
Kansas Geological Survey, Chemical Quality Series 2, originally published in 1975
Next Page--Appendices

Originally published in 1975 as Kansas Geological Survey Chemical Quality Series 3. This publication is also available as an Acrobat PDF file (13 MB).
The principal aquifer in southern Wallace, Greeley, Wichita, Scott, and Lane Counties is the Ogallala formation. Analysis of ground water samples collected from 154 irrigation wells in this 4 1/2 county area during summer of 1974 indicate waters of this region are basically calcium-magnesium bicarbonate in nature. Higher salinities and increased sulfate contents are observed for wells in parts of the Scott Basin area, which appear to be in association with saline soils.
Compiled chemical quality data and chemical quality contour maps for the area of study are presented. This report represents the first phase of a program directed toward establishing base line chemical quality data for irrigation waters in western Kansas.
The primary objective of this study is a set of unified ground water chemical quality data for irrigation wells of the hydrogeologic observational network in west-central Kansas, i.e., southern Wallace, Greeley, Wichita, Scott and Lane Counties (Figure 1). Most of the 154 wells used in this study derive water from the Ogallala formation of Pliocene age, exceptions being wells in southeastern Scott County which are associated with the Niobrara Chalk of Cretaceous age or undifferentiated Quaternary-Ogallala sequences. Bedrock in southern Wallace County is the Pierre Shale of Cretaceous age and the Niobrara Chalk in the remainder of the area of study (Stullken et al., 1974; Keene and Pabst, 1971). Historical chemical quality data exist for about 21% of the wells considered in this investigation, but variations in time-location and sampling conditions make evaluation of potential changes in quality of the ground water system difficult to assess using these data alone.
Figure 1--Index map of Kansas showing area discussed in this report.
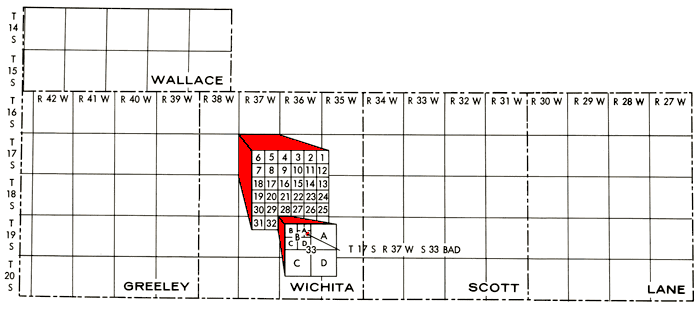
well locations listed are in accordance with the bureau of land management numbering system. the location is composed of the township, the range, and the section number, followed by letters that indicate the subdivision of the section in which the well is located. the first letter denotes the 160-acre tract; the second, the 40-acre tract; and the third, the 10-acre tract (figure 2).
Figure 2--Well location system used in this report.
Ground water samples were collected over a three-day period during the high production portion of the season, July 30-August 1, 1974, from pumping wells which had been in operation for some time prior to sampling. Water samples from the producing wells were found to be free of suspended material, thus eliminating the need for field filtration. Samples were collected at the wellhead whenever possible to minimize the possibility of contamination.
Three types of samples were collected in acid-rinsed polyethylene bottles at each well, with two of the three bottles containing premeasured amounts of hydrochloric acid (HCl). The acidified samples were used in nitrate-phosphate (0.2% in HCl) and trace element--iron, zinc, manganese, and nickel--(1% in HCl) determinations. The unacidified sample was used in the determinations of the major constituents. Fifteen duplicate sets of samples also were collected to provide a measure of analytical reliability. All samples were kept cool in ice chests until they were returned to the field laboratory in Scott City. Measurements of pH, temperature, and specific conductance were also made at each well during the sampling.
Samples returned to the field laboratory were logged, the nitrate-phosphate samples frozen, and the trace element samples refrigerated for return to the Kansas Geological Survey laboratory in Lawrence. Portions were withdrawn from the unacidified samples for analysis, with bicarbonate (HCO3 being determined by potentiometric titration and chloride (Cl) and fluoride (F) by specific-ion electrode measurements. In every case, HCO3 determinations were made the same day the samples were collected. The unacidified samples were then stored at room temperature. After completion of the field work, samples were returned to the Kansas Geological laboratory in Lawrence for the remaining analyses, with the acidified samples being transported in ice chests under ice.
Silica (SiO2), calcium (Ca), magnesium (Mg) and strontium (Sr) were determined in portions of the unacidified samples using standard atomic absorption techniques. Iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn) were determined by atomic absorption spectroscopy in portions of the acidified samples which had been concentrated ten-fold by evaporation. Flame emission spectroscopy was used in the determination of sodium (Na) and potassium (K). Nitrate (NO3) levels were determined by ultra-violet absorption spectroscopy.
In the case of phosphate (PO4), total PO4 determinations were made on 24 samples whose locations were widely dispersed in the area of study. The remaining samples were screened to a 0.12 mg/l ortho-PO4 level and total PO4 measurements were made on those samples showing above the screening level. Analytical methods for the determinations mentioned above are found in Standard Methods (American Public Health Association, 1971).
Sulfate (SO4) determinations were made using a modification of the atomic absorption technique of Dunk et al. (1969). An acidified stock solution containing barium chloride and excess sodium chloride, added as a flame buffer, was added to portions of the unacidified water samples. After standing for 12 hours, relative concentrations of excess barium in the solutions were measured and related to the amounts of SO4 originally present by comparison with standard SO4 solutions treated in a similar manner.
An ammonia specific-ion electrode was used to check for the presence of ammonium (NH4) salts in samples taken from the delivery pipe down-stream from the wellhead where fertilizer contamination would be a possibility.
Compiled chemical quality data for ground waters from the irrigation wells as determined for this study are listed by county and location in Appendix A. Concentration data are given in ppm (parts per million or mg/l) for all species except the trace elements. Concentrations of Fe, Mn, Zn, and Ni are given in ppb (parts per billion or µg/l). Standard deviations for the various analyses as based upon the 15 sets of duplicate samples are given in Table 1.
Table 1--Standard Deviations of Chemical Determinations
| Determination | ±σ |
|---|---|
| SiO2 | 2.8 ppm |
| Ca | 0.7 ppm |
| Mg | 0.5 ppm |
| Na | 0.3 ppm |
| K | 0.03 ppm |
| HCO3 | 1.2 ppm |
| SO4 | 4.0 ppm |
| Cl | 0.6 ppm |
| F | 0.02 ppm |
| NO3 | 0.3 ppm |
| Sr | 0.05 ppm |
| Fe | 7.6 ppb |
| Mn | 0.7 ppb |
| Zn | 17.8 ppb |
| Ni | 4.3 ppb |
N 2
![standard deviation is the square root of [(half the sum of the square of the difference between each point and the mean), all divided by the number of points]](gifs/equ1.gif) ri = range for analysis of sample pairs N = numbers of sample pairs |
|
A computerized plotting program using a weighted octant search routine was used in the interpretation of the ground water chemical quality data (Sampson, 1973). For a grid node to be seen in the program, at least two octants must contain a total of at least five wells, with one of the octants having at least three wells. Further restraints limit the number of wells considered per octant to three, with at least one well in the octant being within 12 miles of the grid node before the octant is considered. The weighting factor used was 1/D6, where D is the distance from the well to the grid node. The distance between grid nodes was taken as 3/4 mile.
The assignment of absolute confidence levels for contour lines at the boundaries of the mapped area or in regions of limited sample data is a difficult problem at best. Generally speaking, the reliability of contour lines on the maps generated by the plotting routine is expected to be a function of the well density distribution, i.e., greatest in regions of high well density. Figure 3 gives the distribution of wells within the area of study. However, the confidence in the contour interval can never exceed that of the analytical data upon which it is based. Data from south-central Scott County indicate waters of widely varying composition exist and can be detected with a sufficient degree of confidence in wells no more than 1-2 miles apart. In this study, regions of relative reliability of the contour intervals have been designated on the basis of several criteria (Figure 4).
Figure 3--Irrigation well distribution in the study area. Principal drainage systems in the area are also indicated.
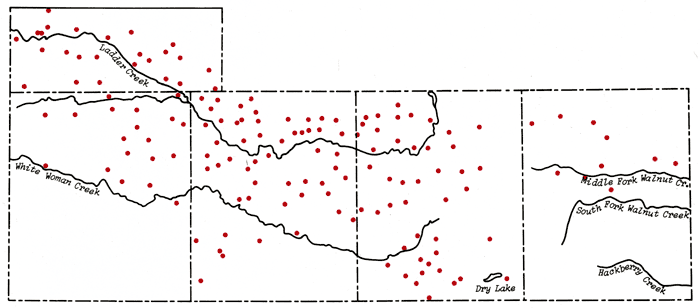
Figure 4--Contour reliability map for the chemical quality contour maps of Appendix B.
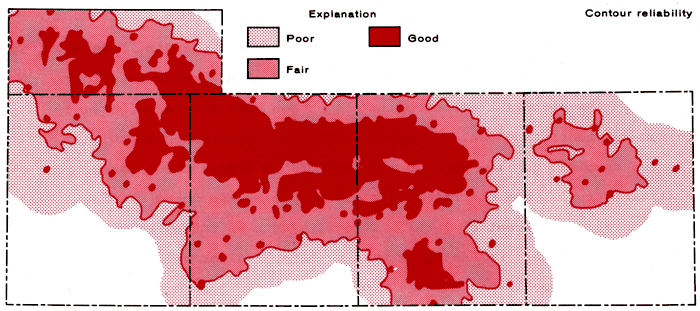
Regions of good reliability are those in which a well is within 2 miles of the grid node and six octants about the grid node contain one well each within a distance of 6 miles of the grid node. In the case of isolated wells, it is assumed that the concentrations are well known in the immediate vicinity of the wells and regions of good reliability exist within areas 1/2 mile from the wells. Regions of fair reliability are taken to be those areas between the defined boundaries for good and poor regions. Regions of poor reliability are assumed to exist when no well is within 4 miles of a grid node or when fewer than three octants about a grid node contain one well each within a distance of 6 miles of the grid node. Contouring is cut-off in regions where the distance from the grid node to the nearest well is greater than 6 miles.
Contour intervals for the chemical quality maps (Appendix B) were chosen so as to divide the total concentration range of each species into four portions. The approximate contour levels chosen were mid-range between minimum and mean, the mean, and mid-range between mean and maximum.
The chemical quality contour maps are of two types, those based upon areal variation in concentration of a given species and those showing areal variation of the percent of total milliequivalents of cation/anion of a given species. The former are useful in gaining a feeling for relative intensities of the chemical species in different portions of the system; whereas the latter show similarities or differences in the nature of the solutes in different parts of the ground water system. Table 2 lists factors for conversion of ppm to milliequivalents per liter.
Table 2--Factors for Conversion of Parts Per Million to Milliequivalents Per Liter
| Species | Multiply by |
|---|---|
| Calcium | 0.04990 |
| Magnesium | 0.08226 |
| Sodium | 0.04350 |
| Potassium | 0.02557 |
| Strontium | 0.02283 |
| Bicarbonate | 0.01639 |
| Sulfate | 0.02082 |
| Chloride | 0.02821 |
| Fluoride | 0.05264 |
| Nitrate | 0.01613 |
Figures 5 and 6 show ground waters for the area of study are basically Ca-Mg-HCO3 waters which grade into SO4-rich waters in localized regions (Piper, 1944; Stiff, 1951; Hem, 1970). The relative importance of the various major chemical species, expressed as percent of the total milliequivalents of cations/anions, in relation to the make-up of the dissolved solids in the ground water system is illustrated in maps of Appendix B. It appears that Ca is prominent along the northern and southern margins of the mapped area, becoming greatest in parts of the southern margin. HCO3 is most pronounced along the northern margin and southwest portion of the study area. Na tends to exhibit a maximum influence in the general area north of the Ladder Creek area (Figure 3). Major contributions from Mg, SO4, and Cl appear to be confined to a general central region between Ladder Creek and White Woman Creek (Figure 3) extending to the southern part of Lane County.
Figure 5--Modified Piper diagram for ground waters in the study area. Well numbers correspond to those found in Appendix A.
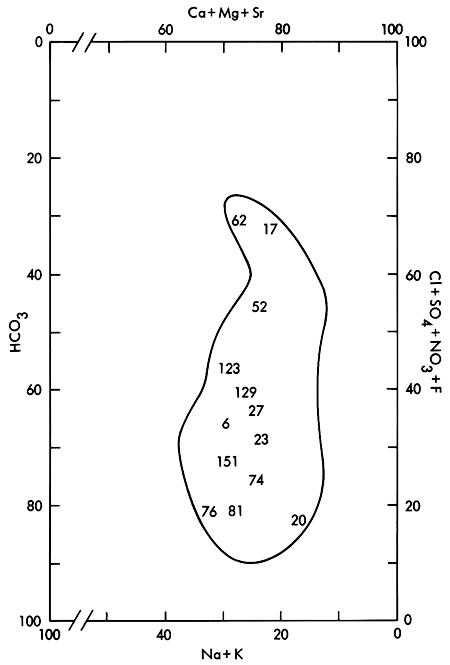
Figure 6--Areal distribution of Stiff diagrams for selected wells. Well numbers correspond to those found in Appendix A.
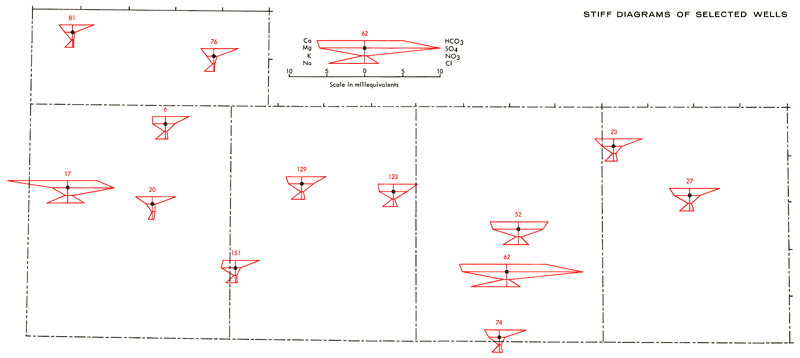
The Stiff diagrams in Figure 6 are positioned over the corresponding well locations on the base map. From these diagrams it is possible to obtain a feeling for the areal variation in both the dissolved solid content and compositional make-up of the dissolved solids in ground water samples from the study area. The amount of dissolved solids in these waters tends to increase in a general northwest and southeast fashion, becoming high locally in portions of the Scott Basin area of south-central Scott County. Well 17 in western Greeley County also appears to be anomalously high. This same overall behavior is noted for most of the areal concentration contour maps of Appendix B. Systematic areal patterns or relationships are not readily apparent for the PO4 data. Locally high PO4 values may reflect fertilizer contamination in wells.
The combining capacities of the various major chemical species, expressed as milliequivalents per liter in the Stiff diagrams, reflect the following compositional trends for the dissolved solids in the bulk of the ground water samples:
Ca > Mg with Na and K contributing lesser amounts
HCO3 > SO4 with Cl and NO3 contributing lesser amounts
Three exceptions to these general trends in composition do occur. Well 17 is a shallow well located along White Woman Creek in Greeley County. The Stiff diagram for this well shows the water there to be a Ca-SO4 rich water, which is atypical of other ground water samples in the study area. The high Ca-SO4 content of this water possibly reflects effects of surface leaching of gypsum from the soil. Historical data for this well from March, 1972, represent basically a Ca-Mg-HCO3 type water with a specific conductance of 710 micromhos, as compared to 1270 micromhos from this study. It is impossible at present to state whether this represents a permanent change in chemical quality or a temporary effect resulting from local recharge during irrigation.
Wells 150, 151, and 154 in southwest Wichita County are associated with HCO3 waters in which the combining capacity of Mg exceeds that of Ca. Waters of this type could arise as a result of loss of Ca by calcite precipitation from saturated solutions or from differential leaching of Mg and Ca in calcareous deposits associated with shallow depressions in the area. Additional field work would be necessary in order to elucidate the origins of these waters. Wells 62, 67, and 69 in the Scott Basin area produce waters in which the cation distribution in the Stiff diagrams is similar to that of ground waters found in the middle portion of the study area (Wells 6, 129, 123, 52, 74, and 27 of Figure 6), but with SO4 exceeding HCO3 by 1.5-2 to 1. There are other well waters, however, within the Scott Basin area which are of lower salinity and have HCO3 as the predominate anion, indicating a localized cause for the higher salinity and SO4 content in these three wells.
Figure 3 shows the termination of White Woman Creek in south-central Scott County, at a point corresponding to the intersection with the Scott Basin depression. A soil survey of Scott County (Sallee and Hamilton, 1965) indicates the presence of saline soils in the Scott Basin area (Figure 7). The regions of saline soil occurrence coincide remarkably well with locations of ground waters having higher salinity and SO4 content. This suggests the possibility that salts accumulated in the soil from waters entering Scott Basin, probably in large part from White Woman Creek, may be responsible for the variable chemistry of the ground waters in this area.
Figure 7--Map showing occurrence of Church silty clay loam and Ulysses silt loam saline soils in Scott County. Modified after Sallee and Hamilton (1965).
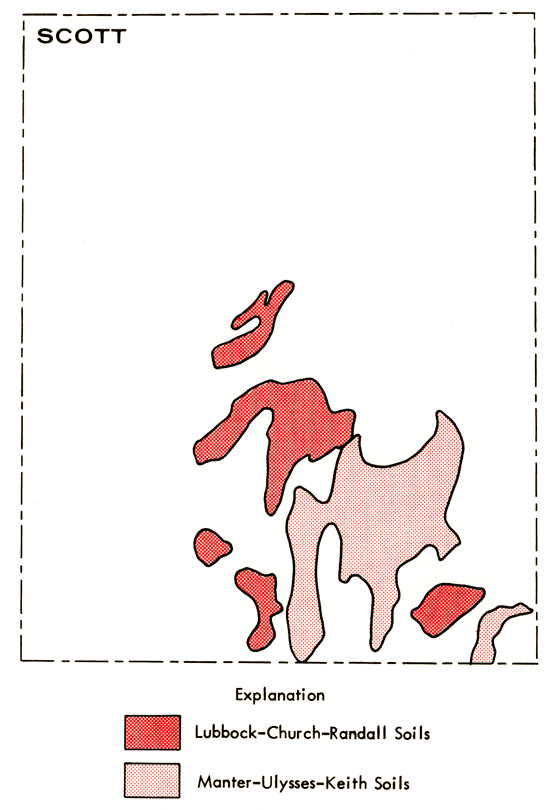
Table 3 represents correlation matrices based upon percent of total milliequivalents of cations/anions for wells in Townships 14 through 18, excluding well 17 in Greeley County, and the 14 wells of the Scott Basin area. Positive correlations significant at the 95% confidence level for wells in Townships 14-18 include Mg-SO4, Mg-Cl, Na-K, NA-HCO3, K-HCO3, and ci-SO4. These relationships suggest close association between Na and K and that both tend to enter this portion of the groundwater system as bicarbonates and not chlorides. Mg apparently enters in association with SO4 and Cl.
Table 3--Percent of Total Milliequivalents of Cations/anions Correlation Matricies
| A. 128 wells located in Townships 14 through 18, exclusive of Well 17 in Greeley County | ||||||||||
| %Ca | %Mg | %Na | %K | %Sr | %HCO3 | %SO4 | %Cl | %F | %NO3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| %Ca | 1.000 | -.434 | -.666 | -.245 | -.251 | .114 | -.279 | .067 | -.136 | .463 |
| %Mg | 1.000 | -.291 | -.219 | .372 | -.570 | .489 | .500 | .041 | -.358 | |
| %Na | 1.000 | .420 | -.032 | .346 | -.188 | -.481 | .108 | -.219 | ||
| %K | 1.000 | -.221 | .636 | -.603 | -.473 | .320 | -.072 | |||
| %Sr | 1.000 | -.262 | .269 | .188 | -.153 | -.142 | ||||
| %HCO3 | 1.000 | -.887 | -.844 | .200 | .080 | |||||
| %SO4 | 1.000 | .554 | -.236 | -.276 | ||||||
| %Cl | 1.000 | -.102 | .020 | |||||||
| %F | 1.000 | -.198 | ||||||||
| %NO3 | 1.000 | |||||||||
| 95% Confidence level = .146 | ||||||||||
| B. 14 Wells located in Scott Basin Area | ||||||||||
| %Ca | %Mg | %Na | %K | %Sr | %HCO3 | %SO4 | %Cl | %F | %NO3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| %Ca | 1.000 | -.862 | -.871 | .732 | -.704 | .436 | -.541 | .605 | -.261 | .180 |
| %Mg | 1.000 | .514 | -.693 | .842 | -.538 | .569 | -.442 | .116 | -.023 | |
| %Na | 1.000 | -.641 | .359 | -.282 | .416 | -.578 | .265 | -.276 | ||
| %K | 1.000 | -.346 | .808 | -.798 | .158 | .284 | .160 | |||
| %Sr | 1.000 | -.240 | .293 | -.507 | .209 | .083 | ||||
| %HCO3 | 1.000 | -.963 | -.096 | .704 | .373 | |||||
| %SO4 | 1.000 | -.125 | -.582 | -.553 | ||||||
| %Cl | 1.000 | -.556 | .216 | |||||||
| %F | 1.000 | .028 | ||||||||
| %NO3 | 1.000 | |||||||||
| 95% Confidence level = .458 90% Confidence level = .365 |
||||||||||
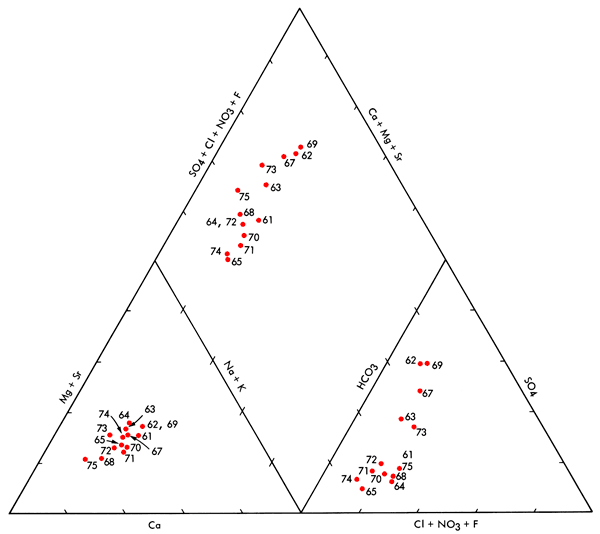
In the Scott Basin area positive correlations significant at the 95% confidence level include Mg-Na, Mg-SO4, K-HCO3, Ca-K, and Ca-Cl. In addition, CA-HCO3 and Na-SO4 are found to be significant at the 90% confidence level. The lack of positive correlation between Na-K, NA-HCO3, and MgCl noted previously and the positive correlations between Mg-Na, Mg-SO4, and Na-SO4 are consistent with the notion that salts enriched in Na-Mg-SO4 content have been added locally to the groundwater system in the Scott Basin area, possibly through leaching of the saline soils found there. The Piper diagram (Figure 8) and general trend of increasing salinity with increasing sulfate content for wells in the Scott Basin area also are consistent with this conclusion.
Figure 8--Piper diagram for ground waters from the Scott Basin area. Well numbers correspond to those found in Appendix A.
The concentration data for the trace elements (Fe, Mn, Zn, and Ni) show positive correlations for Fe-Zn and Mn-Ni for the study area as a whole. These associations are the same as those cited by Jenne (1968) for trace element associations in soils. Conclusions based upon the zinc data may be open to some question, however, due to the large uncertainty involved in the duplicate sets of zinc determinations (Table 1). Problems with zinc contamination in pumping wells have been noted by Carpenter and Miller (1969) in their study of saline waters in Saline County, Missouri, and may be a factor here.
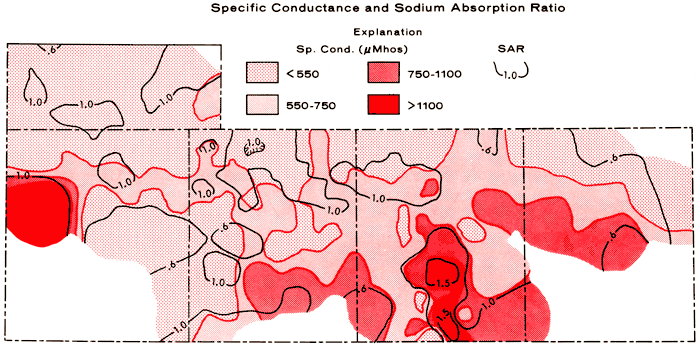
With respect to irrigation quality, groundwaters in the study area are of low alkali hazard, but range from medium to high in salinity hazard (U.S. Salinity Laboratory Staff, 1954). Medium and high salinity hazards are represented by ranges in specific conductance of 250-750 and 750-2,250 micromhos, respectively. Figure 9 is a composite areal plot of specific conductance and the sodium-adsorption ratio (SAR), which is used to define the alkali hazard. The SAR value for a water is determined by:
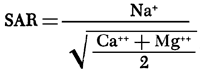
where (Na), (Ca), and (Mg) are the milliequivalents per liter concentrations of the respective species. High salinity hazard is noted for well 17 in Greeley County and parts of the south-central to southeastern portions of the area of study.
Figure 9--Composite contour map of specific conductance and the sodium-adsorption ratio for the study area. Ranges of salinity hazard, based upon conductivity expressed in micromhos/cm at 25°C: low (100-250), medium (250-750), high (750-2,250), and very high (2,250). SAR values correspond to low alkali hazard throughout the study area.
U.S. Public Health Service (1962) recommended maximum values for drinking water of 500 ppm for dissolved solids, 250 ppm for SO4, and 45 ppm for NO3 are approached or exceeded locally within the 4 1/2 county area. The high fluoride background approaches and exceeds the recommended upper limit of 1.7 ppm in the eastern half of the area of study.
Comparison of the data collected during this study with historical data from 1970-1972, shows an average increase of 8% in the specific conductance, excluding well 17 in Greeley County, and 73% in the NO3 content for 21 wells. It is uncertain at this point whether these values represent true changes in chemical quality or reflect only seasonal changes since most of the historical data represents samples collected during the first half of the year, and under unknown pumping conditions. Also, sample preservation represents another unknown factor in the historical NO3 data since the nitrogen balance in water samples may be changed through biological activity (American Public Health Association, 1971).
In conclusion, it should be noted that other factors such as the relief and composition of the bedrock surface probably have important but yet undetermined effects upon the groundwater quality in Scott Basin and other portions of the system. Also, most of the discussion presented in this report has ignored the depth of interval within the aquifer from which the groundwater samples have been obtained, primarily because of uncertainties in the locations of screened intervals of the wells. Additional interpretation of the chemical quality data using methods such as factor analysis may provide useful relationships between quality and a number of features of the system such as well depth, saturated thickness of the aquifer, bedrock composition and relief, drainage patterns, and soil types.
The authors wish to acknowledge the services of personnel from the U.S.G.S.-K.G.S. office in Garden City, Kansas, in the collection of the water samples; and A. M. Diaz of the U.S.G.S. office in Lawrence, Kansas, in the operation of the field laboratory. A special note of appreciation is due Mr. Clarence Williams, Superintendent of Schools, Scott City, Kansas, for making high school facilities available for the field laboratory.
American Public Health Association, 1971, Standard Methods for Examination of Water and Waste Water (13th ed.): Washington, D. C., 874 p.
Carpenter, A. B. and Miller, J. C., 1969, Geochemistry of saline subsurface waters, Saline County (Missouri): Chemical Geology, v. 4, no. 1/2, p. 135-167.
Dunk, R., Mostyn, R. A., and Hoare, H. C., 1969, The determination of sulfate by indirect atomic absorption spectroscopy: Atomic Absorption Newsletter, v. 8, no. 4, p. 79-81.
Hem, J. D., 1970, Study and interpretation of the chemical characteristics of natural water: U.S. Geological Survey, Water-Supply Paper 1473, 363 p.
Jenne, E. A., 1968, Controls on Mn, Fe, Co, Ni, Cu, and Zn concentrations in soils and water; the significant role of hydrous Mn and Fe oxides; in, Trace Inorganics in Water: American Chemical Society Advances in Chemistry Series v. 73, p. 337-387.
Keene, K. M. and Pabst, M. E., 1971, Hydrogeologic data from Gove, Logan, and Wallace Counties, Kansas: Kansas Geological Survey Basic-Data Release No. 2, 76 p.
Piper, A. M., 1944, A graphic procedure in the geochemical interpretation of water analyses: Transactions American Geophysical Union, v. 25, p. 914923.
Salee, K. H. and Hamilton, V. L., 1965, Soil survey of Scott County, Kansas: U.S. Department of Agriculture, Series 1961, no. 33, 66p.
Sampson, R. J., 1973, Users manual for the Surface II graphics system: Kansas Geological Survey.
Stiff, H. A., 1951, The interpretation of chemical water analysis by means of patterns: Journal of Petroleum Technology, v. 3, no. 10, p. 15-17.
Stullken, L. E., Weakly, E. C., Gutentag, E. D., and Slagle, S. E., 1974, Hydrogeologic data from Greeley, Wichita, Scott and Lane Counties, Kansas: Kansas Geological Survey Basic Data Series, Groundwater Release No. 4, 58 p.
U.S. Public Health Service, 1962, Drinking water standards, 1962: U.S. Public Health Service Publication 956, 61 p.
U.S. Salinity Laboratory Staff, 1954, Diagnosis and improvement of saline and alkali soils: U.S. Department of Agriculture Handbook, 60, 160 p.
Kansas Geological Survey
Placed on web Oct. 17, 2012; originally published in 1977.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS4/index.html