
The total hardness of 21 samples of water from Rawlins County ranged from 148 to 1,160 parts per million. Twelve samples had less than 200 parts per million total hardness, and 9 samples more than 200 parts per million. Only one sample had a noncarbonate hardness of more than 50 parts per million.
Iron
Next to hardness, iron is the constituent of natural water that generally receives the most attention. The quantity of iron in ground waters may differ greatly from place to place although the water may be derived from the same formation. If a water contains more than several tenths of a part per million of iron, most of the iron may settle out as a reddish precipitate. Iron, present in sufficient quantity to give a disagreeable taste and to stain cooking utensils and plumbing, may be removed from most water by aeration and filtration, but some water requires the addition of lime or some other substance.
The iron content of 21 samples of water from Rawlins County ranged from 0.06 to 2.9 parts per million. Six samples contained 0.1 part per million or less.
Fluoride
The fluoride content of drinking water is associated with the dental defect known as mottled enamel. Mottled enamel may appear on the teeth of children who drink water containing fluoride in excess of 1.5 parts per million during the period of formation of the permanent teeth. Concentrations of fluoride less than 1 part per million are known to prevent or lessen the incidence of tooth decay, and fluoride is now being added to some municipal supplies (Dean, 1938).
The fluoride content of samples of water from Rawlins County ranged from 0.4 to 2 parts per million. Of the 21 samples analyzed, 3 contained less than 1 part per million, 10 contained between 1 and 1.5 parts per million, and 8 contained more than 1.5 parts per million of fluoride.
Nitrate
The use of water containing an excessive amount of nitrate in the preparation of a baby's formula can cause cyanosis or oxygen starvation. Some authorities advocate that water containing more than 45 parts per million of nitrate should not be used in formula preparation (Metzler and Stoltenberg, 1950). Water containing 90 parts per million of nitrate generally is considered very dangerous to infants, and water containing 150 parts per million may cause severe cyanosis. Cyanosis is not produced in adults and older children by the concentrations of nitrate found in drinking water. Boiling of water containing excessive nitrate does not render it safe for use by infants; therefore, only water that is known to have low nitrate content should be used for infants.
The nitrate content of the water from some wells is somewhat seasonal, being highest in the winter and lowest in the summer (Metzler and Stoltenberg, 1950). In general, water from wells that are most susceptible to surface contamination is likely to be high in nitrate concentrations.
The nitrate content of water from wells sampled in Rawlins County ranged from 0.7 to 80 parts per million. Only one sample contained more than 45 parts per million of nitrate.
Sulfate
According to the U. S. Public Health Service drinking-water standards (1946), sulfate in water supplies used on interstate carriers should not exceed 250 parts per million. Water containing excessive amounts of sodium sulfate (Glauber's salt) or magnesium sulfate (Epsom salt) may have a laxative or other physiological effect and may be unsatisfactory for drinking.
The sulfate content of water samples from Rawlins County ranged from 12 to 983 parts per million. Only one sample contained more than 250 parts per million of sulfate.
Water for Irrigation
This discussion of the suitability of water for irrigation is adapted from Agriculture Handbook 60 of the U. S. Department of Agriculture.
Successful irrigation depends upon not only the supplying of irrigation water to the land but also the control of the saline and alkali conditions of the soil. Quality of irrigation water, irrigation practices, and drainage conditions are involved in salinity and alkali control. Soil that was originally nonsaline and nonalkali may become unproductive if excessive soluble salts or exchangeable sodium are allowed to accumulate because of improper irrigation and soil-management practices or inadequate drainage.
In areas of sufficient rainfall and ideal soil conditions the soluble salts originally present in the soil or added to the soil with water are carried downward by the water and ultimately reach the water table. This process of dissolving and transporting soluble salts by the downward movement of water through the soil is called "leaching." If the amount of water applied to the soil is not in excess of the amount needed by plants, there will be no downward percolation of water below the root zone and an accumulation of mineral matter will form at that point. Likewise impermeable soil zones near the surface can retard the downward movement of water, resulting in water-logging of the soil and deposition of salts. Unless drainage is adequate, attempts at leaching may not be successful, because leaching requires the free passage of water through and away from the root zone.
The characteristics of an irrigation water that seem to be most important in determining its quality are: (1) total concentration of soluble salts; (2) relative proportion of sodium to other cations (magnesium, calcium, and potassium); (3) concentration of boron or other elements that may be toxic; and (4) under some conditions, the bicarbonate concentration as related to the concentration of calcium plus magnesium.
The total concentration of soluble salts in irrigation water can be adequately expressed, for purposes of diagnosis and classification, in terms of electrical conductivity. Electrical conductivity is a measure of the ability of the inorganic salts in solution to conduct an electrical current, and is usually expressed in terms of micromhos per centimeter. The electrical conductivity can be determined in the laboratory, or an approximation of the electrical conductivity may be obtained by multiplying the total equivalents per million (Table 4) of calcium, sodium, magnesium, and potassium by 100, or by dividing the total dissolved solids in parts per million by 0.64. In general, waters with electrical conductivity values below 750 micromhos are satisfactory for irrigation insofar as salt content is concerned, although salt-sensitive crops such as strawberries, green beans, and red clover may be adversely affected by irrigation water having an electrical conductivity value in the range of 250 to 750 micromhos. Waters in the range of 750 to 2,250 micromhos are widely used, and satisfactory crop growth is obtained.under good management and favorable drainage conditions, but saline conditions will develop if leaching and drainage are inadequate. Use of waters with conductivity values above 2,250 micromhos is the exception, and very few projects can be cited where such waters have been used successfully.
In the past the relative proportion of sodium to other cations in irrigation water usually has been expressed simply as percent sodium. According to the U. S. Department of Agriculture, however, the sodium-adsorption ratio, used to express the relative activity of sodium ions in exchange reactions with soil, is a much better measure of the suitability of water for irrigation. The sodium-adsorption ratio may be determined by the formula
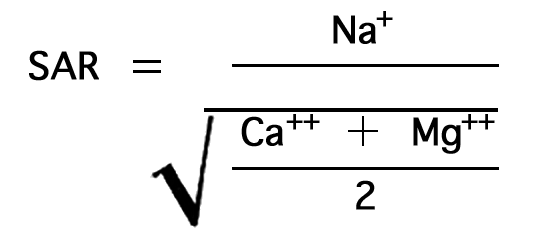
where the ionic concentrations are expressed in equivalents per million. The sodium-adsorption ratio may also be determined by use of the nomogram shown in Figure 11. In using the nomogram to determine the sodium-adsorption ratio of a water, the concentration of sodium expressed in equivalents per million is plotted on the left-hand scale, (A) and the concentration of calcium plus magnesium expressed in equivalents per million is plotted on the right-hand scale (B). The point at which a line connecting these two points intersects the sodium-adsorption-ratio scale (C) determines the sodium-adsorption ratio of the water. When the sodium-adsorption ratio and the electrical conductivity of a water are known, the suitability of the water for irrigation can be determined by graphically plotting these values on the diagram shown in Figure 12. Low-sodium water (S1) can be used for irrigation on almost all soils with little danger of the development of harmful levels of exchangeable sodium. Medium-sodium water (S2) will present an appreciable sodium hazard in certain fine-textured soils, especially under low-leaching conditions. This water may be safely used on coarse-textured or organic soils with good permeability. High-sodium water (S3) may produce harmful levels of exchangeable sodium in most soils and will require special soil management, such as good drainage, high leaching, and additions of organic matter. Very high sodium water (S4) is generally unsatisfactory for irrigation unless special action is taken, such as addition of gypsum to the soil.
Figure 11--Nomogram for determining the sodium-adsorption ratio of water.
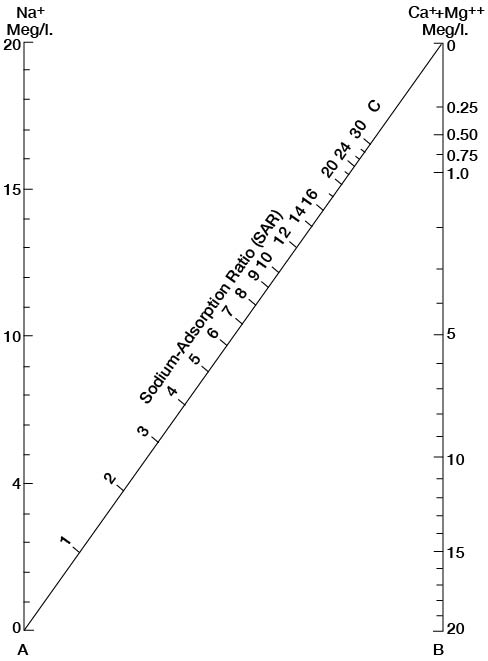
Figure 12--Diagram showing classification of water for irrigation use in Rawlins County.
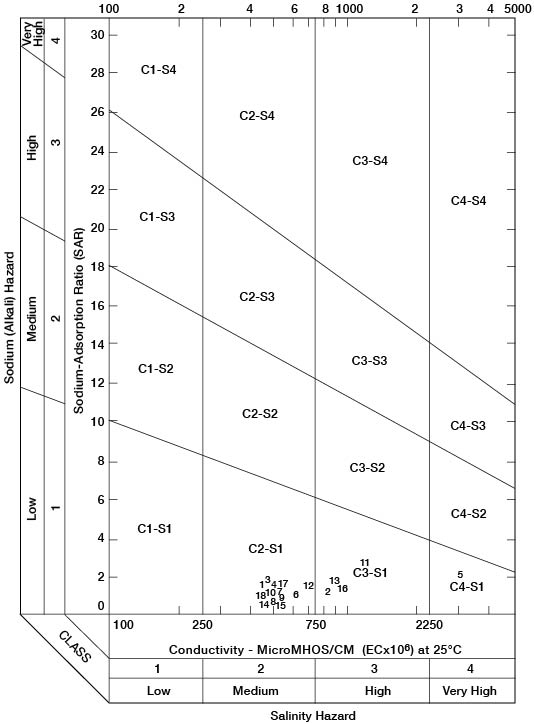
Low-salinity water (C1) can be used for irrigation of most crops on most soils with little likelihood that soil salinity will develop. Medium-salinity water (C2) can be used if a moderate amount of leaching occurs. Crops with moderate salt tolerances, such as potatoes, corn, wheat, oats, and alfalfa, can be irrigated with C2 water without special practices. High-salinity water (C3) cannot be used on soils with restricted drainage. Very high salinity water (C4) can be used only on certain crops and then only when special practices are followed.
Boron is essential to normal plant growth, but the quantity required is very small. Crops differ greatly in their boron tolerances, but in general it may be said that the ordinary field crops common to Kansas are not adversely affected by boron concentrations of less than one part per million. The boron content of water samples from Rawlins County was not determined; however, other investigations in the general area have not found excessive concentrations of boron in the water.
Prolonged use, under adverse conditions, of water having a high concentration of bicarbonate could have an undesirable effect upon the soil texture and plant growth. Ground water in Rawlins County (represented by analyses in Table 3) contained less than 1.25 equivalents per million "residual sodium carbonate" except for water from well 4-31-5bb, which contains 1.74 equivalents per million. Water containing more than 2.5 equivalents per million "residual sodium carbonate" is not suitable for irrigation; water containing 1.25 to 2.5 equivalents per million is marginal, and water containing less than 1.25 equivalents per million is probably safe.
Of the waters sampled in Rawlins County, only one (1-36-5dc) was entirely unsuitable for irrigation (Fig. 12). The high concentration of dissolved solids resulting in a very high salinity hazard in this water is probably due to mineralization of the water by the Pierre shale. Four of the samples collected, those from wells 1-31-36dd, 3-34-1bd1 and 2, 4-31-15bb, and 5-33-12ad, indicate a high salinity hazard and water from these wells should be used for irrigation only on soils having good drainage. The other 13 waters sampled are suitable for irrigation without special practices on most soils.
Table 6--Index numbers of water samples shown on Figure 12, and the sodium-adsorption ratio (SAR) and conductivity of the water samples. (Analyses are given in Table 3.)
| Well Number | Index number on Figure 12 |
SAR | Approximate conductivity |
|---|---|---|---|
| 1-31-7aa | 1 | 1.4 | 466 |
| 1-31-36dd | 2 | 1.4 | 872 |
| 1-33-8ad | 3 | 1.5 | 492 |
| 1-35-13bc | 4 | 1.4 | 524 |
| 1-36-5dc | 5 | 2.0 | 3,000 |
| 1-36-27cd | 6 | 1.1 | 616 |
| 2-31-3aa and db1 | 1.8 | 1,150 | |
| 2-32-5bb | 7 | 1.3 | 512 |
| 2-35-31da | 8 | 0.8 | 504 |
| 3-31-23bb | 9 | 1.2 | 525 |
| 3-33-8db1 and 2 | 2.0 | 1,250 | |
| 3-33-31bc | 10 | 1.2 | 514 |
| 3-34-1bd1 and 2 | 11 | 2.7 | 1,340 |
| 3-35-24cb | 12 | 1.3 | 737 |
| 3-36-21ba | 1.6 | 517 | |
| 4-31-15bb | 13 | 1.9 | 897 |
| 4-32-5ca | 14 | 0.8 | 497 |
| 5-31-23dd | 15 | 0.9 | 517 |
| 5-33-12ad | 16 | 1.6 | 965 |
| 5-34-1bb | 17 | 1.3 | 530 |
| 5-36-32aa | 18 | 1.2 | 465 |
Prev Page--Occurrence, Recharge, Discharge || Next Page--Formations
Kansas Geological Survey, Geology
Placed on web Nov. 17, 2008; originally published Dec. 1956.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Rawlins/05_gw.html