Kansas Geological Survey, Public Information Circular (PIC) 14
![]()
![]()
![]()
Kansas Geological Survey, Public Information Circular (PIC) 14
M. A. Townsend and D. P. Young,
Geohydrology, Kansas Geological Survey
A downloadable PDF version is available here
Ground water is the major source of drinking water for 70% of Kansas residents. In rural areas, 85% of the population relies on ground water. Therefore, contaminants that may cause health problems, such as nitrate, are of significant concern. For owners of private wells, the issue of ground-water contamination is particularly serious. Most private domestic supplies are neither tested nor treated on a routine basis.
Nitrate is the most common inorganic contaminant in Kansas ground water. Previous studies have found that about 30% of domestic wells in Kansas have nitrate levels greater than the Maximum Contaminant Level (MCL) for public drinking water (Steichen et al., 1988; Spalding and Exner, 1993; Townsend and Young, 1995; Townsend, 1996). Concentrations seem to be increasing in many areas of the state (Townsend et al., 1996; Townsend et al., 1997). The purpose of this circular is to describe nitrate, its sources, the extent of the nitrate problem in Kansas, and how ground water can be protected from nitrate contamination.
Nitrogen (N) is an important plant nutrient, absorbed primarily in the form of nitrate (NO3-). The other prominent forms of nitrogen are atmospheric nitrogen gas (N2), organic nitrogen, and ammonium (NH4+), the latter two of which can attach to soil particles. In the soil zone most forms of nitrogen will be converted to nitrate by bacterial processes; this conversion is termed nitrification (fig. 1).
Figure 1--Simplified illustration of the nitrogen cycle.
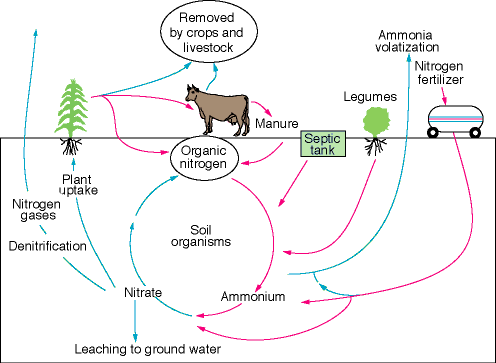
Nitrate readily dissolves in water and, once there, is hard to remove. Nitrate concentration in ground water is commonly reported as "nitrate as nitrogen" (nitrate-N)--that is, only the nitrogen in the nitrate molecule (NO3-) is counted. The U.S. Environmental Protection Agency (EPA) and the Kansas Department of Health and Environment (KDHE) set the MCL for drinking water at 10 milligrams/liter (mg/L) nitrate-N. Nitrate consumption can pose health risks. One of these, methemoglobinemia (or blue baby disease), is caused by bacterial conversion of nitrate to nitrite (NO2-) in the intestinal tract. Nitrite interferes with the oxygen-carrying capability of the blood and the victim appears "blue." This condition can be fatal both to human infants and to some young animals.
Background levels for natural nitrate-N in ground water are nearly always less than 3 mg/L (Madison and Brunett, 1984). Concentrations above 3 mg/L indicate that nitrate from non-natural sources such as human or animal waste or fertilizers has entered the ground water.
Kansas Geological Survey, Public Outreach
Web version July 1999
http://www.kgs.ku.edu/Publications/pic14/pic14_1.html