
Kansas Geological Survey, Mineral Resources Circular 5, originally published in 1937

Originally published in 1937 as Kansas Geological Survey Mineral Resources Circular 5. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (14 MB) is also available.
Rock wool is a tangled mass of very thin glassy fibers made by blowing air or steam through a stream of molten rock of proper composition. The blast of air or steam breaks the molten material into small droplets. These are propelled through the air and become drawn out into long thin threads which freeze into glass fibers. Chemically rock wool is composed of silica and lime in approximately equal proportions. Alumina may be present with the silica and magnesia with the lime, but neither of these two oxides is essential to the formation of rock wool.
The principal use of rock wool is a heat insulating material.
Glass wool has been made from slag for about sixty years (Fryling and White, 1935). The first commercial rock wool was made from a shaly limestone ("woolrock") in a plant in Alexandria, Indiana, in 1897. Over thirty plants including both slag and rock wool producers, are now in operation in the United States. The number of rock wool plants is increasing. At present the industry is mainly concentrated in three states: Indiana, Ohio, and Illinois. The only rock wool plants west of the Mississippi are in Texas and California. One slag wool plant has been erected at Joplin, Missouri.
The next stage in the manufacture of rock wool, which the industry is apparently entering, is the use of mixtures of rocks which will produce the proper proportions of oxides.
This investigation was initiated as a project of the Kansas Geological Survey, in the hope that it would lead to the establishment of a new mineral industry in Kansas. In April, 1936, before commencing the study, a trip was made by Prof. E. D. Kinney, Norman Plummer, and me to the experimental rock wool plant of the Illinois Geological Survey at Urbana, Illinois. Equipment, set-up, and technique were investigated. During the summer of 1936, Mr. Plummer collected samples from all of the promising rock formations in Kansas, which took him into almost all parts of the state. These samples were then tested in an experimental plant which was erected in Fowler Shops on the University of Kansas campus. The fall and winter months have been spent in testing materials and in the preparation of this report.
This investigation owes its success in no small part to the assistance and cordial cooperation of a number of individuals. The Kansas Geological Survey gratefully acknowledges the courtesy shown and the advice given by Dr. Morris M. Leighton, state geologist of Illinois, Dr. J. E. Lamar, geologist and head of the non-fuels division, Illinois Geological Survey, Dr. F. H. Reed, chief chemist, and Dr. Charles F. Fryling, chemist, likewise of the Illinois Geological Survey. The Department of Chemical Engineering at the University of Kansas loaned the electric furnace and other equipment used in the experimental plant, and in addition Prof. E. D. Kinney, chairman of the department, gave considerable time, and advice during the setting up of the experimental plant and the first testing operations. The Department of Machine Construction, through its chairman Prof. A. H. Sluss, permitted the experimental p1ant to be erected in its shops, and cooperated in other ways. Mr. V. M. Smith worked with Mr. Plummer during all of the sample testing and aided materially in the success of the project. Mr. Norman Plummer not only collected the samples and ran the tests, but also has written the following report.
This report will show that the resources of Kansas in raw materials suitable for making rock wool are practically unlimited. Some of the raw material is woolrock, which can be mined, melted, and blown without the addition of any other rock. Most of the Kansas raw material, however, must be mixed, just as the ingredients of cement are mixed, before the molten rock is blown. This step adds very little to the production cost.
The second item of cost is fuel. Coal coke is the fuel used in the Illinois-Indiana-Ohio plants. Kansas has an enormous supply and reserve of natural gas, which is piped into most parts of the state. Industrial consumers purchase this gas at costs as low as 10 and 11 cents per thousand cubic feet. Natural gas at this price is a much cheaper fuel than the coke delivered to the plants in the east.
Kansas has not as great a density of population as the states where the rock wool industry centers at present, but it nevertheless does have a large present and potential market for insulating material. The Kansas City metropolitan area and several parts of eastern Kansas are relatively congested and would afford a steady market. In addition, there are no rock wool plants at present in Missouri, Nebraska, Oklahoma, Colorado, and other near-by states, which may afford a market for Kansas rock wool. A steadily increasing number of new houses and buildings are using rock wool for insulation and older non-insulated houses constitute a very large potential market, for rock wool can be laid between the ceiling joists and blown between the studdings of houses already constructed.
Rock wool is so bulky that a small weight occupies a large space. Furthermore it cannot be packed in shipment to much depth, or crushing, with resultant breakage of the fibers and diminution of insulating ability, takes place. For these reasons only 12 tons of rock wool are loaded in a freight car. The freight rate from the established plants of the East to eastern Kansas points is in excess of $10 a ton. Local production of rock wool in Kansas would result in a great saving in transportation cost, which, if passed on to the consumer, would make insulation cheaper and thereby increase its use.
In conclusion, the prospects for one or more rock wool plants in Kansas are very favorable because of the abundance of raw materials, cheaper fuel costs compared with eastern plants, a much smaller transportation expense between plant and consumer, and the presence of a sufficient market. An ideal arrangement at the start, at least, would be for rock wool to be manufactured as a side line by a cement or brick plant. Such plants already possess: (1) quarries in rock that can be used for rock wool manufacture in most instances; (2) quarrying, crushing, and mixing equipment; (3) available gas at an industrial rate; and (4) a marketing organization in the field of building materials. For these reasons. samples were taken and tested during this investigation from all of the cement quarries and most of the clay pits in the state.
Figure 1--Index map of Kansas, showing location of outcrops sampled for testing for rock wool.
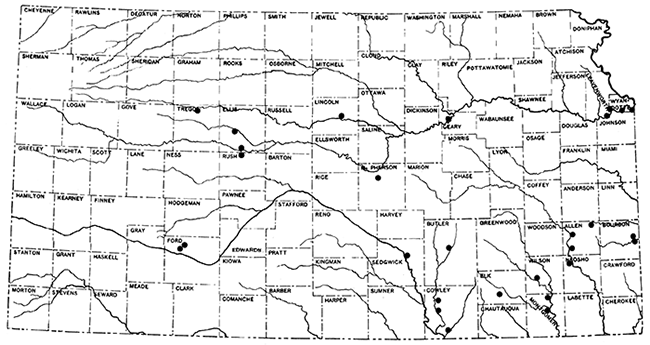
The first consideration in the choice of locations or materials to be tested for their suitability for rock wool production, was economic. This included the availability of cheap fuel, transportation, and a favorable marketing area. A large proportion of the rocks sampled are in or near the gas- and oil-producing areas of the state, and have marketing possibilities within the range of economical transportation. The choice of locations was further limited to the areas having available raw materials, and outcrops suitable for sampling. An attempt was made to include such materials as calcareous shales, and shaly limestones which have hitherto had little economic value, and which are, in the case of the calcareous shales, a handicap to the brick industry. Consideration in the choice of locations was also given to the fact that some established industries, such as cement plants and brick plants, have equipment and facilities which could be employed in the production of rock wool, and which at the same time could use a portion of the raw materials not suitable for their present products. The locations sampled extend from Ford and Trego counties in the western half of the state, to Montgomery, Bourbon, and Wyandotte on the east.
The stratigraphic range extends from the lowest formation of the Pennsylvanian, the Cherokee shale, upward through higher parts of the Pennsylvanian, and through the Permian, Cretaceous, Tertiary, and Quaternary rocks. The eastern Kansas area is limited in general to the Pennsylvanian formations, the central Kansas area to the Permian and the western Kansas area to the Cretaceous and Tertiary. Quaternary deposits, such as chert gravel, loess, and alluvium, were sampled in all three areas. The order of arrangement of both general and local areas as treated in this report follows approximately their position in the stratigraphic column. beginning with the earliest.
The choice of location for sampling in any area was limited to outcrops exposing a depth of rock sufficient for economical quarrying. If the samples which were to be included in rock wool tests for a single location were taken at different exposures, the outcrops had either to be fairly close together, or else the samples had to be stratigraphically continuous so that quarrying as a unit would be possible.
A representative sample was taken at the outcrop, each lithologic unit being sampled separately. Trench samples were taken of the shales (and other materials soft enough to admit of this method) by excavating a U-shaped trench of a uniform depth sufficient to expose unweathered rock through the entire vertical section of the unit to be sampled. A smaller trench of uniform depth inside the larger one was then excavated and the material saved. If large, the sample was quartered at the outcrop.
Limestones and other hard materials were chipped by the hammer from freshly exposed surfaces, and proportioned so that the sample was representative of the entire vertical distance included. Samples taken in this manner were not divided in the field. At some of the cement plants samples were taken of the crushed rock, usually at the weighers or from conveyors.
Each field sample was crushed to pass a 4-mesh screen and thoroughly mixed and "quartered" to provide 5-pound samples for the laboratory. From these samples, portions of 500 grams or less were set aside for the calcination test. These smaller portions were crushed to pass a 20-mesh screen, dried to approximately constant weight at 100° to 110° C, and the final weight recorded as that of the moisture-free sample. The samples were then fired in a muffle kiln for a period of eight hours to a maximum temperature of 1010° C. After cooling to approximately 100° C, each sample was weighed and the weight recorded as that of the calcined sample. The ignition loss was determined as the percentage of loss in weight in the sample by calcination on the moisture-free basis, using the formula:
% CO2, or ignition loss = [(Wgt. of moisture free sample) - (Wgt. of calcined sample)] / (Wgt. of moisture free sample)
This figure was assumed to represent the carbon dioxide content of the sample, although some water and other volatile ingredients would be freed above 100° C.
All weighing was done on a balance sensitive to within 0.1 per cent of the total weight of the smallest sample. Temperatures in the drier were determined by a mercury thermometer, those in the kiln by pyrometric cones (cone 06 down).
The carbon dioxide content was used as an index of the calcium carbonate and magnesium carbonate content of the samples, and is a fairly accurate indication in most instances. When a chemical analysis of a sample was available the carbon dioxide content, as determined by ignition loss, and that determined by calculation from the percentage of calcium carbonate and magnesium carbonate were checked, and found to correspond quite closely for the most part. A representative comparison of the carbon dioxide content, determined by calcination, and the calculated ignition loss of the carbonates, follows: 39.7 per cent, 40.35 per cent; 36 per cent, 36.55 per cent; 43.25 per cent, 42.43 per cent; and 37.9 per cent, 39.2 per cent. On shales and other materials having an ignition loss of 15 per cent or less the comparison was not so close, although the differences are not great enough to be of importance in selecting materials for a wool rock mixture. Carbonaceous material in shales is one source of error.
In accordance with the University of Illinois' experiments (Lamar and others, 1934), 20 to 30 per cent carbon dioxide was chosen as the limits for a wool rock. This corresponds to a calcium oxide content ranging from 31.75 per cent to 54.4 per cent of a calcined rock containing no magnesia.
In selecting the samples from an outcrop to be used in a wool mixture, as great a vertical distance as possible was included. If the most desirable portion, from the standpoint of quarrying, did not fall within the range of a wool rock, an adjustment was made by excluding the portion of the exposure which would not interfere with quarrying, or by the addition of a readily accessible material. To illustrate, one Arkansas City wool rock mixture contained limestone which cannot be sold for building material and which the owner considers waste. To this was added about 56 per cent of a shale which outcrops on his property a few hundred yards east of the limestone quarry. In another instance, all the materials included in the wool rock were sampled in a limestone mine which enters near the base of an exposure. This selection was made because the engineer suggested that these materials could be obtained by blasting an additional seven feet from the mine roof and lowering the floor one foot. The present mine section is nearly pure limestone, much too high in calcium carbonate for wool rock.
The samples range in composition from nearly pure silica (sand or sandstone) to nearly pure calcium carbonate (limestone or chalk), and included a few natural wool rocks.
After determining the correct proportions of the various ingredients to be used, a wool rock mixture totaling one kilogram of raw material was weighed out. This mixture was calcined in fire-clay crucibles in a muffle kiln at a temperature of 1000° C. This preliminary calcination was found advisable in that it hastened the fusion in the induction furnace, and prevented the generation of carbon-dioxide and water with the consequent corrosion of the crucible. When calcined, the sample was then crushed to pass a 20-mesh screen and thoroughly mixed in a ball mill for one hour. The sample was then ready for the blowing test.
A 35 k.v.a. high frequency induction furnace of a standard design was used to melt the wool rock mixture. The charge was placed in a graphite crucible 7 inches deep and 4 inches average inside diameter. The primary coil was protected from the heat of the crucible by a packing of a refractory material. Three layers of heavy sheet mica were used for electrical insulation. The furnace was mounted so that the crucible could be rotated around its pouring lip without disconnecting the electric current of the flow of water through the primary coil. The crucible was tilted manually by a handle which extended over the top of a sheet-iron screen provided for the protection of the operators. A steam gun with a flexible mounting was so placed that the nozzle would be about 4 inches from the molten stream of rock and an equal distance below the pouring lip. The direction of the steam blast was parallel to the axis of rotation of the crucible. The nozzle consisted of a horizontal slit three-fourths of an inch long and three thirty-seconds of an inch wide cut into an iron cap which was screwed onto a short section of 2-inch pipe welded to a section of smaller pipe. A connection to a steam line was made by a length of heavy flexible hose. A steam valve was placed in the line within convenient reach of the operator, and a pressure gauge was mounted directly behind the nozzle of the steam gun. The gun was aimed and operated through a hole in the protection screen, the steam gauge and about 8 inches of the gun protruding through the screen. Two graphite crucible covers were used. One was provided with a small opening at the lip for pouring. The other had a larger hole in the center to receive a graphite thermocouple well.
Figure 2--Experimental blowing equipment showing high frequency current generator hydrogen cylinder and jars, water-cooling pipes for generator and furnace, and mounting of steam gun.
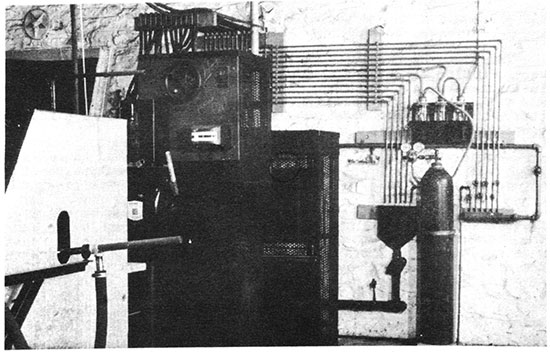
Figure 3--Blowing rock wool with experimental equipment. Tilted furnace and crucible, steam gun and gauge, and a portion of the generator are shown.
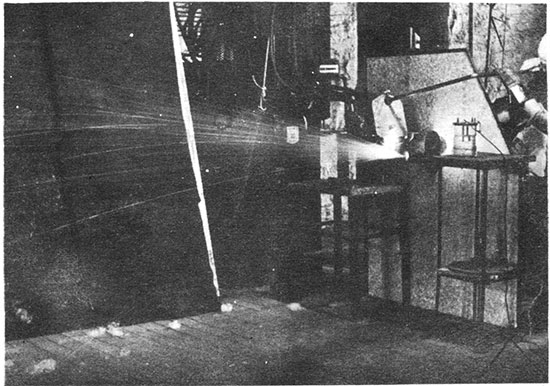
Temperatures were measured with a platinum-platinum 10 per cent rhodium thermocouple which was protected by a one-fourth inch diameter alundum tube, a larger mullite refractory tube, and the graphite thermocouple well. The triple protection of the thermocouple was necessary, since excessive reduction in the crucible made the metal brittle.
A sheet-iron house, 7 by 16 feet, was constructed to receive the wool. The front of this was placed 2 feet from the pouring lip so that a maximum blowing distance of 18 feet was provided.
Before turning on the power to the high frequency induction furnace, the hydrogen was bubbled through the mercury interrupter for 5 minutes and an explosion test made at the end of the period to be sure no air was mixed with the hydrogen. The charge was then placed in the crucible and covered, the flow of water adjusted for cooling and the power turned on. The power was adjusted to 6-10 kw. for the first period of heating. When the charge began to slump the power was raised to 10-14 kw., and when nearly liquid reduced to about 10 kw. The graphite well and thermocouple were then inserted, and a time-temperature-power record kept until the charge was completely fused. The molten glass was held for about 10 minutes at the maximum temperature attained, to fine the charge before pouring. After the first few blowing tests, the thermocouple was withdrawn at 1500° C. and temperatures above this point were estimated from the time-temperature-power curve constructed from the records of the earlier tests. Judging from the composition of the wool rocks blown, these estimates of the higher temperatures may be as much as 25° C too high. The first heating period, from the time the crucible was charged until the thermocouple was inserted, averaged 30 minutes when starting with a hot crucible. An additional 15 to 30 minutes heating was required before pouring the glass.
Before pouring, the steam was turned on to heat up the line, and the pouring cover, with an opening at the lip, put in place. Then the steam gun was swung into position, and the molten glass poured into the blast of steam by slowly tilting the crucible. The time required to pour a charge varied from 45 seconds to over 2 minutes, depending on the amount of glass resulting from a full crucible of calcined rock. The steam pressure, just back of the nozzle, was usually at 60 pounds, although variations from 45 to 70 pounds occurred. It was discovered that moisture in the steam affected the quality of the wool.
The actual conversion of molten rock to wool occurs too rapidly to be observed. After the molten rock hits the steam blast, small incandescent particles are visible for a short distance as they are projected into the collecting room. The wool was picked up by hand and the collecting rooms swept after each blowing test.
Fiber diameters were determined with a microscope equipped with a micrometer eye-piece. Wool samples for examination were taken at random. The average was arrived at by measuring twenty consecutive oriented fibers as the field was moved. Maximum and minimum diameters were determined by a rapid examination of the whole slide, and the measurement of several of the larger and smaller fibers. The minimum and maximum measurements are quite accurate, the average is only approximately correct. Diameters are reported in microns (0.001 millimeter). Satisfactory wool rocks range in average fiber diameter from 2 to 10 microns.
In general the reports on the various rock wool samples are arranged according to the position of the outcrops in the stratigraphic column, beginning with the lowest formation. The eastern Kansas outcrops sampled are all in the Pennsylvanian rocks and extend from upper Cherokee shale, the lowest formation of the Pennsylvanian subsystem, to the Calhoun formation in the upper Shawnee group.
Table 1--Rock formations in eastern Kansas; formations sampled for rock wool are underlined.
| System and Series | Group | Formation | Lithologic character | Thickness (feet) |
|
|---|---|---|---|---|---|
| Quaternary | Recent | Stream alluvium, sand, gravel | |||
| Pleistocene | Loess, glacial drift | ||||
| Unconformity | |||||
| Pennsylvanian | Virgil | Wabaunsee | 28 formations not sampled | Shale, sandstone, and chiefly thin limestones | 80-500 |
| Shawnee | Topeka ls. Calhoun sh. Deer Creek ls. Tecumseh sh. Lecompton ls. Kanwaka sh. Oread ls. |
shale and limestone | 275-325 | ||
| Douglas | Lawrence sh. Haskell ls. Stranger fm. |
Shale, sandstone, and limestone; predominantly shale and sandstone | 150-700 | ||
| Unconformity | |||||
| Missouri | Pedee | Iatan ls. Weston sh. |
5-22 ft. ls. 0-140 ft. sh. |
160 | |
| Lansing | Stanton ls. Vilas sh. Plattsburg ls. |
Shale and limestone, predominantly ls. | 75-150 | ||
| Kansas City | Bonner Springs sh. Wyandotte ls. Lane sh. Iola ls. Chanute sh. Drum ls. Westerville ls. Wea sh. Block ls. Fontana sh. |
Shales and unusually thin limestone | 80-200 | ||
| Bronson | Dennis ls. Galesburg sh. Swope ls. Ladore sh. Hertha ls. |
Shales and limestones; limestone predominating | 100-150 | ||
| Bourbon formation | Chiefly sandy to silty shale | 100-150 | |||
| Unconformity | |||||
| Des Moines | Nowata sh. Altamont ls. Bandera sh. Pawnee ls. Labette sh. Fort Scott ls. |
Shales and limestones | 100-200 | ||
| Cherokee sh. | Chiefly shale | 400 | |||
| Unconformity | |||||
| Mississippian | |||||
Location of Outcrop Sampled. A quarry one-half mile west of Morris, Johnson County, and 3 miles west of Kansas City, Kansas, in the escarpment south of the Kansas river. The quarry is operated by The American Sand Company, Turner, Kansas.
Stratigraphy. The rocks sampled at Morris include the Bethany Falls limestone member of the Swope formation the Galesburg shale, and the Stark shale member of the Dennis formation. All of these rocks belong to the Bronson group of the Missouri series.
The Bethany Falls limestone is being mined for road and building materials by the room and pillar method, entering on a level a little above the flood plain on the south side of Kansas River. The Galesburg shale forms the roof of the mine; sample No. 1 is a shale-limestone mixture from the floor of the mine.
It was impossible at this place satisfactorily to sample 50 to 60 feet of limestone and shale that are exposed in a vertical cliff above the Stark shale in the quarry. Most of this stratigraphic section, however, was sampled from other locations and produced satisfactory wools in the blowing tests.
Results of Tests. All the rocks represented by the samples selected tor-testing can be taken from the mine. Use of the rock represented by samples No. 3, 4, and 5 would entail an increase in the height of the mine 7 feet, to the base of the Winterset limestone. Similarly, use of the rock represented by sample No. 1, would require the lowering of the floor 1 foot.
Wool rook A is a mixture of the Galesburg shale and the Bethany Falls shaly limestone (No. 1) of the mine floor. These samples were mixed in the proportion of the outcrop thickness of the two rocks; that is, five parts Galesburg shale to one part Bethany Falls shaly limestone. A fairly fine white wool was produced in the blowing test.
The Galesburg shale (No. 3) has a carbon-dioxide content of 21.3 per cent, and is, therefore, within the limits for a wool rock, and was blown without admixture. A slightly coarse white wool was produced. (Rock Wool B.)
Figure 4--Stratigraphic section of outcrop at the Morris limestone mine, showing locations of samples No. 1 (CO2 28.36 per cent), No. 2 (CO2 41.17 per cent), No. 3 (CO2 21.3 per cent), No. 4 (CO2 1.16 per cent), and No. 5 (CO2 10 per cent), and wool rocks A, B, and C.
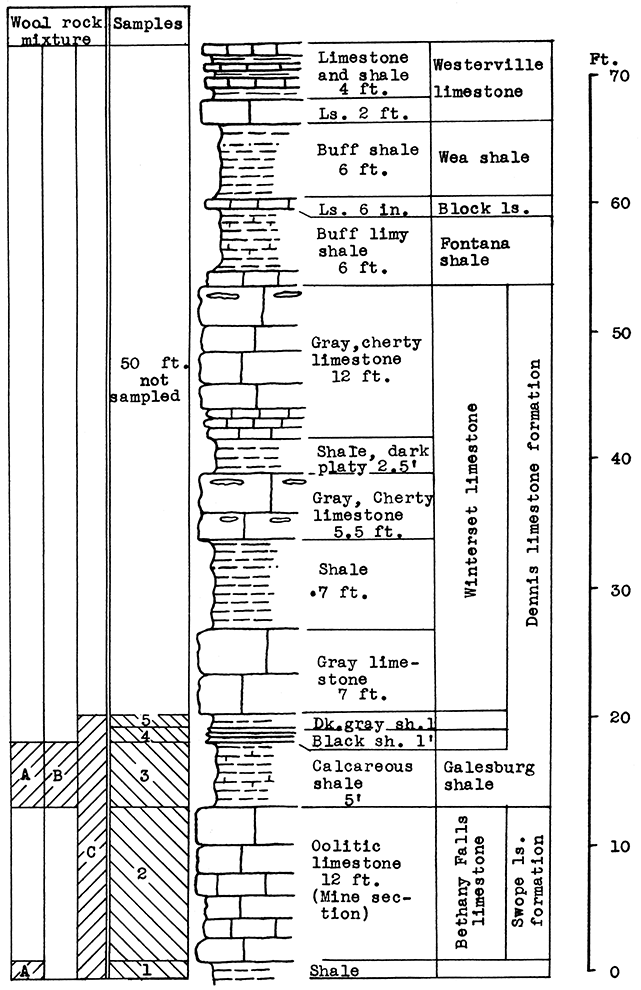
Wool rock C is a mixture including all the samples taken from the Morris quarry. Those above and below the Bethany Falls limestone are included. in proportion to their thickness at the outcrop. The Bethany Falls limestone makes up 38 per cent of the total weight of the mixture in the raw rock. This selection would cause no difficulty in the mining operation since the Bethany Falls limestone would be mined and removed first. The balance could be mined without attention to proportions, and the 38 per cent Bethany Falls limestone could be added at the plant. The above mixture fused easily in the crucible and produced a fine, white wool in the blowing test.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1550° C | 60 | 2 | 68 | 7 | White | 22.74 |
| B | 1575° C | 64 | 2 | 56 | 10 | White | 21.3 |
| C | 1525° C | 60 | 3 | 30 | 5 | White | 27.01 |
Remarks. The Morris outcrop is near the Santa Fe railroad. The city limit of Kansas City, Kansas, is less than 3 miles distant. The gas supply for this area is obtained from local fields in Wyandotte and Johnson counties, and is also piped in from the southwest.
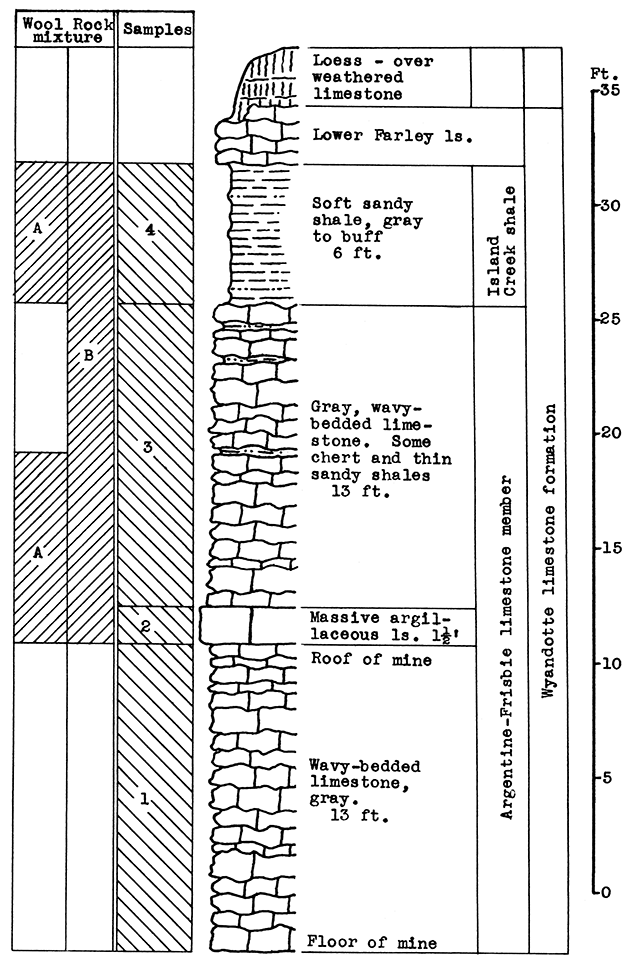
Location of Outcrop Sampled. Limestone mine and quarry are near the corner of Rainbow Avenue and Douglas, near Southwest Boulevard.
Stratigraphy. The entire outcrop sampled at this location is in the Wyandotte limestone formation. The exposure includes the Argentine-Frisbie limestone. the Island Creek shale, and the lower part of the Farley limestone members. The lower 13 feet of the Argentine-Frisbie limestone is mined and crushed for road and building material. Above the weathered portion of the Farley limestone is a fairly thick mantle of red-brown loess.
Results of Testing. The samples selected for wool rock A include 8 feet of the Argentine-Frisbie limestone above the roof of the mine, and the 6 feet of the Island Creek shale; or in terms of raw weights, 8.5 per cent No. 2 sample, 47.5 per cent No. 3 sample, and 44 per cent No. 4 sample. The carbon-dioxide content of the mixture is 23.1 per cent. In the blowing test a rather coarse white wool was produced. By raising the temperature of the melt a fine wool could be obtained.
A more easily quarried mixture and one with a lower fusion temperature is recommended. One such mixture is indicated on the section in Fig. 5 as Wool Rock B. It includes the entire section from the top of the mine (the base of sample No. 2) to the top of the Island Creek shale (sample No. 4), a total vertical distance of 33 feet. The carbon-dioxide content of this mixture is 28.11 per cent. The slight depth of Farley limestone and the sandy loess above the Island Creek shale might be included in the batch if frequent checks were made on the carbon-dioxide content.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1500-2S° C | 60 | 3 | 148 | 10-15 | White | 23.1 |
Remarks. Since this location is within the limits of Kansas City, Kansas, it is very favorably situated in respect to market, transportation facilities, and fuel.
Figure 5--Stratigraphic section of quarry near Rainbow Ave. and Douglas, Kansas City, showing locations of samples No. 1 (CO2 40.45 per cent), No. 2 (CO2 33 per cent), No. 3 (CO2 39.4 per cent), No. 4 (CO2 3.34 per cent), and wool rock A and recommended wool rock B.
Location of Outcrop Sampled. Quarry of the Union. Pacific Railroad, west of Loring, in the SW sec. 13, T. 12 S., R. 22 E.
Stratigraphy. The rocks sampled at the Loring quarry include the Wyandotte limestone and the Bonner Springs shale formations of the Kansas City group, and the Plattsburg limestone or the Lansing group. The vertical section of 60 feet included in the above is made up almost entirely of thick limestones, interbedded with a few thin beds of shale. Two of the shales (Island Creek and Bonner Springs) are about 10 feet in thickness in nearby outcrops.
In addition, a sample was taken from red-brown loess, a Pleistocene deposit of irregular thickness which nearly covers the Vilas shale at the top of the section. This material was probably "derived from the river silt of the valley bottoms, deposited by the overloaded streams during the ice retreat, and later blown by the wind onto the neighboring uplands" (Newell and Jewett, 1935).
Sand from the Kansas river, a few hundred yards south of the outcrop, was also sampled.
Results of Tests. The entire section of 28 feet from the base of the Bonner Springs shale to the top of the Spring Hill limestone, is included in sample No. 1. The carbon-dioxide determination for this sample is 35 per cent, too high for a wool rock. For rock wool mixture A, 30 per cent by weight of Sample No. 2 (loess), or the equivalent of 12 feet of loess, was added to Sample No. 1. The carbon-dioxide content of the mixture was 26 per cent. A very fine white wool was produced at a moderate temperature in the blowing test.
Wool rock B is a mixture of 70 per cent No. 1, 16 per cent No. 2 (loess), and 14 per cent No. 3 (sand). The percentages are based on the weights of the raw materials. This mixture had a carbon-dioxide content of 25.65 per cent, and produced a wool almost identical in appearance to rock wool A.
The proportions of the three samples used in the tests are given according to weights of the raw materials because the mixtures would have to be made up by that method in the operation of a plant at this location. The loess (sample No. 2) is of varying thickness, this thickness increasing with the distance back from the quarry face. The sand would have to be obtained from the river bed. Both the loess and sand are valuable additions to a wool rock mixture, since either material contributes silica with a low percentage of alumina. By lowering the alumina content the fusion temperature is lowered, and consequently, the consumption of fuel is reduced.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1520 °C | 60 | White | 26.00 | |||
| B | 1550 °C | 60 | 3 | 30 | 7 | White | 25.65 |
Remarks. It will be noted in Fig. 6 that 23 feet of Vilas shale occurs stratigraphically above the top of the quarry face. Although this shale is almost completely covered with the loess deposit at present, increasing thickness of the Vilas beds will be uncovered if the loess is removed for use in wool rock mixture. In this event it would probably be advisable to include the Vilas shale in the mixture, with possibly a portion of the Farley limestone to bring the calcium carbonate content up to the required percentage.
This quarry is operated by the Union Pacific railroad, which has a double track passing the quarry floor. The rock is crushed and loaded upon the cars at this point. There is a gas pipe line just north of the quarry.
Figure 6--Stratigraphic section of Union Pacific quarry at Loring, Leavenworth County, showing locations of samples No. 1 (CO2 35 per cent) and No. 2 (CO2 4.1 per cent), and wool rocks A and B; 6 feet of No. 2 is included in B, and 14 per cent of Kansas river sand (Sample No. 3).
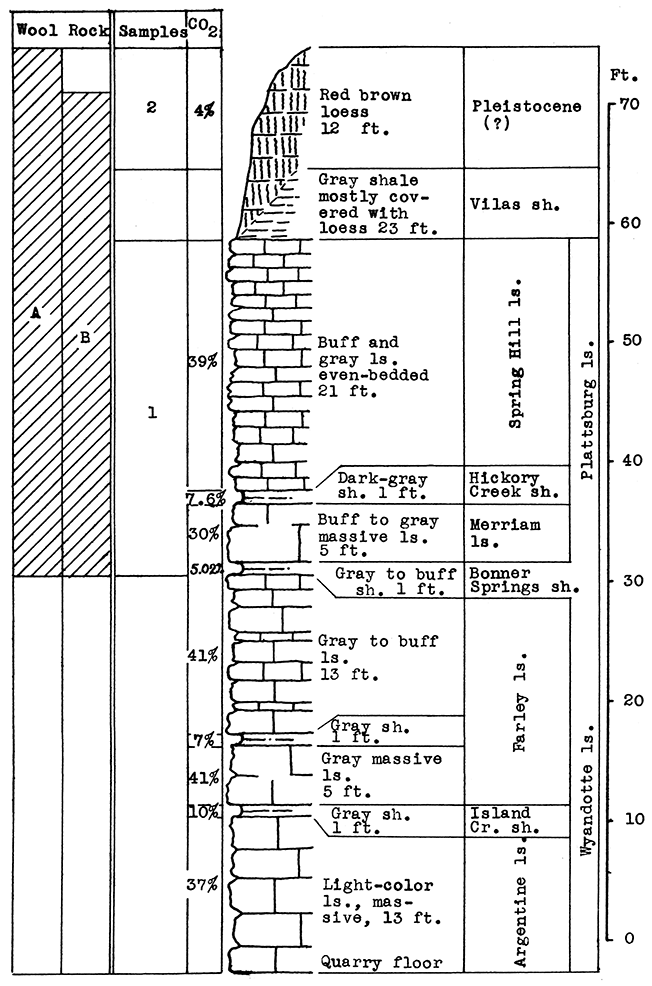
Location of Outcrop Sampled. Quarry of the Lone Star Cement plant, east of Bonner Springs, Leavenworth County.
Stratigraphy. The outcrop sampled at Bonner Springs is almost identical, both in stratigraphic section represented and topographic position, to that at Loring a few miles west and south. The quarry at Bonner Springs is cut into a steep bluff south of the flood plain of Kansas River. The formations exposed include the greater part of the Wyandotte limestone, the Bonner Springs shale, and the Plattsburg limestone. There is a striking difference in the thickness of the Bonner Springs shale exposed at the two outcrops. At Loring this shale is but 1 foot thick; at Bonner Springs it is 25 feet.
An abundance of sand is available in the Kansas River to the south of the outcrop.
Results of Tests. Sample No. 1 was taken as raw slurry at the cement plant. This is made up of the Argentine limestone from the base of the quarry to the top of the member, the Island Creek shale, and the Farley limestone, with enough of the Bonner Springs (about 2 per cent) to bring the mixture to the correct composition for cement.
Wool rock A is composed of 75 per cent slurry to 25 per cent river sand (Sample No. 5), or on the basis of calcined weights: 65 per cent calcined slurry (clinker) to 35 per cent sand. The carbon-dioxide determination for the mixture is 26.82 per cent. A fine textured white wool was blown from this mixture.
Wool rock B contains 60 per cent slurry and 40 per cent raw shale (Sample No. 2), or 50 per cent clinker (calcined slurry) to 50 per cent calcined shale, and has an average carbon-dioxide content of 23.62 per cent. This mixture produced a white wool as fine in texture as A, and at a lower temperature.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1575 °C | 60 | 2 | 40 | 5-8 | White | 26.82 |
| B | 1550 °C | 60 | 3 | 30 | 7 | White | 23.62 |
| Chemical Composition of Samples and Wool Rocks | |||||
|---|---|---|---|---|---|
| Sample No. 1 (Slurry) (percent) |
Sample No. 2 (uncalcined) (percent) |
Wool Rock A calcined composition (percent) |
Wool Rock B calcined composition (percent) |
||
| Silica | 13.50 | 59.40 | 41.80 | 48.50 | |
| Alumina | 3.40 | 19.00 | 12.64 | 3.40 | |
| Ferric oxide | 1.75 | 6.20 | 4.63 | 1.75 | |
| Calcium oxide | 43.43 | 3.40 | 35.30 | 43.43 | |
| Magnesium oxide | 2.75 | 2.12 | 2.75 | ||
| Ignition loss CO2 | 36.00 | 5.50 | |||
| Kansas river sand, No. 3 sample, assumed to be 100 per cent silica. | |||||
Remarks. The Union Pacific and the Kansas City, Kaw Valley and Western (electric) railroads, and Kansas Highway 32 connect Bonner Springs with Kansas City, to the east, and Lawrence and other points, to the west. Gas is produced in Wyandotte county and adjoining Leavenworth and Johnson counties.
Figure 7--Stratigraphic section of cement plant quarry at Bonner Springs showing locations of samples No. 1 (CO2 36 per cent) and No. 2 (CO2 5.5 per cent).
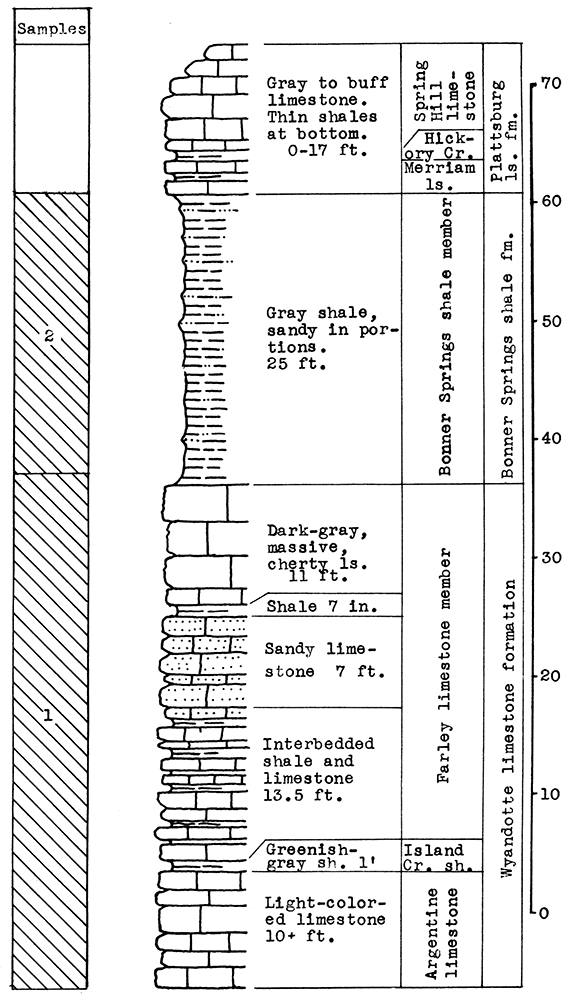
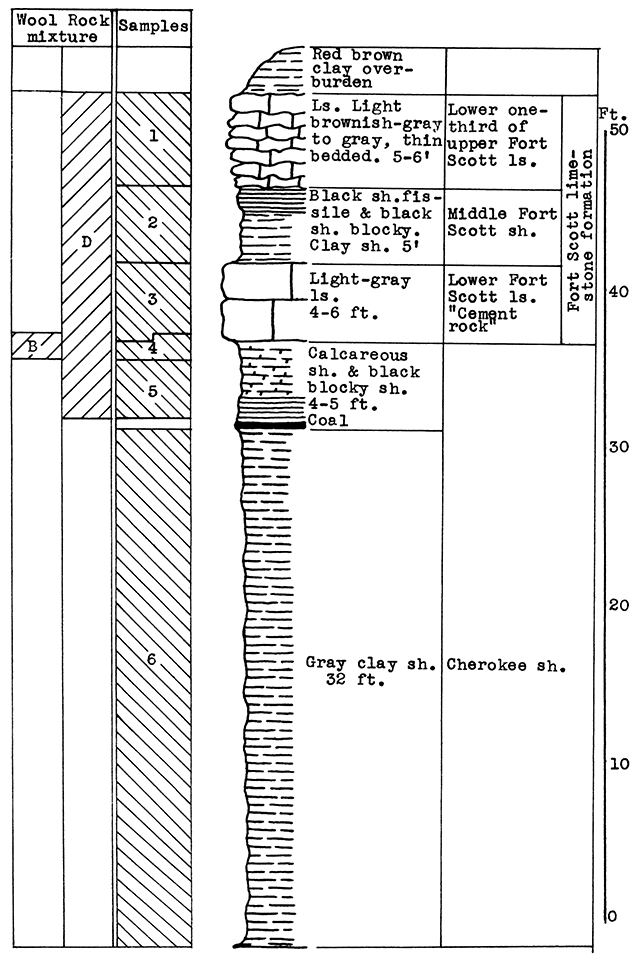
Location of Outcrop Sampled. Quarry of the Fort Scott Hydraulic Cement Company, north of Fort Scott; and the shale pit of Western Shales Products Company, Fort Scott, Bourbon County, Kansas.
Stratigraphy. The Cherokee shale and the Fort Scott limestone formations, which were sampled in this area, are the oldest formations described in this report. The Cherokee shale is the lowermost formation in the Kansas Pennsylvanian. The lower member of the Fort Scott formation is quarried and used in the manufacture of natural cement. It was necessary to secure samples from two outcrops in order to include the stratigraphic section described.
Results of Tests. Three wool rock mixtures were tested. The materials composing wool rock A can easily be quarried in separate units and combined by weights, since the separation of these materials in quarrying is necessary under present circumstances. Percentages of the samples included based on weights of the raw materials follow: Sample No. 1 (upper Fort Scott limestone) 32 per cent; sample No. 2 (middle Fort Scott shale) 40 per cent; sample No. 4-5 (upper Cherokee shale, directly under the "cement rock") 28 per cent. This in terms of vertical distance of rock would include 4 feet of the upper Fort Scott limestone, the total 5 feet of the middle Fort Scott shale, and 3 1/2 feet of the upper Cherokee shale; or in other words, 9 feet of rock above. and 3 1/2 feet below the "cement rock." This mixture produced a rather coarse, grayish wool in the blowing test. The carbon-dioxide content averages 21.8 per cent. If a greater proportion of the upper limestone were included in this mixture a finer wool could be produced. A longer heating period might result in a lighter-colored wool.
Wool rock B is composed of 1 foot of shaly limestone and calcareous shale at tile base of the "cement rock. The "bottom rock" is now considered to have no value and must be disposed of in quarrying. This is the only wool rock tested in the series having a carbon-dioxide content lower than 20 per cent. A very coarse dark-gray wool was produced from this material at a pouring temperature of 1550 °C.
Wool rock C is composed of 68 per cent of sample No. 3 ("cement rock"), and 32 per cent of sample No. 6 (Cherokee shale), percentages based on weights of uncalcined materials. The Cherokee shale used was that sampled below the "red" coal seam. The shale above the coal seam and below the base of the "cement rock" was excluded, because it was concluded that the carbonaceous material and pyrite occurring in it were responsible for the gray or brownish color in the other two wools blown. In the blowing test, wool rock C produced a very fine-fibered wool. nearly white in color. The carbon-dioxide content of 29.4 per cent approaches the upper limit for a wool rock.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1550 °C | 60 | 2 | 40 | 15 | Gray | 21.8 |
| B | 1550 °C | 60 | 20 | 80 | 40 | Gray | 19.0 |
| C | 1550 °C | 60 | 3-4 | White | 29.4 | ||
Remarks. The Missouri Kansas and Texas, the Frisco, and two branches of the Missouri Pacific railroads serve this area. The main highways are U. S. Highways 54 and 69, and Kansas Highway 7. Gas is produced near Fort Scott. The operator of the cement plant has considered coking the locally mined coal to use as fuel in the production of rock wool.
Figure 8--Stratigraphic section including outcrops at Fort Scott Hydraulic Cement plant quarry, and pit of Western Shales Products Company, showing samples No. 1 (CO2 42 per cent), No. 2 (CO2 9.11 per cent), No. 3 (CO2 35 per cent), No. 4 (CO2 19 per cent), No. 5 (CO2 15 per cent), No. 6 (CO2 5.54 per cent), and wool rocks B and D.
Location of Outcrop Sampled. Sample No. 1; clay bank in Verdigris River valley just east and north of cement plant; Sample No. 2, limestone in quarry of cement plant, NE sec. 5, T. 33 S., R. 16 E., Montgomery County, Kansas.
Stratigraphy. The Corbin City member of the Drum limestone is exposed across the entire vertical distance of the outcrop in the cement plant quarry southeast of Independence. The maximum thickness quarried is about 40 feet. The lower member of the Drum formation, the Cement City limestone, is not exposed at this outcrop. A silty alluvial clay from the Verdigris River valley, just east and north of the cement plant. is used also in making the cement at the Independence plant. and was sampled for use in making a wool rock mixture.
Results of Tests. The Drum limestone (sample No. 2) approximates the following composition after calcination: Silica 11.5 per cent, alumina 3.02 per cent, ferric oxide 2.14 per cent, calcium oxide 82.59 per cent, magnesium oxide 0.84 per cent. The carbon-dioxide content is 39.82 per cent of the raw rock. The calcined clay (sample No. 1) contains 70.20 per cent silica, 15.31 per cent alumina, 7.70 per cent ferric oxide, 2.62 per cent calcium oxide, and 1.56 per cent magnesium oxide. [Chemical analysis made by the Universal Atlas Cement plant, Independence.] The carbon dioxide content is 7.00 per cent of the raw clay.
A rock wool mixture of 60 per cent calcined clay, and 40 per cent calcined limestone has the following composition: Silica 46.71 per cent, alumina 10.39 per cent. ferric oxide 5.47 per cent, calcium oxide, 34.62 per cent, magnesium oxide 1.26 per cent. The carbon dioxide content of this mixture is 23.52 per cent of the uncalcined mixture. A light-colored wool, slightly coarse in texture, was produced from this mixture in the blowing test at a pouring temperature of 1500 ° C. A higher temperature, a longer heating period, or a small addition of limestone to the mixture should result in a fine-textured wool.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1500 °C | 60 | 2 | 40 | 10-15 | White | 23.52 |
Remarks. Transportation facilities include the Union Pacific and the Santa Fe railroads, and paved highways radiating from Independence. U. S. Highways 160 and 166 and Kansas Highway 96 serve east and west traffic. North and south traffic follows U. S. Highway 75 or Kansas Highway 16.
Location of Outcrop Sampled. Quarry of cement plant half a mile northwest or Mildred in the center of the south half of sec. 23, T. 23 S., R. 20 E., Allen County, Kansas.
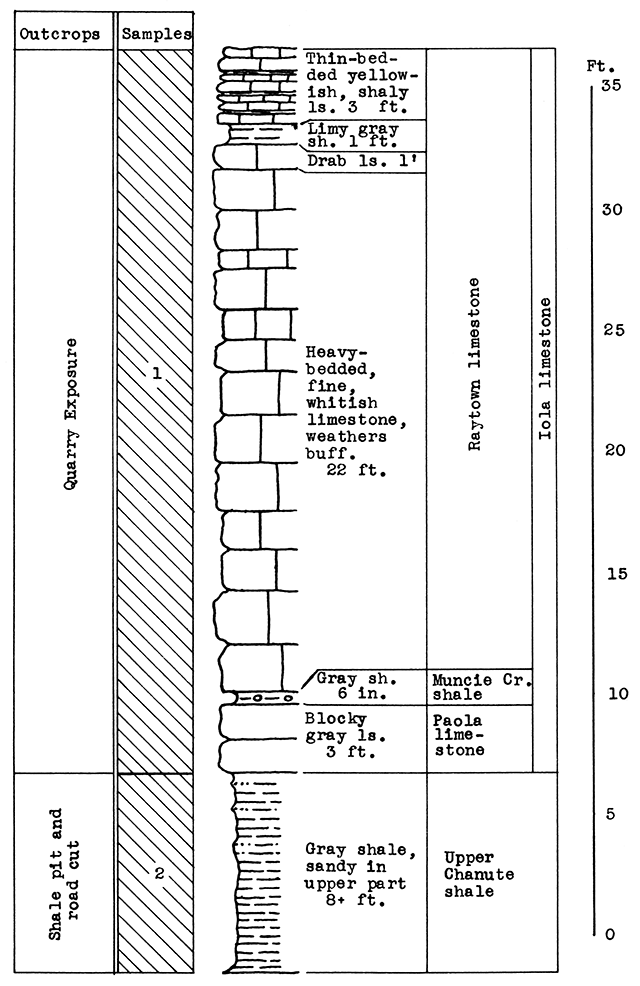
Stratigraphy. The rocks exposed at and near the quarry of the cement pian northwest of Mildred include the Paola limestone, the Muncie Creek shale, and the Raytown limestone, all in the Iola limestone formation; and the upper 8 feet of the Chanute shale. The limestone sample (No. 1) includes the thin shales in the Iola limestone and 3 feet of shaly limestone at the top of the exposed Raytown limestone.
Results of Tests. For the blowing test, a mixture was chosen of 45 per cent raw limestone (Sample No. 1) to 55 per cent raw shale (Sample No. 2). The carbon dioxide content of this wool rock is 22.5 per cent. The presence of sulphur, derived from the pyrite concretions of the upper Raytown limestone, is responsible for the light-brown color of the wool produced from this sample, a character which could probably be overcome with a longer heat1ng period. With a pouring temperature of 1530 °C, a wool of uneven quality was produced, the first part of the sample poured being fine, the later part coarse. A longer heating period or a slightly higher temperature would correct this. A slightly higher proportion of limestone would make a lower pouring temperature possible.
From analyses furnished by the Consolidated Cement Corporation. Fredonia, the calculated chemical composition of the calcined wool rock mixture tested, is as follows: Silica, 40.99 per cent, alumina 13.12 per cent, ferric oxide 5.09 per cent, calcium oxide 31.07 per cent, magnesium oxide 2.94 per cent.
The Chanute shale (Sample No. 2) is sandy in the upper part. and rather high in alumina in the lower portion sampled. The first foot contains 68.5 per cent free and combined silica. At 8 feet the silica content is 54.5 per cent. Within the same vertical distance the alumina content ranges from 11.5 per cent to 19 per cent.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | |||||
| 1530 °C | 60 | 3 | 50 | Brown | 22.5 | |
Remarks. Mildred is in a gas-producing area. Pipe-line connections extend to the Anderson and Linn County fields. The cement plant is on the Missouri, Kansas and Texas railroad. Kansas Highway 6, which passes through Mildred, joins U. S. Highway 54 a few miles to the south at Moran, and U. S. Highway 59 at Welda, about 15 miles northwest of Mildred.
Figure 9--Stratigraphic section of quarry and shale pit of cement plant at Mildred, showing locations of samples No. 1 (CO2 42 per cent) and No. 2. (CO2 6 per cent). Measured by N. D. Newell.
Location of Outcrop Sampled. No. 1 Sample, flood plain north of Neosho River, east of U. S. Highway 59; No. 2 Sample, brick plant shale pit 5 miles southwest of cement plant; No. 3 Sample, cement plant quarry. Neosho County. Kansas.
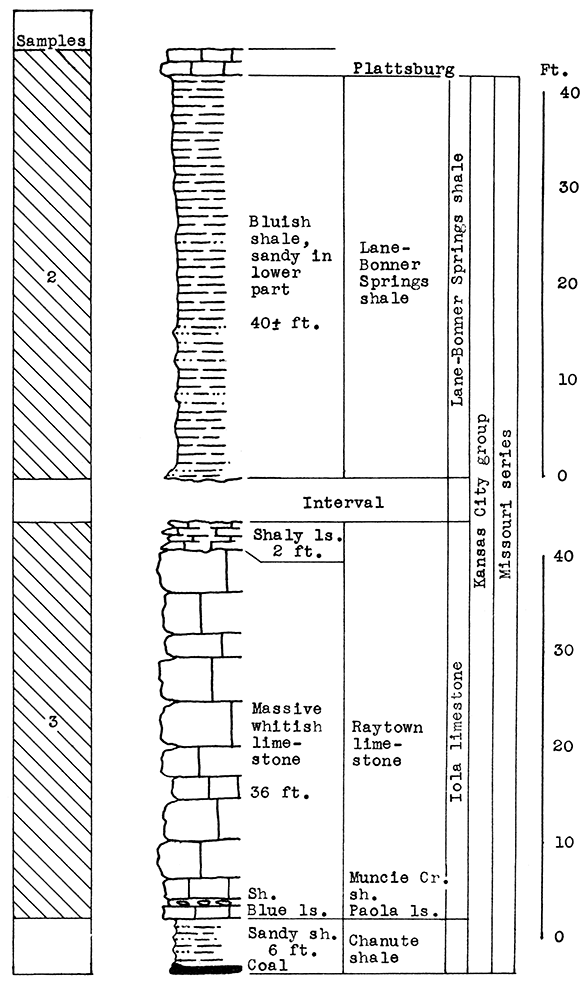
Stratigraphy. The samples for this area were taken from three separate outcrops. Sample No. 1 is a sandy alluvial clay from the flood plain of the Neosho River. Sample No. 2 is the Lane-Bonner Springs shale which was obtained from a brick plant pit. Sample No. 3, rock quarried near the cement plant, is made up chiefly of the massive portion of the Raytown limestone member. This, with a few inches of the Muncie Creek shale and 1 foot of the Paola limestone, totals 37 feet of the Iola formation exposed at the quarry outcrop. At the base of the Paola limestone, 6 feet of the Chanute shale is exposed. The cement mixture for the Ash Grove plant includes materials from all three of the outcrops sampled.
Results of Tests. The proportion of ingredients used in the wool rock tested was determined both by chemical composition and carbon-dioxide content. The mixture used, based on raw, dry weights, follows: No. 1, 20 per cent; No. 2, 33 per cent; No. 3, 47 per cent. If calcined materials are combined, the mixture would contain 25 per cent No. 1, 40 per cent No. 2, and 35 per cent No. 3. The average carbon-dioxide content is 23.85 per cent. According to analyses furnished by the Ash Grove Cement Plant, the chemical composition of the calcined samples and wool rock follows:
| No. 1 (per cent) |
No. 2 (per cent) |
No. 3 (per cent) |
Wool Rock Mixture (per cent) |
|
|---|---|---|---|---|
| Silica | 64.60 | 58.00 | 1.76 | 39.94 |
| Alumina | 17.20 | 20.00 | 2.08 | 13.03 |
| Iron Oxide | 5.90 | 6.82 | 2.75 | 5.15 |
| Calcium Oxide | 2.15 | 5.40 | 88.80 | 33.74 |
| Magnesium Oxide | 1.07 | 1.60 | 4.60 | 2.51 |
| Sodium Oxide | 3.30 | |||
| Potassium Oxide | 5.20 | 3.40 |
This mixture produced a slightly coarse white wool in the blowing test, at a pouring temperature of 1550 °C. A longer heating period would probably have produced a finer wool at the same temperature. However, a mixture slightly higher in limestone content would likely be more satisfactory.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1550 °C | 60 | 2 | 72 | 10 | White | 23.85 |
Figure 10--Stratigraphic section of shale pit and quarry of the Ash Grove cement plant at Chanute. Sample No. 2 from shale pit (CO2 7.6 per cent). Sample No. 3 from quarry (CO2 43 per cent). Measured by N. D. Newell.
Location of Outcrop Sampled. Samples for these tests were taken from the-Monarch Cement Plant quarry, located at the south edge of Humboldt in Allen County, Kansas.
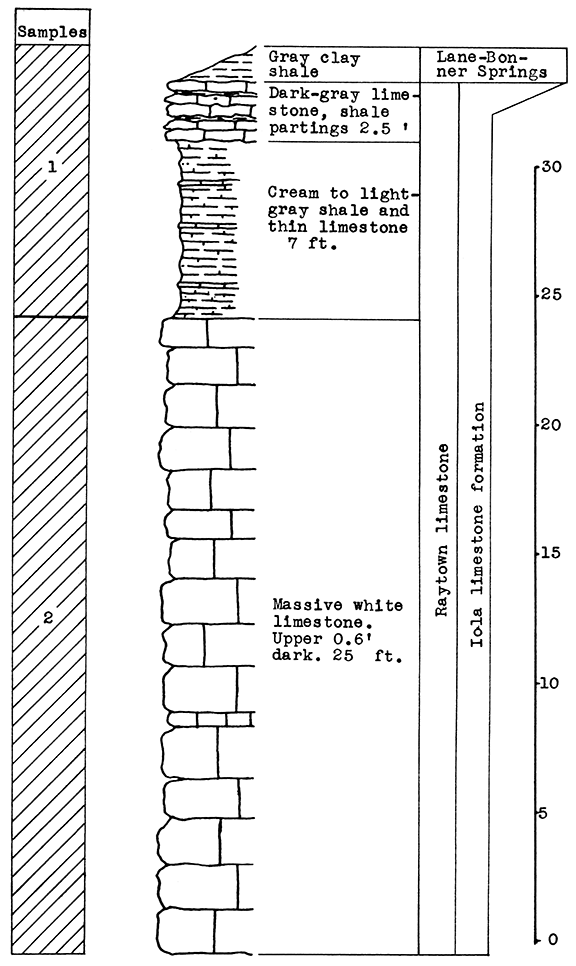
Stratigraphy. With the exception of a thin remnant of the Lane-Bonner Springs shale at the top, the entire section sampled at the Monarch Cement Plant quarry is in the Raytown limestone. Sample No. 1 includes 9 1/2 feet of shale and shaly limestone in the upper part of the exposure. Sample No. 2 includes only the 25 feet of massive limestone of this member.
Results of Tests. Two rock wool mixtures were subjected to the blowing test. Wool rock A contains 66 per cent of No. 1 sample, and 34 per cent of No. 2 sample, based on the weights of the raw, dry materials. Using calcined samples, the proportions are 75 per cent of No. 1 to 25 per cent of No. 2. The carbondioxide content is 21.22 per cent. In the blowing test this mixture produced a very coarse lavender wool at a pouring temperature of 1525 °C. Since the crucible burned out in the process of pouring, this test is not conclusive.
On the basis of raw weights, wool rock B contains 55 per cent of sample No. 1, and 45 per cent of sample No. 2. Using calcined materials the percentages are: 65 per cent No. 1 to 35 per cent No. 2. The carbon-dioxide content of the mixture is 26 per cent. At a pouring temperature of 1575 °C. an extremely fine wool, slightly lavender in color, was produced.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1530 °C | 60 | 3 | 250 | 3 | Lavender | 21.22 |
| B | 1575 °C | 60 | 1 | 20 | Pale Lavender | 26.00 | |
Remarks. Humboldt is in an oil- and gas-producing area. Gas is used at the cement plant. The Sante Fe and the Missouri, Kansas and Texas railroads, and U. S. Highway 59 are available for transportation.
Figure 11--Stratigraphic section of quarry at the Monarch Cement plant, Humboldt, showing locations of samples No. 1 (CO2 12 per cent) and No. 2 (CO2 43 per cent). Measured by N. D. Newell.
Location of Outcrop Sampled. The quarry of the LeHigh Portland Cement Company is located at the south edge of Iola, county seat of Allen County, Kansas.
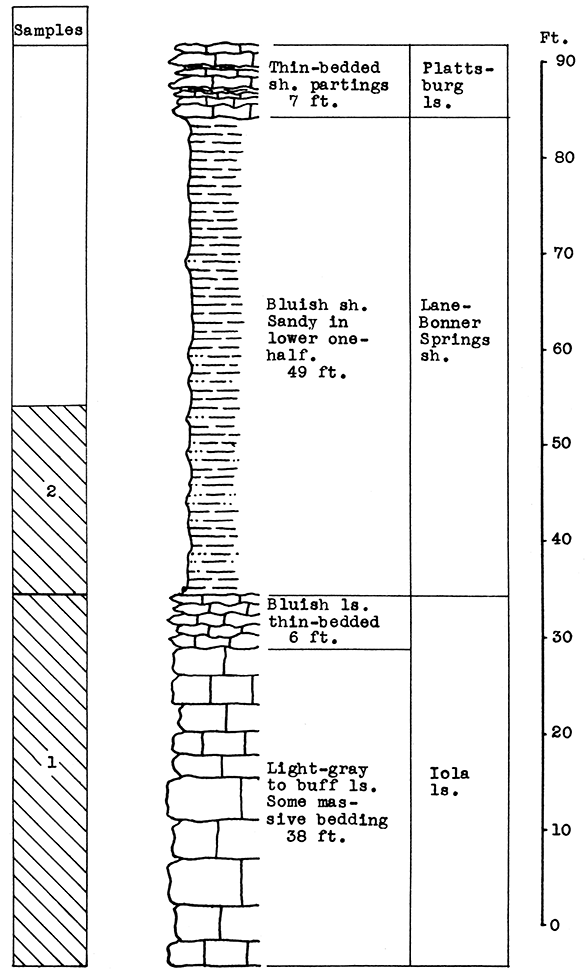
Stratigraphy. The samples taken were from the Lane-Bonner Springs shale and the Iola limestone.
Results of Testing. The carbon-dioxide content of the Iola limestone (Sample No. 1) is 42 per cent of the weight of the uncalcined rock. The chemical composition of the calcined sample, according to an analysis made by the LeHigh Portland Cement Company, is approximately: 4.76 per cent silica, 2.14 per cent ferric oxide, 0.46 per cent alumina, 87.50 per cent calcium oxide, 3.40 per cent magnesium oxide. The Lane-Bonner Springs shale (Sample No. 2) contains after calcination 58.50 per cent silica, 8.35 per cent ferric oxide, 20 per cent alumina, 8 per cent calcium oxide, and 2.7 per cent magnesium oxide. The carbon-dioxide content is 9 per cent of the weight of the uncalcined rock.
For the blowing test the samples were mixed in the proportions of 46.4 per cent, No. 1 (limestone) to 54.6 per cent, No. 2 (shale) of the raw, dry materials. Using calcined samples the proportions would be 35 per cent No. 1 to 65 per cent of No. 2 for the same wool rock mixture. The chemical composition is estimated to be: 39.66 per cent silica, 13.16 per cent alumina, 6.18 per cent ferric oxide, 35.80 per cent calcium oxide, and 2.96 per cent magnesium oxide. This mixture (Iola A) produced a rather coarse wool in the blowing test at a pouring temperature of 1500-25 °C. A fine-fiber wool could be produced at a slightly higher temperature, or at the same temperature. with a slightly greater proportion of limestone in the mixture. Suggested proportions for this alternative are 50 per cent limestone to 50 per cent shale, based on raw weights.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1500-25 °C | 60 | 3 | 120 | 20 | White | 24.4 |
Remarks. Pipe-line connections are established from the Allen County, Woodson County, and other nearby gas fields. Transportation facilities include the Santa Fe and Missouri Pacific railroads, a north and south highway, U. S. 59, and an east and west highway, U. S. 54.
Figure 12--Stratigraphic section at quarry of the LeHigh Portland Cement Company, Iola, showing locations of samples No. 1 (CO2 42 per cent) and No. 2 (CO2 9 per cent). The shale is quarried from a 20-foot face. Measured by N. D. Newell.
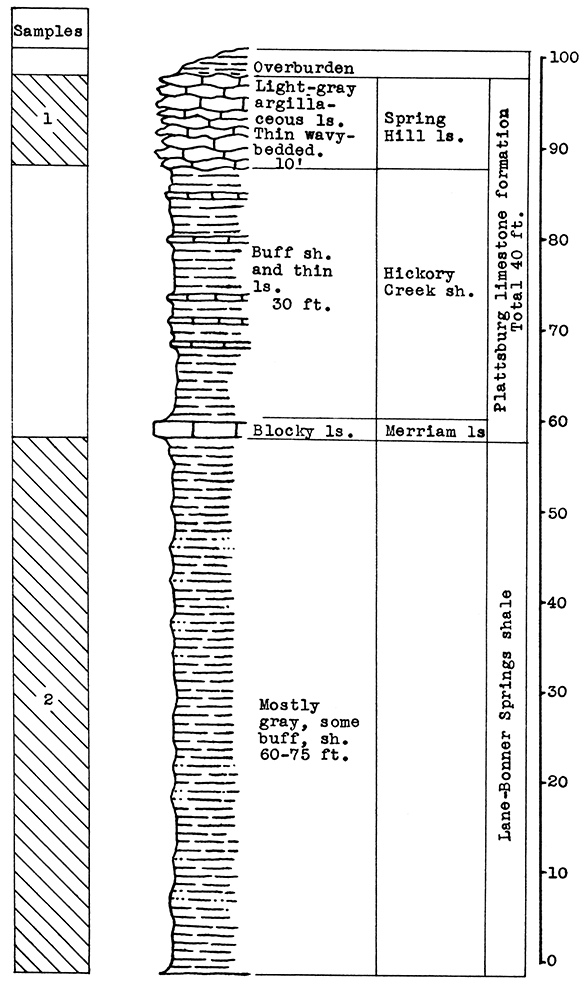
Location of Outcrops Sampled. The Verdigris Valley Vitrified Brick ana Tile plant, south of Neodesha, Wilson County, Kansas.
Stratigraphy. The samples tested from this area were sent in by the superintendent of the Verdigris Valley Vitrified Brick and Tile plant south of Neodesha. The formations exposed in the shale pit, from which the samples were taken, include the Lane-Bonner Springs shale which is used in the manufacture of bricks, and the Plattsburg limestone which forms the major portion of the overburden. The Plattsburg formation includes the Merriam limestone, the Hickory Creek shale, and the Spring Hill limestone members. Probably only the latter member was sampled from the Plattsburg formation.
Results of Tests. The Spring Hill limestone, sample No. 1, has a carbon-dioxide content of 40 per cent. The carbon-dioxide content of the Lane-Bonner Springs shale, sample No. 2, is 7.5 per cent. Two wool rock mixtures were made from varying proportions of these samples. Wool rock A, on the basis of raw weights, contains 47.5 per cent of sample No. 1, and 52.5 per cent of sample No. 2. The weight of the carbon dioxide in this mixture is 23 per cent of the total weight of the raw rooks. A coarse white wool was produced from this wool rock in the blowing test, but at a slightly higher temperature it should produce a satisfactory wool.
Wool rock B contained 53 per cent of sample No. 1 and 47 per cent of sample No. 2 on the basis of raw weights. The carbon-dioxide content was 24.6 per cent. In the blowing test this mixture produced a slightly coarse, greenish wool at a pouring temperature of 1475 °C. With a longer heating period. or at a slightly higher temperature, a fine wool could be blown. Wool rock B fused more readily than A, as can be expected from the carbon-dioxide determination.
The composition of calcined wool rook A, as calculated from typical analyses of the rocks sampled, approximates 37.49 per cent silica, 13.31 per cent alumina, 5.29 per cent ferric oxide, 32.8 per cent calcium oxide 2.76 per cent magnesium oxide, 3.27 per cent potassium oxide, 2.06 per cent sodium oxide. Theoretically a wool rock of this composition should produce a fine wool at a pouring temperature of 1500 °C.
Mr. Charles Laird, superintendent of the plant from which the samples were sent. has produced a fine wool from a mixture of the Lane-Bonner Springs shale and the Spring Hill limestone, using proportions similar to those of wool rocks A and B. His experiments were made in anticipation of constructing a plant for commercial production. The rock is fused in a gas-fired furnace with a low-pressure air blower. Air is used to blow the wool.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1520 °C | 60 | 2 | 60 | 17 | White | 23 |
| B | 1475 ° C | 60 | 3 | 30 | 10 | Green | 24.6 |
Remarks. Cheap gas is available since Neodesha is situated in a large gas-producing area. The brick plant is on the edge of the Larimer-Sycamore field. Transportation facilities include the Missouri Pacific railroad, which connects the plant with the Frisco line at Neodesha, and the Sante Fe at Independence, U. S. Highway 75, which joins Kansas Highway 34 at Neodesha, U. S. Highway 160, and Kansas Highway 96 at Independence.
Figure 13--Stratigraphic section of shale pit at the Verdigris Valley Vitrified Brick and Tile Company south of Neodesha, showing locations of samples No. 1 (CO2 7.5 per cent) and No. 2 (CO2 40 per cent).
Location of Outcrops Sampled. Outcrop A, brick plant shale pit just east of Fredonia; outcrop B, cement plant quarry, south edge of Fredonia. Wilson County, Kansas.
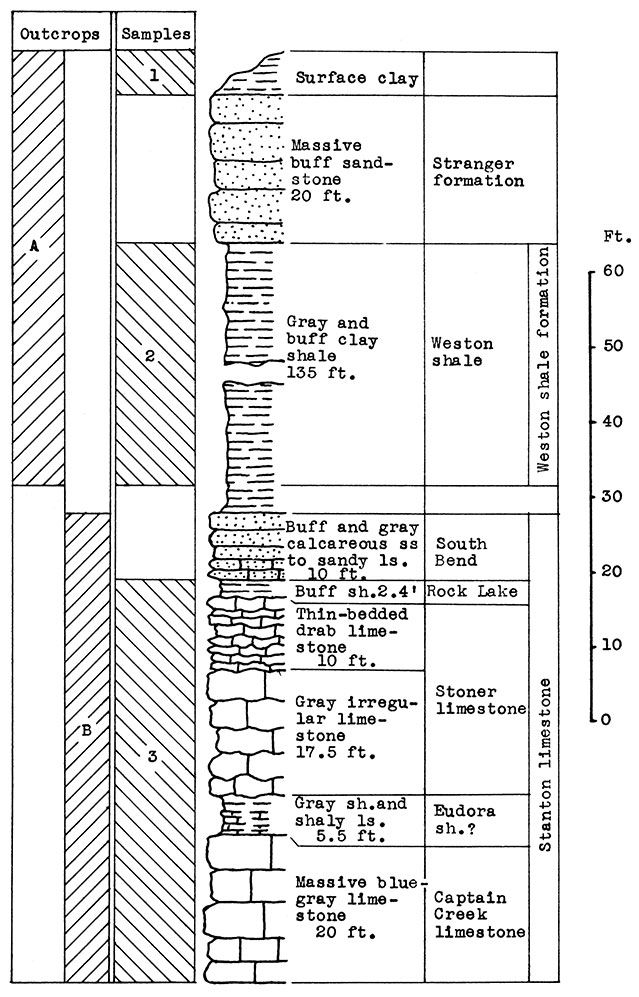
Stratigraphy. Two outcrops were sampled in this area. At location A, in the shale pit of the brick plant east of Fredonia, the Weston shale and 20 feet of sandstone in the lower part of the Stranger formation are exposed. Above the sandstone is a surface clay, probably composed of weathered sandstone and loess.
At location B in the cement plant quarry, is a nearly complete exposure of the Stanton limestone formation, which includes the Captain Creek limestone, the Eudora shale, the Stoner limestone, the Rock Lake shale, and the South Bend limestone. The latter member varies from calcareous sandstone to sandy limestone at this exposure.
Results of Tests. Cement clinker was used as a basis for a wool rock mixture in the laboratory preparation of the samples. To 50 parts by weight of clinker was added 25 parts calcined clay (sample No. 1), and 25 parts calcined shale (sample No. 2). On the basis of raw weights the proportions would be 59 parts dried slurry, and 41 parts clay and shale. This mixture, in terms of the original materials, includes approximately 50 per cent raw limestone, (sample No. 3) and 50 per cent raw shale and clay (sample No. 1 and No. 2). The chemical composition of the calcined mixture was estimated as 45 per cent silica, 13 per cent alumina, 5.3 per cent ferric oxide. 34 per cent calcium oxide, and 2 per cent magnesium oxide. [Chemical analyses of limestone and clay made by the Consolidated Cement Corporation, Fredonia.] The carbon-dioxide content of the raw mix was 24 per cent.
A slightly coarse, white wool was produced in the blowing test. If the maximum temperature had been held for a longer period before pouring, a finer wool would have resulted.
If a larger proportion of the sandy South Bend limestone or of the Stranger sandstone were included in a wool rock mixture, the silica content could be maintained with a minimum amount of alumina, and the viscosity of the glass lowered.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1550 °C | 60 | 3 | 50 | 12 | White | 24 |
Remarks. Fredonia is on Kansas Highways No. 47 and No. 96. Three railroads serve this area; the Missouri Pacific, the Sante Fe, and the Frisco. The town is in one of the large gas- and oil-producing fields of southeastern Kansas.
Figure 14--Stratigraphic sections at outcrops A and B at Fredonia, showing locations of samples No. 1 (CO2 7 per cent), No. 2 (CO2 10 per cent) and No. 3 (CO2 38 per cent). Measured by N. D. Newell.
Location of Outcrop Sampled. Quarry of the Solvay Process Company east of Moline in sec. 12, T. 31 S., R. 10 E., Elk County, Kansas.
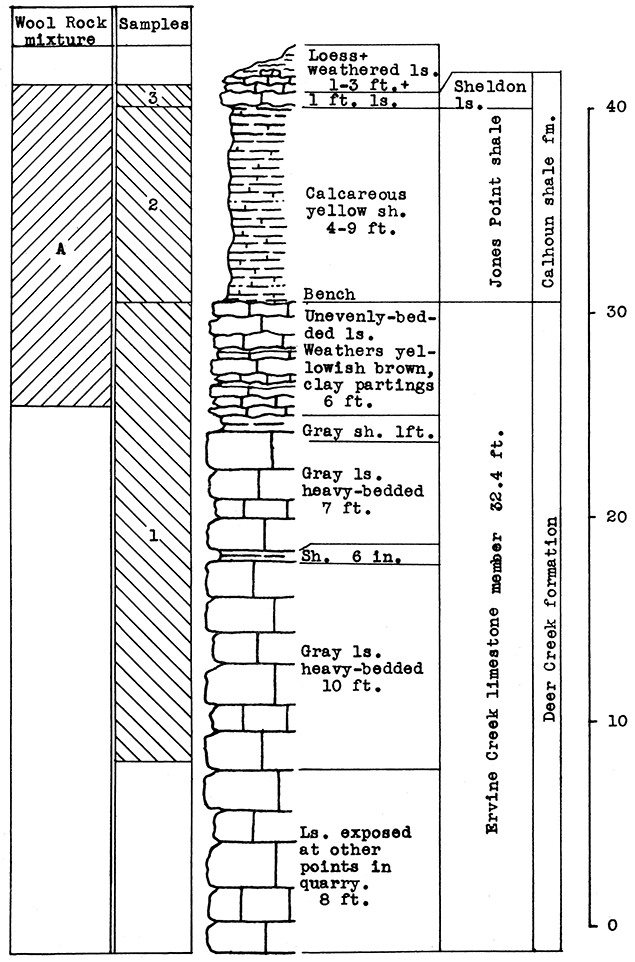
Stratigraphy. The samples taken for this area include the Ervine Creek limestone, upper member of the Deer Creek formation, and the Jones Point shale and a portion of the Sheldon limestone of the Calhoun shale formation. At the point sampled in the quarry a little over 24 feet of the Ervine Creek member is exposed. Some horizontal variation was noted in the thickness of the Jones Point shale. A greater thickness of the Sheldon limestone would probably be encountered beneath the surface deposits of loess and weathered materials north and east of the outcrop.
Results of Tests. In selecting a wool rock mixture from the samples taken in this outcrop, an attempt was made to use the portions of the rock exposed which are least desirable for other uses. The 1 foot of limestone and 9 feet of calcareous shale above the quarry bench, is merely overburden in the quarrying of limestone. The 5 feet of limestone below the bench, which was included in the mixture, contains clay or shale partings. This rock has a carbon-dioxide content of 38 per cent. The carbon-dioxide content of the Jones Point shale above the bench is 13.5 per cent. The 1-foot limestone above this shale has a carbon-dioxide content of 36 per cent. The wool rock mixture, which represents a complete section from the top of the Sheldon limestone to 5 feet below the top of the Ervine Creek limestone, has a carbon-dioxide content of 23.17 per cent.
In commercial production of rock wool, some variations in the method of securing the mixture used would probably be desirable. The loess and weathered limestone overburden could be included by increasing the calcium-carbonate content with a larger amount of the limestone from the main quarry. Possibly it would be easier to add the Ervine Creek limestone portion by weighing the crushed limestone in which is included the entire section of this member. In this event the mixture by weight of raw rock would contain 75 per cent of the Jones Point shale and Sheldon limestone (nine parts shale to one of limestone) which is exposed above the quarry bench, to 25 per cent of the main quarry limestone (Ervine Creek).
This wool rock mixture produced a white wool of excellent quality in the blowing test. The wool contained a very small proportion of coarse fibers and shot.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1550 °C | 60 | 1 | 20 | 4-5 | White | 23.17 |
Remarks. An abundant gas supply is available in this area from local production in Elk and Chautauqua counties. The quarry is on a branch line of the Sante Fe running east and west. Another branch line runs north from Moline.
Figure 15--Stratigraphic section in northeast corner of the Solvay Process Company quarry, Moline, showing locations of wool rook A, and of samples No. 1 (CO2 38 per cent), No. 2 (CO2 13.5 per cent). No. 3 (CO2 36 per cent). The upper 5 feet of sample 1 1s included in wool rock A. Measured by R. C. Moore.
The outcrops included in this division of the report are presented in the order of their stratigraphic position beginning with the lowest formation. These extend from the Blue Springs shale member of the Matfield formation to the upper Wellington shale in the Permian system, and also include Tertiary and Quaternary deposits.
Table 2--Rock formations in central Kansas; formations sampled for rock wool are underlined.
| System and Series | Group | Formation | Member | Lithologic character | Thickness (feet) |
|
|---|---|---|---|---|---|---|
| Quaternary | Recent | Loess | Sandy clay wind deposits | |||
| Alluvium | Sand, silt, and clay | |||||
| Pleistocene | McPherson fm. | Sand, gravel, and clay | 200 | |||
| Tertiary | Pliocene | Ogallala fm. | "Mortar beds," sand, and gravel, cemented by calcium carbonate | |||
| Unconformity | ||||||
| Permian | Big Blue series | Sumner Group | Wellington sh. | upper sh. Carlton ls. Buckeye sh. |
Gray and red sh. and thin ls. | 645 |
| Donegal ls. | Strickler ls. Newbern sh. Hollenberg ls. |
Flaggy ls. and calcareous sh. | ||||
| Perl sh. | Gray and red shales | |||||
| Nolans ls. | Herington ls. Paddock sh. Krider ls. |
White, buff ls. Calcareous sh. Ls. and calcareous sh. |
||||
| Odell sh. | Drab, gray, maroon sh. | |||||
| Chase Group | Winfield ls. | Luta ls. Cresswell ls. Grant sh. Stoval ls. |
Ls. and calcareous sh. with thin ls. | 260 | ||
| Gage sh. Towanda ls. Holmesville sh. |
Gray, green, and red sh. Flaggy ls. Gray and red sh. |
|||||
| Barneston ls. | Fort Riley ls. Oketo sh. Florence ls. |
Massive ls. Calcareous sh. Flint ls. |
||||
| Blue Rapids sh. | Green and red sh. | |||||
| Kinney ls. | Ls. and Sh. | |||||
| Wymore sh. | Gray and red sh. | |||||
| Wreford ls. | Schroyer ls. Havelsville sh. Threemile ls. |
Cherty ls. Calcareous sh. Cherty ls. |
||||
| Council Grove Group | No samples from this group. | 300 | ||||
| Admire Group | No samples from this group. | 115-230 | ||||
| Unconformity | ||||||
| Pennsylvanian | ||||||
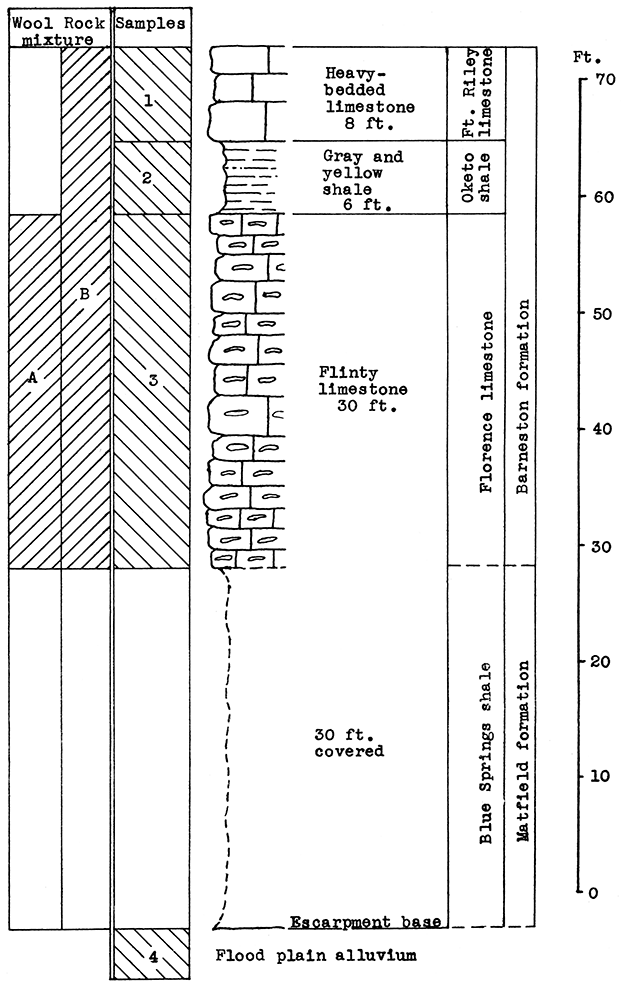
Location of Outcrops Sampled. Quarry in bluff north of the Smoky Hill River, about 3 miles southwest of Junction City, near the Union Pacific tracks. in the SW sec. 21. T. 12 S., R. 5 E. Flood-plain alluvium was sampled on the north bank of the Smoky Hill River about half a mile west of quarry, Geary County, Kansas.
Stratigraphy. The main sampling for this area was made in an abandoned quarry about 3 miles southwest of Junction City, on the escarpment north of the Smoky Hill River. A sample was taken of the entire vertical section of the rocks exposed, which includes 30 feet of the cherty Florence limestone, 6 feet of the Oketo shale, and 8 feet of the Fort Riley limestone in the Barneston formation. The Oketo member is a calcareous shale (carbon-dioxide content, 27.9 par cent). Above the shale 8 feet of massive limestone was sampled.
Below the lowest point sampled in the Florence limestone, there is a vertical section of about 30 feet covered with debris from the quarry. Most of this section, if exposed, would reveal the upper Blue Springs shale. An outcropping of the Blue Springs shale was observed in a road cut about a half mile west of the quarry.
In addition to the quarry outcrop the sandy alluvium of the flood plain of the Smoky Hill River was sampled. This material extends nearly to the foot of the quarry escarpment.
Results of Tests. Two blowing tests were made from the materials sampled, The 30 feet of Florence "flint" (sample No. 3) had the mean carbon-dioxide content (25 per cent) within the limits defined for a wool rock, and was therefore used without additional material for rock wool A. The first portion of the melt poured in the blowing test, produced a fine, white wool. The remainder produced a somewhat coarser wool because of a temperature drop in the crucible.
If a change in the proportioning of ingredients should be found advisable in plant operation, the calcium carbonate content could be raised, if needed, by the addition to the mixture. of the Fort Riley limestone or the Oketo shale. Either the flood-plain alluvium or the underlying Blue Springs shale could be the source of additional silica.
Rock wool B was a mixture made of the complete vertical section sampled in the quarry, including 30 feet of the Florence flint, the 6 feet of Oketo shale, 8 feet of the Fort Riley limestone, and an additional 12 per cent by weight of the floodplain alluvium. This mixture produced a very fine, white wool with little shot.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1575 °C | 60 | 2 | 32 | 6 (selected) |
White | 25 |
| B | 1525 °C | 43 | 1 | 20 | 3 | White | 26 |
Remarks. The quarry is near the Union Pacific tracks. A siding extends from the main line to the quarry site. Highway U. S. 40 is about a mile north. The gas fields nearest this area are in Morris County, about 25 miles south of Junction City. A pipe-line from the southwest, which connectS Junction City with the McPherson gas fields, passes within a short distance of the outcrop sampled.
Figure 16--Stratigraphic section of quarry southwest of Junction City showing locations of samples No. 1 (CO2 39.82 per cent), No. 2 (CO2 27.92 per cent), No. 3 (CO2 25 per cent), No. 4 (CO2 7.65 per cent), and wool rocks A and B. Wool rock B includes 12 per cent of No. 4.
Location of Outcrop Sampled. Dolese Brothers' quarry 2 miles east of El Dorado. The Holmesville shale is exposed in W2 and SE, sec. 18, T. 25 S, R. 6 E; NE, sec. 32, T. 24 S., R. 6 E. The Gage shale is exposed in N. side sec. 19, T. 26 S., R. 4 E.; S2, sec. 28, T. 26 S., R. 4 E; S2, sec. 24, T. 25 S., R. 4 E; W2, sec. 18, T. 25 S.. R. 6 E; NW, sec. 5, T. 25 S., R. 5 E., Butler County, Kansas.
Stratigraphy. The exposed rocks in this area belong to the Chase group. Detailed consideration was given only to the Barneston formation, all three members of which were sampled. These samples included the lower Fort Riley limestone, the Oketo shale, consisting of shaly limestone and calcareous shales, and slightly over 20 feet of the upper Florence limestone. Since no portion of the outcrop sampled has a calcium carbonate content low enough for it to fall within the range of wool rocks, other rocks stratigraphically below and above the Barneston formation must be taken into consideration. The Holmesville shale, which is exposed several places within a few miles of El Dorado, occurs above the Fort Riley limestone. Although this shale was not sampled in the El Dorado area, a typical sample taken in the Arkansas City area was used successfully in making a mixture.
In the Junction City area the flint-bearing Florence limestone proved to be a natural wool rock with a carbon-dioxide content of 25 per cent. This same limestone could be obtained from below the present quarry floor at El Dorado.
Results of Tests. No portion of the outcrop has a carbon-dioxide content low enough to fall within the limits of a wool rock. The upper 6 feet of the Oketo shale tested the lowest in carbon-dioxide, 33 per cent. The average for the entire section is 37.6 per cent.
Since the entire vertical section, including the Holmesville and Gage shales, outcrops at various points within a few miles of El Dorado. and since at these locations the Fort Riley limestone, Oketo shale, and Florence limestone could be quarried below the base of the Holmesville shale, a wool rock mixture was made, using 43 per cent of the Arkansas City sample No. 1 (Holmesville shale) to 57 per cent of the Fort Riley limestone from El Dorado sample (No. 1). This mixture produced a fine, white wool in the blowing test.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1550 °C | 60 | 1 | 40 | 4 | White | 27.4 |
Remarks. El Dorado is situated in an important oil- and gas producing area. The Dolese quarry is on the Missouri Pacific railroad. The Sante Fe railroad and U. S. Highways 54 and 77 pass through El Dorado.
Figure 17--Stratigraphic section of Dolese Bros. quarry, El Dorado, showing location of samples No. 1 (CO2 37.6 per cent) and Arkansas City No. 1 (see fig. 18). Vertical variation in carbon dioxide content indicated by a 36 per cent, b 33 per cent, c 40.9 per cent.
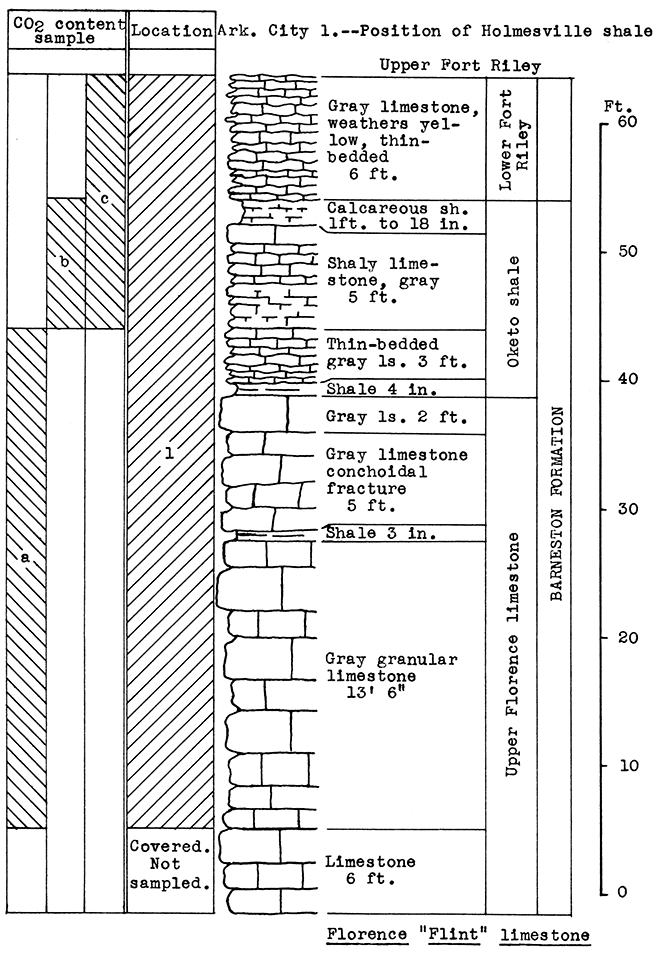
Location of Outcrop Sampled. Quarry of Silverdale Limestone Company, north of Silverdale and about 7 miles east of Arkansas City in the E2 sec. 29, W2 sec. 28, T. 34 S., R. 5 E., Cowley County, Kansas.
Stratigraphy. All samples from this area were taken on the property of he Silverdale Limestone Company, an important source of building stone for this state. The 40 feet of limestone exposed at the plant site is made up of the Florence limestone, the Oketo shale, the Fort Riley limestone. and the lower limy portion of the Holmesville shale. The "J-rock" and "White cap" of the upper Fort Riley limestone, represent waste in the quarrying operation, and a large amount has been thrown aside during the past years. Other portions of the 40 foot section are not used, but do not interfere with quarrying. If a rock wool plant should be established by this company, the proprietor plans to use all the rock not suitable for building stone, and also broken stone too small for other use.
The Holmesville shale outcrops in a road cut east of the quarry. Although only 12 feet of the clayey portion of this shale was sampled at the outcrop, a much greater thickness is available.
Large amounts of flint gravel are quarried from irregular deposits east and north of the quarry. An unused railroad track leads from these deposits to a washer just east of the quarry.
Results of Tests. The mixture for rock wool A was prepared with the purpose in mind of utilizing as much of The waste "J-rock" and "White cap" (Sample No. 3) as possible. The Holmesville shale (Sample No. 1) was selected to supply the silica and alumina content of the mixture. The proportions determined upon were 44 pounds of Sample No. 3 to 56 pounds of Sample No. 1. The proportions were based on the weights of raw, moisture-free materials, since in actual plant operation these materials would have to be quarried separately and mixed by weights.
A white wool of excellent quality was blown from this mixture. The glass was readily fusible and poured freely from the crucible.
A much larger proportion of the outcrops sampled was used in the mixture for rock wool B. It included all of the 40 feet of Fort Riley limestone exposed, the Holmesville shale, and flint gravel (Sample No. 4). The proportions of raw, dry materials used were: 28 per cent of Sample No. 1, 55 per cent of Sample No. 2, and 17 per cent of Sample No. 4. In the blowing test this mixture produced a fine white wool which contained very little shot. The glass fused easily.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1525 °C | 60 | 1 | 30 | 5 | White | 25.65 |
| B | 1550 °C | 60 | White | 24.78 | |||
Remarks. The Missouri Pacific, the Frisco, and the Sante Fe railroads pass through Arkansas City, 7 miles west of the Silverdale quarry. A branch line of the Missouri Pacific has a spur road extending to the quarry site. U. S. Highways 77 and 166 serve this Arkansas City district. Arkansas City is in a gas- and oil-producing area. At present the gas pipe-line connection does not extend to the quarry.
Figure 18--Stratigraphic section of Silverdale quarry and outcrops near the quarry in the Arkansas City area showing locations of samples No. 1 (CO2 14.2 per cent), No. 2 (CO2 38 per cent), No. 3 (CO2 40.5 per cent) and No. 4 (CO2 none). The Holmesville shale and the flint are exposed on the road east of the quarry.
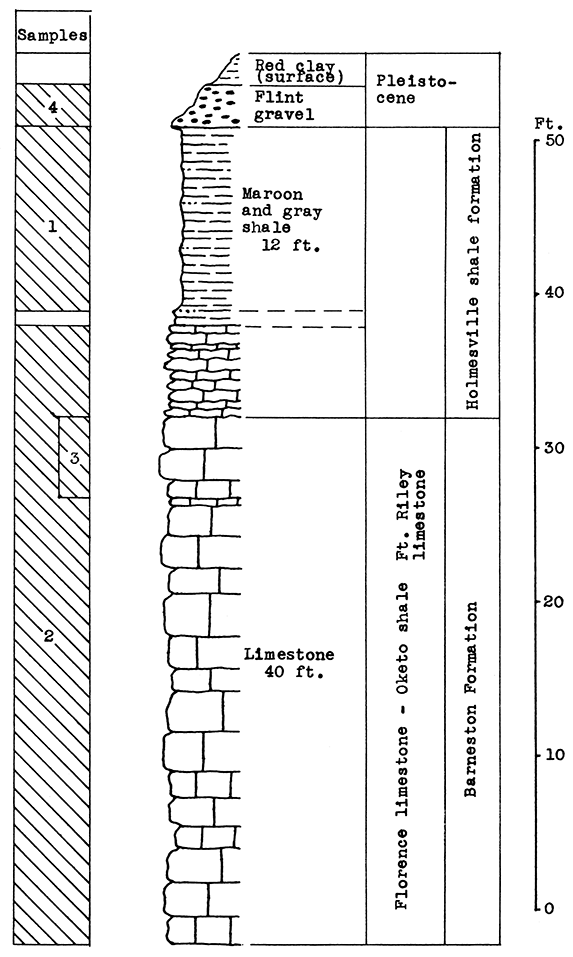
Location of Outcrops Sampled. Location W-A: An abandoned quarry one mile east of Winfield, south of Highway 160, on east bank of Black Crook Creek, in the center of the N2 sec. 26, T. 32 S., R. 4 E. Location W-B: Escarpment east of Walnut Creek 4.2 miles north of Winfield on U. S. Highway No. 77 in the center of sec. 32, T. 31 S., R. 4 E, Cowley County, Kansas.
Stratigraphy. Two outcrops were sampled in the Winfield area. At location W-A, a mile east of Winfield, two samples were taken. One included 10 feet of thin-bedded to shaly, platy limestone in the upper Oketo shale member, and 3 feet of the heavy-bedded Fort Riley limestone of the Barneston formation. The other was taken from the loess deposits of late Pleistocene age, which partially cover the slopes above the bench formed by the Fort Riley limestone. The loess is several feet in depth a short distance from the limestone outcrop sampled.
Above the Fort Riley limestone, the Holmesville shale underlies the loess and weathered surface materials, and, including the next higher Towanda limestone and Gage shale, extends up the slopes of Black Crook Creek valley to the base of the Winfield limestone, which caps the buttes on either side. In the event of actual production of rock wool at this location, the Holmesville shale could be included in the mixture by quarrying at a point higher up on the slope. A greater thickness of the Florence limestone might also be economically included.
At location W-B, 4 miles north of Winfield, a nearly complete section of the Grant shale, member of the Winfield limestone, was sampled at the outcrop, from 2 feet above the top of the Stovall limestone member to the base of the Cresswell limestone member (Winfield formation).
Results of Tests. The 13 feet of Fort Riley limestone (Sample No. 1) sampled at location W-B tested too high in calcium carbonate for a wool rock, as indicated by a carbon-dioxide content of 37 per cent. The silica and calcium carbonate of the wool rock mixture blown was adjusted by the addition of 50 per cent loess, (Sample No. 2). A fairly fine, white wool was blown from this mixture. The outcrop of Grant shale at location W-B, which is made up of thin limestones and limy shales, is a natural wool rock with a carbon-dioxide content of 27 per cent. In the blowing test this material produced a very fine, white wool. The glass was markedly fluid and poured easily from the crucible.
| Data on Tests | ||||||||
|---|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Location | Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||||
| A | W-A | 1525 ° C | 60 | 2 | 70 | 10 | White | 21.35 |
| B | W-B | 1550 ° C | 60 | 1 | 20 | 4 | White | 27.00 |
Remarks. Two branches of the Sante Fe, the Missouri Pacific, and the Frisco railroads pass within a half mile south of location W-A, and the Sante Fe within less than a half mile west of outcrop W-B. U. S. Highway 77 and U. S. Highway 160 pass through Winfield. Abundant oil and gas resources are available in this area. Gas wells of the Winfield field are producing in section 26 where outcrop W-A is located.
Figure 19--Stratigraphic section of outcrops in the Winfield area at locations W-A and W-B, showing position of samples No. 1 (CO2 37 per cent) and wool rock B. The upper 10 feet of the Grant shale is included in wool rock B.
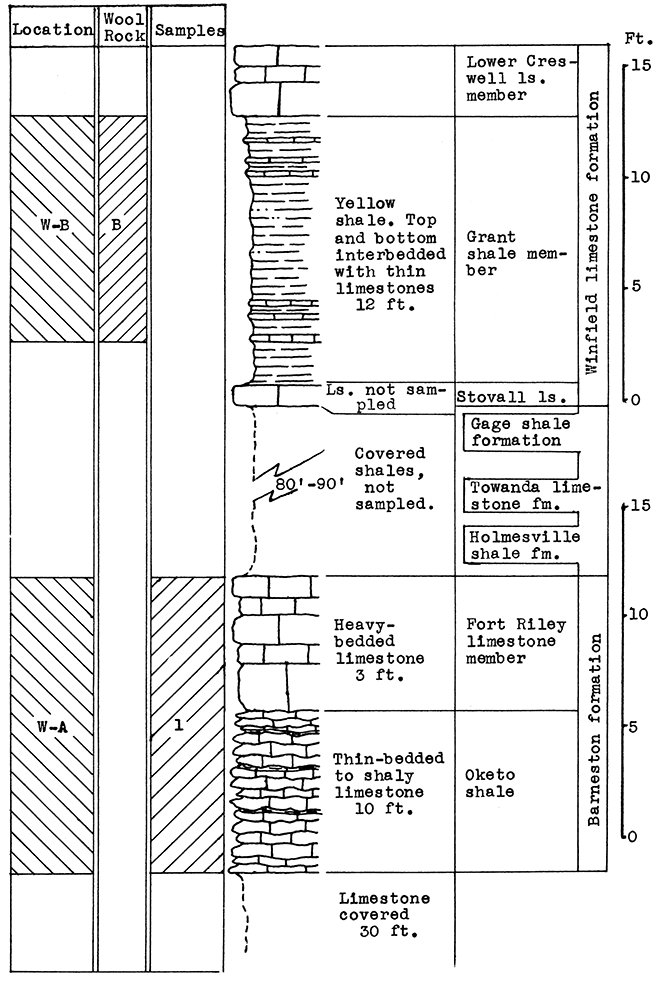
Location of Outcrop Sampled. Shale pit of United Brick and Tile Company, 53000 Broadway; north of Wichita on U. S. Highway No. 81 on the east side of the road, Sedgwick County, Kansas.
Stratigraphy. With the exception of 18 inches of "mortar beds" in the Ogallala formation of the Tertiary system, the rocks sampled for this area consist of buff or gray calcareous shale from the lower portion of the upper Wellington shale, and from shales and thin limestones of the Carleton member of the lower Wellington shale in the Big Blue series of the Permian system.
Results of Tests. The portions of the outcrop which were selected for testing constitute a waste in the ordinary operation of the brick plant. The samples were for the greater part limestones or highly calcareous and impure portions of the shale. Because of the method of selection, a continuous vertical section of the exposure could not be used in the mixture for rock wool A. Percentages by weight of the raw, moisture-free samples used in A follow: No. 1, 13 per cent; No. 2, 30 per cent; No. 3, 35 per cent; No. 6, 22 per cent. The average carbon-dioxide content of the mixture was 23.68 per cent. A fairly fine, white wool was produced in the blowing test at a pouring temperature of 1575 °C. A lower temperature could be used for the melt if the percentage of Sample No. 3 was lowered.
The carbon-dioxide determination of 27 per cent for Sample No. 4 placed it in the category of a natural wool rock. At a pouring temperature of 1550 °C this sample produced an exceedingly fine, white wool (Rock Wool B.)
Quarry operations would be facilitated by including all of the Carleton limestone member, a vertical section from the base of Sample No. 3 to the base of, and including, Sample No. 4, in a wool rock mixture (Wool rock C in Fig. 20). The boundaries would be easily defined since the top of the section is a 1 foot limestone and the base an 8 inch limestone. The average carbon-dioxide determination for this 10-foot section is 24 per cent.
It is not likely that this outcrop would prove profitable for rock-wool production unless operated in connection with the brick plant; otherwise the cost of removing and disposing of the overburden would be prohibitive.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| A | 1575 °C | 58 | 3 | 60 | 10 | White | 23.68 |
| B | 1500 °C | 60 | 1 | 15 | 4 | White | 27. |
Remarks. This outcrop is near the Sante Fe and the Frisco railroads and U. S. Highway 81. The Rock Island, Missouri Pacific, Sante Fe, and Frisco railroads radiate from Wichita. Wichita is situated in a gas-producing area.
Figure 20--Stratigraphic section of shale pit at the United Brick and Tile Company, Wichita, showing locations of samples No. 1 (CO2 12.28 per cent), No. 2 (CO2 41 per cent), No. 3 (CO2 16.14 per cent), No. 4 (CO2 27 per cent), No. 5 (CO219 per cent), and wool rocks B and C.
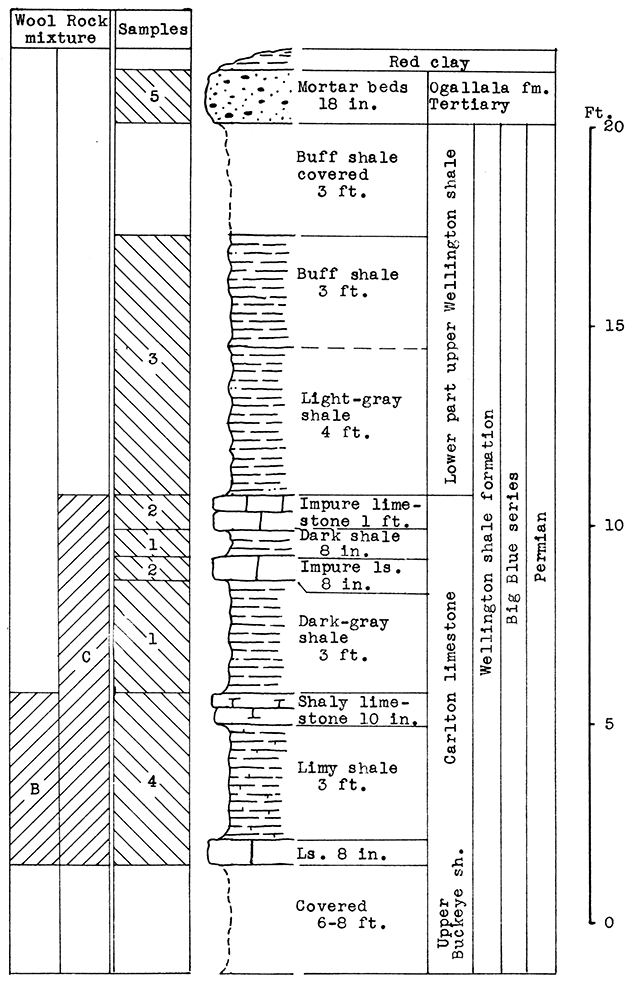
Location of Outcrops Sampled. Eq 1; 7 miles north of McPherson on the east side of U. S. Highway 81; samples No. 1, No. 7, and No. 8, 7 miles north and 1 mile west of McPherson, on west bank of Indian Creek at Johnstown, just north of bridge, SE, sec. 8, T. 18 S., R. 3 W., McPherson County, Kansas.
Stratigraphy. The principal surface rock in McPherson County is the McPherson formation or Equus beds, consisting of Pleistocene clay, sand, and gravel, deposited by the Smoky Hill River when it turned south at Lindsborg and joined the Arkansas. The greater part of this valley was cut into the Upper Permian system. Where sampled north of McPherson the Equus beds rest on the upper Wellington formation of the Big Blue series.
The McPherson deposits are not horizontally uniform, either in thickness or composition. The limy clay, similar to sample Eq 1, was sampled for ceramic tests in 1935 at an exposure in the SW, sec. 29, T. 18 S., R. 3 W, where it ranges from 15 to 20 feet in thickness, but this clay averages much lower than Eq 1 in lime content. It is possible that a deposit of this clay can be found having a calcium-carbonate content Within the range of a wool rock and having a thickness sufficient for rock-wool production. Evidently this material was deposited as a mixture of clay and lime pebbles. The limestone portion is disintegrated, and very likely greatly leached in exposures near or on the surface. Auger borings would probably yield samples higher in calcium carbonate than those taken.
Results of Tests. Since sample Eq 1 had a carbon-dioxide content of 22.3 per cent, and so fell within the range of a wool rock, a separate blowing test was made of this material, producing a white wool at a pouring temperature of 1550 ° C. The average of the fiber diameters was slightly over 10 microns. Owing to an unavoidable shortening of the heating period, and low steam pressure (51 pounds) the wool was coarser than could be expected under more favorable conditions.
Wool rock A is a mixture having no relationship to the thickness of the rocks sampled. On the basis of raw weights the mixture is composed of 43.5 per cent of Sample No. 1, and 56.5 per cent of Sample No. 7. A fine, white wool was produced in the blowing test at a pouring temperature of 1525 ° C.
Wool rock B includes Samples Eq 1, and No. 1 in proportion to the vertical distance sampled (1 foot each), and 8 inches of No. 7 and No. 8 shales combined. On the basis of raw weights, the mixture includes 38 per cent of No. Eq 1, 38 per cent of No. 1, 16 per cent of No. 7, and 8 per cent of No. 8. This wool rock produced an extremely fine white wool in the blowing test. Although the steam pressure was but 50 pounds this factor was compensated by raising the temperature to 1575 ° C, thus bringing the glass to a very fluid state.
| Data on Tests | |||||||
|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| Eq 1 | 1550 °C | 51 | 3 | 4:5 | 10 + | White | 22.3 |
| A | 1525 °C | 60 | 1 | 50 | 5 | White | 23.56 |
| B | 1575 °C | 50 | 1 | 15 | 3 | White | 26.73 |
Remarks. These outcrops are just north and west of the important gas and oil-producing area around McPherson. The nearest pipe-line is 3 to 4 miles east of the outcrops. The Johnstown outcrop is near a branch line of the Union Pacific railroad from McPherson, and slightly over a mile west of U. S. Highway 81. Sample Eq 1 was taken on a road cut on this highway and directly east of Johnstown.
Figure 21--Stratigraphic section of outcrops near Johnstown in the McPherson area, showing locations of samples Eq 1 (CO2 22.3 per cent), No. 7 (CO2 9.32 per cent), No. 8 (CO2 10.68 per cent), and No. 1 (CO2 42.1 per cent).
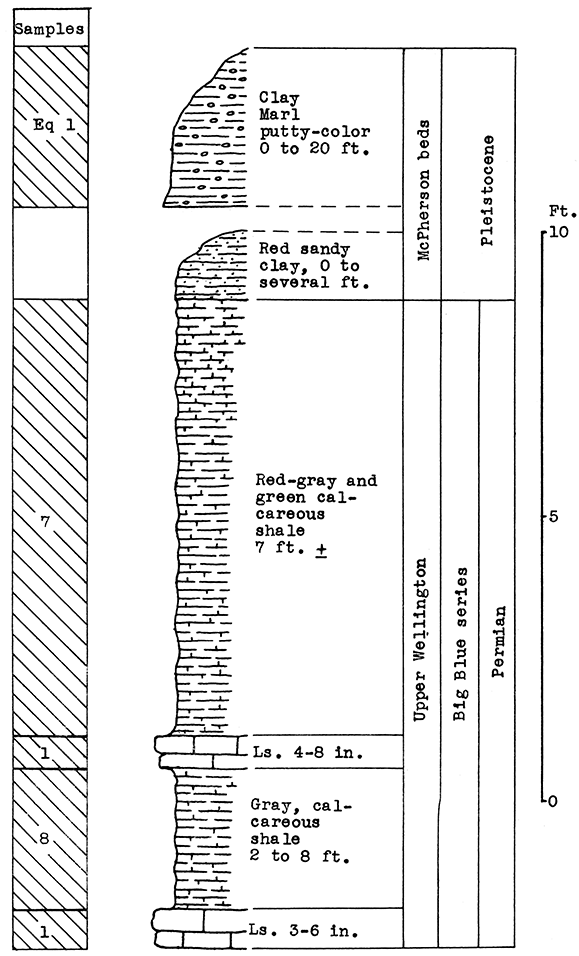
The outcrops sampled in western Kansas include Cretaceous, Tertiary, and Quaternary deposits. The order of presentation is based on the position of the major portion of the outcrop in the stratigraphic column.
Table 3--Rock formations in western Kansas; formations sampled for rock wool are underlined.
| System | Series | Group | Formation | Member | Lithologic character | Thickness (feet) |
|---|---|---|---|---|---|---|
| Quaternary | Recent | Alluvium | Sand, gravel, silt | |||
| Loess | Sand and silt | |||||
| Tertiary | Pliocene | Ogallala fm. | Unconsolidated sediments, "mortar beds" | 14 | ||
| Unconformity | ||||||
| Cretaceous | Colorado | Niobrara chalk | Lower Smoky Hill Chalk | Chalk and chalky sh. | 30 | |
| Fort Hays Ls. | Massive chalk | 60 | ||||
| Unconformity | ||||||
| Carlisle sh. | Blue Hill sh. | Clay sh. | 200 | |||
| Fairport sh. | Chalky sh.;thin ls. | 103 | ||||
| Greenhorn ls. | Pfeifer sh. | Chalky ls. and sh. | 18 | |||
| Jetmore chalk | Limestone, chalky sh. | 17 | ||||
| Hartland sh. | Chalky sh., thin ls. | 22-27 | ||||
| Lincoln ls. | Chalky sh., thin ls. | 25 | ||||
| Graneros sh. | Blue-gray sh. with sandy streaks | 26 | ||||
| Dakota | Dakota sandstone | Sandstone and sh., "quartzite," near Lincoln | 26 | |||
| Unconformity | ||||||
| Permian | ||||||
Location of Outcrop Sampled. L-l, quarry of Quartzite Stone Company, just south of Lincoln, in escarpment north of Saline River flood plain; L-2, road cut on Kansas Highway No. 1, north escarpment of Smoky Hill River, near north boundary of Ellis County, Kansas.
Stratigraphy. The only outcrop sampled at Lincoln was the so-called "quarzite" which is quarried south of town. This is a dense, light-gray sandstone cemented by calcium carbonate, and closely resembles true quartzite in appearance. It is Dakota sandstone, belonging in the Upper Cretaceous series.
After laboratory tests had revealed that this sample contained too little calcium carbonate to be a satisfactory wool rock, a sample from an outcrop of the Fairport and Pfeifer shale members was added to make a wool rock mixture. This sample (L-2) includes the "Fencepost" limestone at the top of the Pfeifer member of the Greenhorn formation, and 14 feet of the Fairport shale immediately above the "Fencepost" limestone. The Fairport shale is the lowermost member of the Carlile shale formation.
Although sample L-2 was taken near the south boundary of Ellis County, these shales are so nearly uniform in calcium carbonate content over large areas that the results of testing would probably have been the same if the samples had been taken from outcrops of these members which are exposed a few miles north of the town of Lincoln.
Results of Tests. The Lincoln "quartzite" (Sample L-1) has a carbon-dioxide content of 15.4 per cent, too low for a wool rock. By mixing 30 per cent of sample L-2 to 70 per cent of L-1 (based on weights of uncalcined material), the carbon-dioxide content was raised to 22 per cent. This mixture (wool rock A) produced a wool which satisfactorily indicates its usability. Owing to the fact that the viscosity of the melt was not completely uniform, a portion of the wool was rather coarse. The portion poured from a completely fused glass produced a fine-fibered wool slightly lavender in color.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1500-20 °C | 60 | 3 | 40 | 10-12 | Lavender to White | 22. |
Sample L-2 includes the lower 14 feet of the Fairport sh., and the "Fencepost" ls. at top of Pfeifer member. CO2 36.7 per cent.
Sample L-1, 30 feet of dense gray Dakota sandstone. So-called "quartzite." CO2 15.4 per cent.
| Table of Rock Formation Showing Stratigraphic Position of Outcrops used in Lincoln Wool Rock A | ||||
|---|---|---|---|---|
| Formation and member | Description | Thickness in feet |
||
| Upper Cretaceous | Carlile shale | Fairport shale member | Chalky sh. with thin ls. | 103 |
| Greenhorn ls. | Pfeifer member ("Fencepost" ls. at top) | Chalky ls. and shale | 18+ | |
| Jetmore member | "Shell" ls. and chalky sh. | 17 | ||
| Hartland member | Blur-gray chalky sh. and thin ls. | 22+ | ||
| Lincoln member | Chalky sh. and thin ls. | 25 | ||
| Graneros sh. | Dark blue-gray sh. | 26 | ||
| Dakota sandstone | Sandstone and sh. | 30+ | ||
Remarks. Transportation facilities in the Lincoln area include a branch line at the Santa Fe railroad from Salina to Stockton, the Union Pacific railroad from Salina to Colby, and Kansas Highways 18 and 14. Gas pipe-lines from the south and from the southwest pass through this area.
Location of Outcrop Sampled. No. 1, road cut one-half mile north of Smoky Hill River on Kansas Highway No. 1 in the W2 sec. 27, T. 15 S., R. 18 W; No. 2, Smoky Hill River on Kansas Highway No. 1, one-half mile south of outcrop No. 1; Rush and Ellis counties, Kansas.
Stratigraphy. The outcrop sampled for this area includes 25 feet of cha cream-colored shales and thin limestones. The lower 9 feet of the exposure is the uppermost portion of the Pfeifer shale member in the Greenhorn formation. This member has the "Fencepost" limestone at its top, above which 16 feet of the lower Fairport shale member of the Carlile formation were sampled.
This particular outcrop was chosen for sampling because of the unusual thickness of the section exposed. However, the same material could be quarried for several miles in any direction from this outcrop. The "Fencepost" limestone, which occurs at the top of the Greenhorn formation, is exposed in many places south of the Smoky Hill River and between LaCrosse and Otis. Furthermore, material quarried for wool rock need not be confined to the stratigraphic section sampled. Both the Fairport and the Pfeifer shales are highly calcareous above and below the limits of the sampling.
A sample was taken of sand from the Smoky Hill River directly south of the outcrop described. A small amount of shale was found in this sample.
Results of Tests. Sample No. 1, Which includes 25 feet of the Pfeifer and Fairport shales, contained 35.65 per cent carbon dioxide, indicating a calcium carbonate content much too high for a wool rock. Sand from the Smoky Hill River (sample No. 2) was added to bring the silica and lime to the correct proportions in the mix. This wool rock contained 82 per cent of sample No. 1, and 18 per cent of sample No. 2, based on the weights of uncalcined materials. The carbon-dioxide content is 29.5 per cent, very close to the upper limit for a wool rock. This mixture produced an excellent quality of white wool at a pouring temperature of 1525 °C.
It is possible that the sand might prove a somewhat costly addition to a wool rock in commercial production. Surface deposits of sandy loam or loess could be used to add silica to the mixture.
| Data on Test | ||||||
|---|---|---|---|---|---|---|
| Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||
| 1525 °C | 60 | 1 | 30 | 3 | White | 29.5 |
Remarks. The outcrops are on Kansas Highway 1, which joins Kansas 96 at Otis, and U. S. 40 S. at Hays. The railroad connections are the Missouri Pacific at LaCrosse, and the Union Pacific at Hays. Gas is produced north of Bison, and south of Otis. A pipe-line extends to LaCrosse.
Figure 22--Stratigraphic section of road cut outcrops on Kansas Highway 1, north of Rush-Ellis county boundary. The total section of 25 feet is included in sample No. 1 (CO2 35.65 per cent). The carbon dioxide content of portions of the section indicated by a 34 per cent, b 42 per cent, c 34 per cent, d 38 per cent.
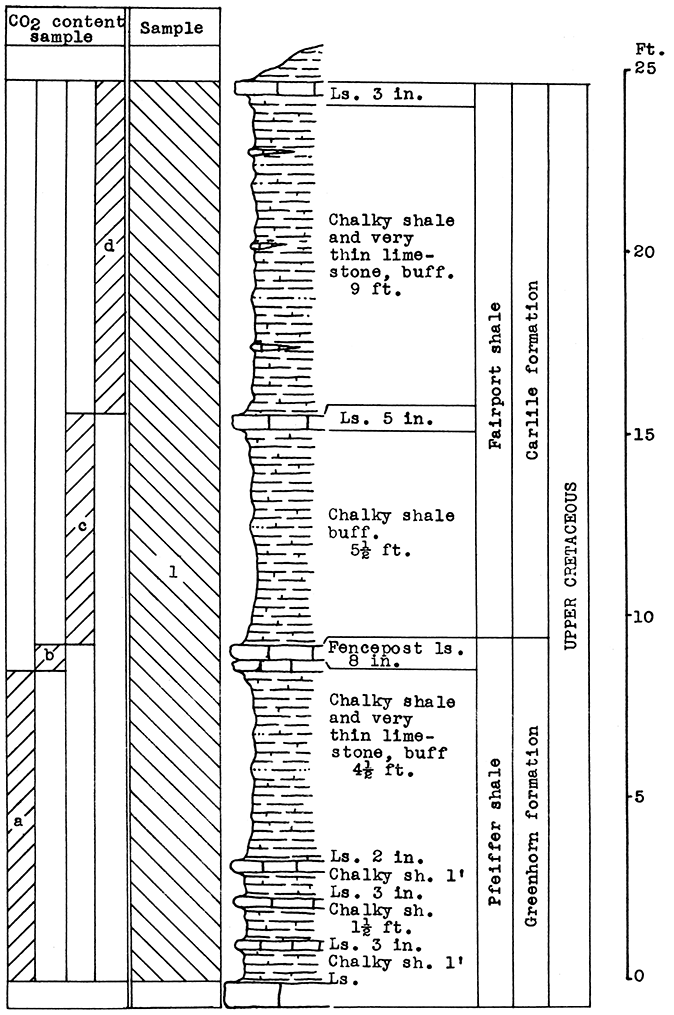
Location of Outcrop Sampled. Quarry of dismantled cement plant at Yocemento, 5 1/2 miles northwest of Rays on U. S. Highway 40 S., in the center of W2, sec. 21, T. 13 S., R. 19 W., Ellis County, Kansas.
Stratigraphy. The outcrops sampled for this area are exposed in the quarry of the dismantled cement plant at Yocemento. The quarry is located in the north-facing escarpment capped by the resistant Fort Hays limestone, and overlooking Big Creek valley. The quarry is benched at the base of the Fort Hays limestone. Over 40 feet of the Blue Hill shale is partially exposed below the bench. The upper 20 feet of this shale is banded by thin yellow sandstone partings. Below this the typical dark Blue Hill shale is exposed.
Results of Tests. The Fort Hays member (Sample No. 1) is a chalky limestone containing a high percentage of calcium carbonate, as indicated by the carbon-dioxide content of 40.8 per cent. The Blue Hill shale (Sample No. 2) lost about 6 per cent of its weight on ignition.
The wool rock mixture tested from these samples contained 56 per cent of sample No. 1, and 44 per cent of sample No. 2. Percentages are based on weights of raw rock. This mixture approximates, in the proportion of samples included, a complete vertical section from the top of the quarry face to 15 feet below the base of the Fort Hays limestone.
This wool rock (Yocemento A) produced a very fine white wool in the blowing test. The glass was extremely fluid and poured easily at a temperature of 1550 °C.
| Data on Test | |||||||
|---|---|---|---|---|---|---|---|
| Vertical Distance Included |
Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | |||||
| 40 ft. | 1550 °C | 44 | 3 | 24 | 4 | White | 25.72 |
Remarks. Yocemento is on U. S. Highway 40 S. and the main line of the Union Pacific railroad. A gas pipe-line from the southwest passes through Yocemento, and oil is produced a short distance to the north.
Figure 23--Stratigraphic section of cement plant quarry at Yocemento, showing locations of samples No. 1 (CO2 40.8 per cent), No. 2 (CO2 6.23 per cent), No. 3 (CO2 6 per cent and the approximate section included in wool rock A.
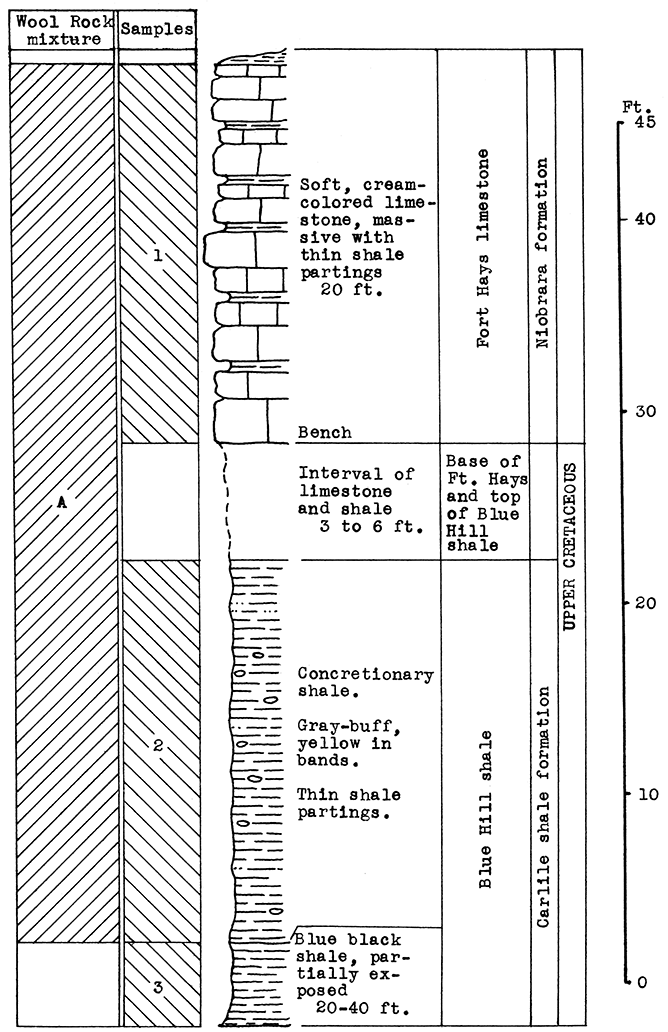
Location of Outcrops Sampled. Location A, one-fourth mile east of Kansas Highway No. 283, on the Harry Reekie farm, 14 miles northeast of Dodge City 1n sec. 30, T. 25 S., R. 25 W. Location B, cut on west side of winding road in escarpment south of Duck Creek branch of Sawlog creek, 7.2 miles northeast of Dodge City, in the W2 sec. 33, to E2 sec. 32, T. 25 S., R. 24 W. Location C, road cut on the east side of road, approximately 3 miles South of Sawlog creek, 1 mile east of Duck creek, and 11.5 miles northeast of Dodge City, in the w2 sec. 14, T. 25 S., R. 24 W., Ford County, Kansas.
Stratigraphy. The wool rocks sampled from this area were taken from the "mortar beds" of the Ogallala formation in the Tertiary system, and from the Fairport and Pfeifer shale members of the Upper Cretaceous system. The "mortar beds" consist of resistant beds of sand and grit that are cemented by calcium carbonate derived from ground water. This part of the formation rests unconformably on the lower Fairport shale member of the Carlile shale or the upper Pfeifer shale member of the Greenhorn limestone in the area sampled.
Although the samples suitable for rock wool were taken along the escarpments of Duck Creek and Sawlog Creek, 7.2 and 11.5 miles, respectively, northeast of Dodge City, it should be possible to quarry these materials between the sampled locations and Dodge City, since the "mortar beds" cap the uplands between the Sawlog and its tributaries, and the Arkansas River valley. The thickness of the "mortar beds" and the overburden, and the calcium-carbonate content of both the "mortar beds" and the underlying shales and limestones would have to be determined by core drilling before a location could be chosen for quarrying operations.
Results of Tests. Three samples were taken from location A. The upper sample, consisting of 12 feet of "mortar beds" had a carbon dioxide content of 10.8 per cent. Ten feet of covered material separates the "mortar beds" from the middle sample, 18 inches of red sandstone with a negligible carbon-dioxide content which rests on a gray Cretaceous shale. The lowest sample was from the top 4 feet of this shale, Which contains no calcium or magnesium carbonates.
The carbon-dioxide content of none of these samples was high enough for a wool rock so no blowing tests were made. However, it is possible that the calcium carbonate has been leached from the weathered exposure from which the "mortar beds" were sampled, and that the rock several feet back from the face of the exposure would prove suitable for the manufacture of rock wool.
An exposure of rocks approximately 16 feet in thickness was sampled at location B. "Mortar beds" capping the escarpment comprised the upper 11 feet of this sample. This portion rests on 1 foot of the lower Fairport shale, below which 4 feet of the upper Pfeifer shale member is exposed. The overburden is very thin several feet back from the edge of the escarpment.
A blowing test was made of the entire sample as taken from the exposure. It yielded an extremely fine, white wool. A separate blowing test was not made of the "mortar beds" sampled at this location, but the carbon-dioxide content of 21 per cent indicates that this portion would be suitable for rock wool if used alone. The "mortar beds" at location C have a carbon-dioxide content of 21 per cent and produced a good wool.
The outcrop at location C includes 14 feet of "mortar beds" resting on a part of the upper Pfeifer shale member of which 9 feet 7 inches is exposed. Although this sample represents a stratigraphically continuous exposure of the shale, the lower 5 feet 7 inches was taken 150 feet east of the upper 4 feet. This lower portion includes two thin limestones. The overburden on top of the "mortar beds" is very thin at this exposure.
Two blowing tests were made from the samples at this location. A fine, white wool was blown, using the "mortar beds" alone (Rock Wool C-A). But by including the entire section at the outcrop in a blowing test (Rock Wool C-B) the carbon-dioxide content was raised from 21 per cent to 26.1 per cent, resulting in a more readily fusible mixture which produced an extremely fine, white wool.
| Data on Tests | ||||||||
|---|---|---|---|---|---|---|---|---|
| Rock Wool No. |
Location | Approx. Temp. Blown |
Steam Blast Lbs. per sq. in. |
Fiber Diameters in Microns |
Color | Percentage of CO2 |
||
| Minimum | Maximum | Average | ||||||
| B-C | B | 1550 °C | 60 | 1 | 10 | 3 | White | 27.3 |
| C-A | C | 1525 °C | 60 | 1 | 30 | 5 | White | 21.0 |
| C-B | C | 1550 °C | 55 | 1 | 20 | 3 | White | 26.1 |
Remarks. All-weather roads connect locations B and C with Dodge City, which is served by the main line of the Sante Fe and a branch line of the Rock Island. Other transportation facilities include U. S. Highways Nos. 50 S, 154, and 283, and Kansas Highway No. 45. An 8-inch pipe-line connects Dodge City with the Hugoton gas field.
Figure 24--Stratigraphic section of outcrop at location B, Dodge City area. The entire section is included in wool rock B-C. The carbon dioxide of units indicated: a 39.3 per cent, b 37.1 per cent, c 40.7 per cent, d 41.8 per cent, e 21 per cent
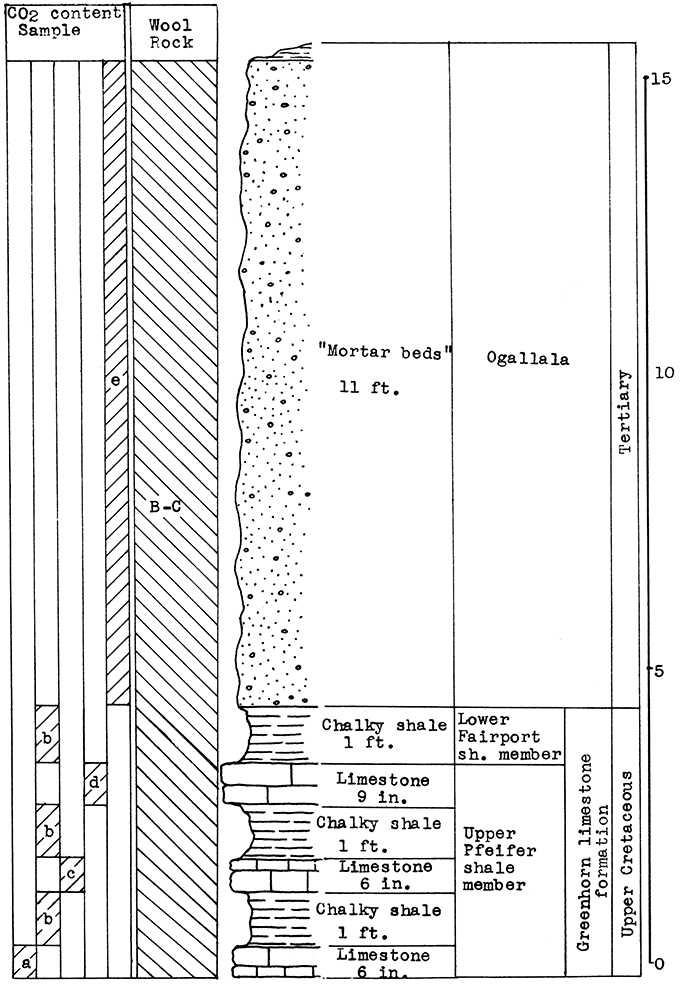
Figure 25--Stratigraphic section of outcrop at location C Dodge City area, showing portions included in wool rock C-A and C-B. The carbon dioxide contents of units indicated: a 40.75 per cent, b 30.3 per cent, c 41.72 per cent, d 33.5 per cent, e 21 per cent.
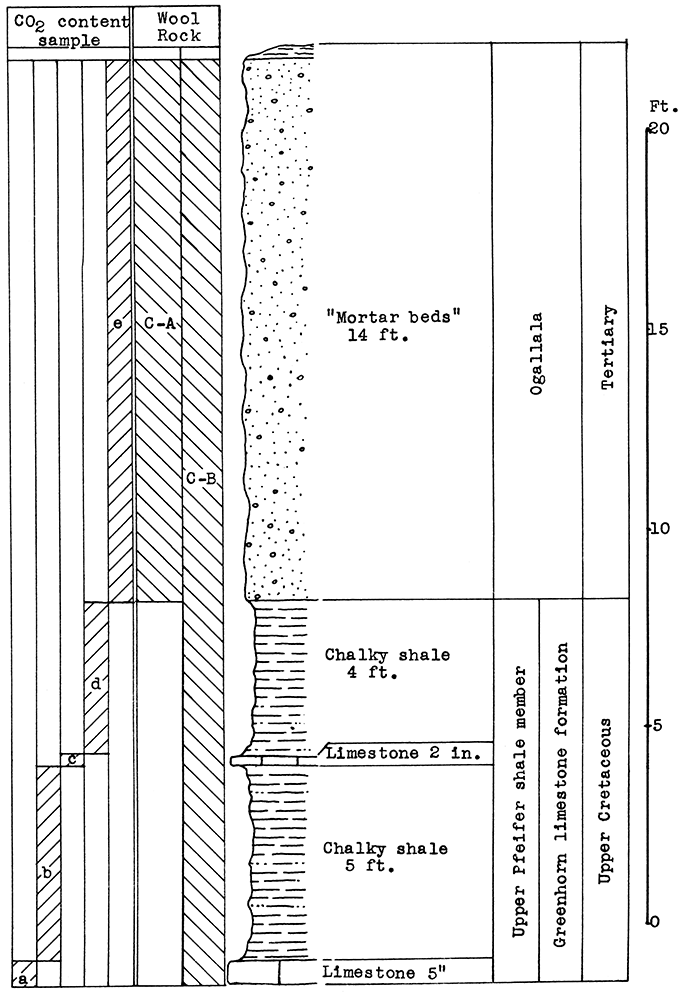
Before summarizing the results of the experiments, factors influencing the accuracy and validity of the tests must be considered. The equipment used for the experimental blowing of rock wool probably only roughly approximates the conditions which would be encountered in commercial production. The steam used varied from dry to wet, even during the brief interval of pouring; the pressure of 60 pounds per square inch was probably too low, necessitating pouring the molten rock in a thin stream, with consequent too rapid cooling. Although the size and shape of the steam nozzle was apparently satisfactory, no experiments were made on other designs.
The pouring of the molten rock was accomplished by tilting the crucible by hand. The rate of pouring was determined solely by judging the size of the stream of glass and the shearing action of the steam blast. Consideration of the time which elapsed in pouring had little value since the rate of pouring was variable, and the amount of glass resulting from a full crucible of calcined rock was not uniform. Considerable inaccuracy was possible in the determination of pouring temperatures. The thermo-couple could not be u$ed continuously at temperatures over 1500 °C; therefore, temperatures above this point had to be calculated from, time-temperature-power curves constructed from a few test runs. Factors such as surges of power, removal of the crucible lid for inspection of the charge, boiling over of the molten glass, and rapid disintegration of the crucible after the couple was removed introduced effects that cannot be calculated. Furthermore, the rate of heating in the crucibles decreased with their use, and the areas of heating in the crucibles were not uniform from time to time. When the temperature at the depth of the couple was 1500 °C, it was observed that the top of the crucible would vary from dark-red with one batch to white heat with another. The graphite crucibles introduced other factors influencing the accuracy of the results. Excessive reduction of the glass, due to oxidation of the graphite, not only affected the color of the wool, but undoubtedly had some influence on the fusibility of the glass, since on occasions the ferric oxide was reduced to an iron button in the bottom of the crucible. In addition to the factor of reduction, fairly large amounts of flake graphite became mixed with the glass, due to the corrosion of the crucible. The aggregation of graphite particles could be observed as nodules in the molten stream before it hit the steam blast, and in the shot after the glass had cooled. Unexplained variations occurred in the quality of the rock wool according to the number of melts which had been run in the crucible. The first melt in a new crucible usually caused trouble, but subsequent melts were increasingly better until rapid disintegration of the crucible began. This phenomenon was possibly due to the variations in the extent of reduction, and the amount of graphite included in the glass. The crucible was most thoroughly protected by glass during the period of best operation.
The choice of mixtures for wool rocks was, for the majority of the samples, based on the carbon-dioxide content. Although this method is fairly accurate, it gives no positive indication of the proportions of the major components other than the carbonates. The proportion of alumina to silica, as well as the ratio of acids to bases, affects the fusibility of the rock. A high alumina content raises the fusion temperature and increases the viscosity of the glass. Other ingredients such as the alkalies, sulphur and manganese, while usually small in amount, undoubtedly have some effect on the fusion, and certainly the sulphur and manganese affect the color of the wool if present in appreciable quantities. However, it is apparent in Figs. 26, 27, and 28, that the carbon dioxide determination correlates rather closely with both the average size of the rock wool fibers in thirty-seven wools blown; also, that the ratio of acidic to basic oxides correlates with both the carbon dioxide content calculated from the percentages of calcium carbonate and magnesium carbonate determined by analysis, and the carbon-dioxide content determined by calcination. The results of.comparisons indicate that the carbon-dioxide content, as determined in the tests, ran from 1 per cent to 2 per cent above the actual amount.
Figure 26--Relationship of CO2 content to average fiber diameters, and the influence of temperature variations in thirty-seven rock wools.
Figure 27--Graph showing relationship of chemical composition, carbon-dioxide content, temperature, and average diameter of fibers.
Figure 28--Correlation of per cent carbon-dioxide determined by calcination, per cent carbon-dioxide calculated from per cent of oxides, and acid to base ratio, using seven rock wool mixtures.
A/B = (gram mols of SiO2 and Al2O3 in 100 grams of material) / (gram mols of CaO and MgO in 100 grams of material)
The average fiber diameters given are merely approximations, since the samples selected for measurement were not necessarily representative. Some of the finer fibers were lost in the blowing, because they clung to the walls and floor of the collecting room and passed out of the openings arranged for the escape of steam. Some of both the finer and coarser fibers and shot were lost by handling. Furthermore, variations in operating conditions, especially the dryness of the steam, were so great that it is probable that even the coarser wools would have been satisfactory in another blowing test. No attempt was made to determine the size or percentage of shot since a large amount was lost in handling. The color of the wool was subject to a great deal of variation. White, green, and brown wools were blown from mixtures of the same rocks. The amount of reduction, or oxidation, and the length of time the rock is heated have decided effects on the color of the wool. Ordinarily ferric oxide is reduced to ferrous oxide, which causes a pale-green color not noticeable in the wool but evident in the shot. If sulphur is not burned out the resulting combination with iron produces a brownish color.
The rapid calcination test for the determination or the suitability of materials for wool rocks was verified by the results of the series of tests. The percentage of carbon dioxide, based on the weight of the uncalcined material, varied from 19 per cent to nearly 30 per cent. The sixteen wool rocks having a carbon-dioxide content of 25 per cent or more produced wools with an average fiber diameter of 8 microns or smaller. This is well Within the range of 3 to 10 microns which includes commercially suitable rock wools. The six wool rocks having a carbon-dioxide content of over 27 per cent produced wools with average fiber diameters from 3 to 5 microns. Half of the twenty-two wool rocks, having a carbon-dioxide content under 25 per cent, produced wools within the 3 to 10 micron limits; half produced wools with fiber diameters averaging over 10 microns. Seven of the former had, a carbon-dioxide content of 23 per cent or more, six of the latter contained less than 23 per cent carbon-dioxide. (Fig. 26 and Table No. 4).
Table 4--Data on all rock wool blown.
| Rock Wool |
Approx. Temp. Blown °C |
Steam Blast Lbs. per sq. in. |
Average Fiber Diam. in Microns |
Color | Percentage of CO2 |
|---|---|---|---|---|---|
| Fort Scott A | 1550 | 60 | 15 | Gray | 21.8 |
| Fort Scott B | 1550 | 60 | 40 | Gray | 19.0 |
| Fort Scott C | 1550 | 60 | 3-4 | White | 29.4 |
| Morris A | 1550 | 60 | 7 | White | 22.74 |
| Morris B | 1575 | 64 | 10 | White | 21.30 |
| Morris C | 1525 | 60 | 5 | White | 27.01 |
| Kansas City A | 1500-25 | 60 | 10-15 | White | 23.1 |
| Bonner Springs A | 1575 | 60 | 5-8 | White | 26.82 |
| Bonner Springs B | 1550 | 60 | 7 | White | 23.62 |
| Loring A | 1520 | 60 | 26.00 | ||
| Loring B | 1550 | 60 | 7 | White | 25.65 |
| Independence A | 1500 | 60 | 10-15 | White | 23.52 |
| Mildred A | 1530 | 60 | 3-50 | Brown | 22.5 |
| Chanute A | 1550 | 60 | 10 | White | 23.85 |
| Humboldt A | 1530 | 60 | 3-250 | Lavender | 21.22 |
| Humboldt B | 1575 | 60 | 3 | Lavender | 26.00 |
| Iola A | 1500-25 | 60 | 20 | White | 24.,4 |
| Neodesha A | 1520 | 60 | 17 | White | 23.0 |
| Neodesha B | 1475 | 60 | 10 | Green | 24.6 |
| Fredonia A | 1550 | 60 | 12 | White | 24.0 |
| Moline A | 1550 | 60 | 4-5 | White | 23.17 |
| Junction City A | 1575 | 60 | 6 | White | 25.0 |
| Junction City B | 1525 | 43 | 3 | White | 26.0 |
| Eldorado A | 1550 | 60 | 4 | White | 27.4 |
| Arkansas City A | 1525 | 60 | 5 | White | 25.65 |
| Arkansas City B | 1550 | 60 | 4-6 | White | 24.78 |
| Winfield A | 1525 | 60 | 10 | White | 21.35 |
| Winfield B | 1550 | 60 | 4 | White | 27.00 |
| Wichita A | 1575 | 58 | 10 | White | 23.68 |
| Wichita B | 1500 | 60 | 4 | White | 27.00 |
| McPherson Eq 1 | 1550 | 51 | 10 plus | White | 22.30 |
| McPherson A | 1525 | 60 | 5 | White | 23.56 |
| McPherson B | 1575 | 60 | 3 | White | 26.73 |
| Lincoln A | 1500-20 | 60 | 10-12 | Lavender | 22.00 |
| Rush-Ellis Co. A | 1525 | 60 | 3 | White | 29.5 |
| Yocemento A | 1550 | 44 | 4 | White | 25.72 |
| Dodge City B-C | 1550 | 60 | 3 | White | 27.3 |
| Dodge City C-A | 1525 | 60 | 5 | White | 21.0 |
| Dodge City C-B | 1550 | 55 | 3 | White | 26.1 |
The optimum percentage of carbon-dioxide as indicated by these tests is around 27 per cent, with a range of 24 per cent to 30 per cent.
An acid to base ratio of slightly over 1.0 is indicated as the average for a suitable wool rock. This ratio was determined by dividing the gram mols of silica and alumina in a hundred grams of material, by the gram mols of calcium and magnesium oxide in a hundred grams. (Figs. 27 and 28; Table No. 5).
Table 5--Data on seven rock wools, showing relationship of chemical composition to other characters.
| No. | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | A CO2 by Calcination |
B CO2 Calculated |
A Average Diameter Fiber |
B Average Diameter Fiber |
A/B | Temp. °C |
Rock Wool |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48.50 | 3.40 | 1.75 | 43.43 | 2.75 | 26.82 | 26.80 | 3 | 5 | 1.00 | 1520 | Bonner Springs A2 |
| 2 | 46.77 | 10.39 | 5.47 | 34.62 | 1.26 | 23.52 | 22.12 | 4 | 20 | 1.10 | 1500 | Iola A2 |
| s | 37.49 | 13.31 | 5.29 | 32.28 | 2.76 | 23.00 | 22.15 | 6.5 | 17 | 1.15 | 1520 | Neodesha A2 |
| 4 | 41.80 | 12.64 | 4.63 | 35.30 | 2.12 | 23.62 | 22.90 | 4 | 7 | 1.20 | 1550 | Bonner Springs B2 |
| 5 | 39.66 | 13.03 | 5.15 | 33.74 | 2.51 | 23.85 | 22.12 | 6.5 | 10 | 1.20 | 1550 | Chanute A2 |
| 6 | 45.00 | 13.00 | 5.3 | 34.00 | 2.00 | 24.00 | 22.00 | 7.5 | 12 | 1.33 | 1550 | Fredonia A2 |
| 7 | 46.77 | 10.39 | 5.47 | 34.62 | 1.26 | 23.52 | 22.00 | 7.5 | 15 | i.36 | 1550 | Independence A |
| CO2 A = percentage of carbon-dioxide determined by calcination. CO2 B = percentage of carbon dioxide determined by calculation from percentage of calcium oxide and magnesium oxide in mixture. Average Fiber Diameter A = Fiber diameters of rock wools blown at 1550 °C with the compositions shown. From averages given in Ill. Geol. Survey Bulletin 61. Average Fiber Diameter B = Average diameter of fibers in rock wools listed above. A/B = Acid to base ratio. |
||||||||||||
Wool rocks having a relatively large proportion of silica to alumina fuse more readily and produce a less viscous glass. This would indicate the value of the use of sand, sandstone, or sandy clay or shale in a rock wool mixture. (Table No. 5).
In the experimental production of rock wool pouring temperatures ranging from 1475 °C. to 1575 °C were used. To produce a good quality of wool it is necessary to use dry steam or air. The average steam pressure of 60 pounds which is used is probably too low, even for experimental purposes.
The commonest raw materials available for the production of rock wool are limestones and shales, but chalk, sand, sandstone, clays, or materials intermediate in character are equally suitable. In most localities a mixture of a calcareous rock such as limestone or chalk, and a siliceous material, such as shale or sand, would be necessary in order to obtain the correct composition. However, a good many natural wool rocks are available in Kansas. These include calcareous shale, flinty limestones, and similar materials.
In quarrying for rock wool production a greater thickness of rock can usually be included than that sampled at specific locations. It is also possible that better raw materials can be found than those tested, since sampling was restricted to outcrops. Furthermore, the area of suitable wool rocks is not confined to the specific locations sampled.
The most important factors to be considered in the production of rock wool are the availability of markets, fuel, and transportation. Consideration of these factors indicates four generalized areas in the state, in each of which one or two rock wool plants could operate. The numbers and size of the plants in any area would depend upon developments of the rock wool industry in overlapping trade territories and general economic conditions.
The Kansas City metropolitan district would afford a market for a fairly large production. This is the most densely populated area of the state and contains many small towns and cities connected by the railway and highway systems radiating from Kansas City. Gas is obtained locally and by pipe-line from the important gas and oil fields to the southwest. Sufficient resources of limestone, shale, sand. and sandstone are available for raw materials.
Southeast Kansas is an especially favorable area for the production of rock wool because of its abundant gas and oil resources. The marketing possibilities are good in this area, which ranks second only to the Kansas City metropolitan district in density of population and buying power. Railway and highway transportation facilities are well developed in this part of the state. A plentiful supply of raw materials may be found in Pennsylvanian limestones, shales, and sandstone which outcrop in this area.
Within the central Kansas area there is a rather wide variation in factors pertaining to rock wool production. The Wichita trade territory would offer a large market with excellent transportation facilities. The supply of raw materials, while not large in the immediate vicinity of Wichita, is available in abundance within a reasonable distance. The wool rock resources are limited around McPherson, but they could possibly be utilized to advantage in connection with the manufacture of brick and tiles, since the raw materials for both industries could be quarried together. This combination would furnish a profitable outlet for the large supply of gas available in that area. The market should be sufficient for a small plant. Transportation facilities are adequate. In contrast to the McPherson district a large supply of excellent raw materials is available in the Junction City area, but the gas supply would have to be obtained from the southwest. The area includes cities with fairly large marketing possibilities. Railway and highway transportation facilities are well developed.
The western half of the state includes a wide marketing area which would extend into eastern Colorado. Suitable raw materials were found as far south as Dodge City, and in Trego, Rush, and Ellis counties. Gas or oil for fuel are available in either territory. Transportation facilities are adequate, but the relative cost would be higher than in the other areas mentioned because long hauls would be necessary to supply the widely scattered market.
Because of the abundant supply of gas and oil in Kansas, a furnace using this type of fuel would probably be more practicable than the usual coke-fired cupola. Since very little information is available on the use of gas and oil as fuel in the production of rock wool, it would be advisable for the prospective manufacturer to set up a small experimental plant. A reverberatory furnace using gas or oil and air under pressure is suggested, to be constructed somewhat like a frit furnace, into which the raw material is fed continuously upon the sloping floor of the combustion chamber where it melts and runs to the lowest level to be tapped for blowing. The blowing could also be a continuous operation if correctly regulated. Little difficulty should be encountered in reaching the desired temperatures, but no doubt the refractory linings which come in contact with the molten glass will need to be carefully chosen and handled. Either dry steam or air at around 100 pounds per square inch can be used for blowing the wool.
Other equipment needed will include that necessary to quarry and convey the raw materials to the plant, a crusher, and possibly some means of calcining or sintering the raw materials. Also a collecting room into which the wool may be blown, means for collecting the wool, and equipment for cleaning and preparing the wool for the market, such as shredding machinery and wool compressors, will have to be considered. Cement and brick plants have much equipment that could be used in manufacturing rock wool. This includes equipment for quarrying and conveying materials to the plant, crushers, and kilns for calcining the rock.
Although the unit of production with the coke-fired cupola is usually 1000 pounds per hour, it should not be necessary to use a gas- or oil-fired furnace of such large capacity for initiating production. One experimental furnace in the state has a total capacity of one cubic foot of raw material, and is tapped in three places for blowing. The use of small capacity furnaces at the beginning of production is recommended, since the cost per ton of wool should not be materially increased thereby, and it would have the advantage of both greater flexibility in adapting production to market and a smaller investment. Very small capacity plants could probably be operated profitably, and seem preferable to the over-capitalization which has marked the development of other industries in the state in the past. This is especially true in the rock wool industry with its high freight rates on the finished product, with consequent inability to compete on distant markets.
Rock wool is prepared for market in a variety of forms: Loose fluffy, loose granulated, and bats. It is sewn into quilts between paper or wire netting, and fabricated into plasters, bricks, and rigid boards. In any of these forms it is convenient for use in the building industry.
Loose fluffy rock wool requires little processing other than the removal of coarse fibers and shot. Loose granulated wool is broken into shorter fibers by means of shredders, or similar machines. Bats are formed by compression and the application of heat. Rock wool which is added to plaster may be sold in sacks or fabricated into a rigid board. Asphalt and other materials are used as binders in the manufacture of rigid forms, the process depending upon the material used as a binder for the fibrous material.
The chief use of rock wool is in the insulation of dwellings and other buildings. This material may be used in existing structures. Granulated or fluffy wool is placed between the ceiling joists, and bats or quilts between the rafters in the attic; granulated material is blown into closed spaces between inner and outer walls and between ceilings and the floor above by means of special equipment. In new structures the walls are usually insulated by placing bats or quilts between the studding. [Rock Wool from Illinois Mineral Resources: Illinois State Geol. Survey, Bull. 61, 1934.]
The maximum of efficiency is obtained from a summer cooling system when rock wool is used as insulation. The necessity of insulation of this kind is recognized as the first step preceding the installation of an air-conditioning plant. The growing demand for air-cooling in houses and other structures should insure a future demand for insulating materials.
Rock wool is used as a protection against the transfer of heat in many ways other than the insulation of buildings. It is effective in preventing house pipes and underground pipes from freezing; its use prevents loss of heat from pipes carrying steam, molten sulphur, hot oil, etc. It is valuable in the insulation of ovens, furnaces, and stoves, including steam boilers, fractionating columns, towers. annealing, baking and enameling ovens, retorts, and metallurgical and chemical furnaces. It is used in the insulation of refrigerators, ice boxes, water coolers, and refrigerator cars.
For sound control this material is used in the form of acoustic tiles, and mats placed under carpets. in walls and in partitions. It has been used extensively for insulating broadcasting studios.
Miscellaneous uses for rock wool cover a wide range. These include its use as packing for acid carboys filter mediums for corrosive fluids, as an air filter in hot-air heating, a component of insulating cement, for prevention of the entrance of vermin, fire prevention, a constituent of polishing wheels, lining between planking and metal sheathing of ships, etc.
Materials competing with rock wool include fiber boards, plaster boards, balsam wool, aluminum foil-covered paper, paper felts, diatomaceous earth bricks, and cork board slabs. Rock wool has many advantages compared with these heat-insulating materials.
Among these advantages the following are quoted (Fryling and White, 1935).
"On the basis of its coefficient of heat transfer, mineral wool ranks among the best commonly available heat insulating materials.
"Deterioration is practically nil. It is quite usual to find it unimpaired after 25 years or more of service.
"In contrast with certain organic materials, it is inert toward moisture and, consequently, its insulating properties show less tendency to fluctuate with changing weather conditions.
"It is thoroughly fireproof and vermin proof. This should be of importance from the standpoints of health and insurance rates.
"It can be fabricated in forms convenient for use in the building industry, such as loose fill, rigid board, and quilts.
"It is an excellent acoustical insulation material ...
"It is relatively inexpensive and the indications are that it can be produced more cheaply than it is at present."
Notice: It is planned to issue a supplement to this bulletin on or about October 1, 1937. The supplement will include tests run on rock samples collected since September 1, 1936.
Fryling, Charles F., and White, Orval, 1935, Considerations in developing a mineral wool industry: Chem. and Met. Eng., vol. 42, no. 10, Oct.
Lamar, J. E., Willman, H. B., Fryling, C. F., and Voskuil, W. H., 1934, Rock wool from Illinois mineral resources: Illinois Geol. Survey, Bull. 61, 282 p.
Newell, Norman D., and Jewett John M., 1935, The Geology of Johnson, Miami, and Wyandotte counties Kansas: Kansas Geol. Survey, Bull. 21 [available online: Johnson and Miami; Wyandotte]
Kansas Geological Survey, Rock Wool Resources of Kansas
Placed on web July 31, 2015; originally published March 1, 1937.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/MRC5/index.html