Kansas Geological Survey, Chemical Quality Series 12, originally published in 1991
Prev Page--Report Start ||
Next Page--Summary, References
Table 3 shows the concentrations of TFP and TOC in the untreated samples. The concentrations of the four individual THM species are given in µg/L and then summed to give TFP in µg/L and micromoles per liter (µM). The percentage chlorine (%Cl) is the percentage of the halogen atoms in the THMs composed of chlorine, the balance being bromine. The yield, in µmol of TFP per mg of TOC, is a calculated value. The chlorine demand is the amount of free chlorine consumed during the 96-hr incubation period in the subsample used for TFP analysis.
In table 3, as well as tables 4-6, a number of the values reported are below the detection limit. In computing the mean, sample standard deviation (SD), geometric mean (G. Mean), and median for a constituent, values less than the detection limit were assumed equal to one half of the detection limit. Geometric means and medians are reported because the concentrations of many constituents were not normally distributed.
Table 3--TFP and TOC in untreated ground-water samples.
| Sample | CHCl3 µg/L |
CHCl2Br µg/L |
CHClBr2 µg/L |
CHBr3 µg/L |
TFP | TOC mg/L |
Yield µmoles/mg |
Chlorine Demand mg/L |
||
|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | µM | %Cl | ||||||||
| 1 | 8.9 | 12.0 | 8.7 | 1.3 | 30.9 | 0.195 | 71 | 0.69 | 0.282 | 1.71 |
| 2 | 85.2 | 14.1 | 3.4 | <0.1 | 102.7 | 0.816 | 95 | 3.31 | 0.247 | 10.61 |
| 3 | 91.2 | 22.8 | 7.2 | <0.1 | 121.2 | 0.938 | 93 | 2.56 | 0.366 | 7.20 |
| 4 | 9.0 | 3.7 | 1.6 | <0.1 | 14.3 | 0.106 | 88 | 0.36 | 0.294 | 1.95 |
| 5 | 1.7 | 2.2 | 9.0 | 12.7 | 25.6 | 0.121 | 31 | 0.48 | 0.252 | 7.20 |
| 6 | 7.5 | 16.2 | 28.5 | 19.4 | 71.7 | 0.375 | 47 | 1.04 | 0.361 | 1.71 |
| 7 | 36.1 | 32.2 | 24.4 | 4.9 | 97.6 | 0.635 | 74 | 1.90 | 0.334 | 2.44 |
| 8† | 27.9 | 21.9 | 30.7 | 15.8 | 96.3 | 0.577 | 64 | 1.37 | 0.421 | 1.77 |
| 9 | 26.7 | 28.4 | 23.3 | 5.6 | 83.9 | 0.530 | 71 | 2.19 | 0.242 | 2.81 |
| 10 | 8.3 | 13.4 | 13.1 | 3.7 | 38.4 | 0.228 | 63 | 1.03 | 0.221 | 3.72 |
| 11 | 37.0 | 28.6 | 18.7 | 3.3 | 87.7 | 0.588 | 78 | 1.90 | 0.309 | 7.44 |
| 12 | 3.4 | 4.5 | 4.5 | 2.4 | 14.8 | 0.087 | 62 | 0.41 | 0.212 | 0.37 |
| 13 | 3.2 | 2.5 | 3.0 | 2.7 | 11.4 | 0.067 | 62 | 0.31 | 0.217 | 0.24 |
| 14 | 4.0 | 3.2 | 2.3 | 0.2 | 9.7 | 0.065 | 77 | 0.30 | 0.217 | 1.22 |
| 15 | 4.3 | 7.3 | 7.6 | 3.0 | 22.2 | 0.129 | 60 | 0.85 | 0.152 | 0.61 |
| 16 | 27.3 | 20.1 | 14.2 | 2.5 | 64.1 | 0.429 | 78 | 1.52 | 0.282 | 2.14 |
| 17 | 4.1 | 13.6 | 24.9 | 19.1 | 61.6 | 0.312 | 42 | 1.06 | 0.294 | 1.65 |
| 18 | 9.5 | 8.7 | 5.6 | 1.0 | 24.8 | 0.163 | 76 | 0.87 | 0.188 | 6.83 |
| 19 | 6.1 | 3.9 | 3.9 | <0.1 | 14.0 | 0.094 | 78 | 0.45 | 0.209 | 1.34 |
| 20* | 64.2 | 29.2 | 12.7 | 0.8 | 106.9 | 0.780 | 87 | 2.84 | 0.275 | 9.88 |
| 21 | 1.2 | 5.3 | 27.0 | 44.9 | 78.4 | 0.349 | 21 | 1.20 | 0.291 | 3.17 |
| 22 | 3.4 | 6.7 | 12.4 | 4.4 | 26.9 | 0.146 | 52 | 0.58 | 0.252 | 0.98 |
| 23 | 9.1 | 10.2 | 8.7 | 2.0 | 30.0 | 0.188 | 70 | 0.83 | 0.227 | 3.90 |
| 24 | 2.8 | 4.4 | 4.8 | 2.3 | 14.3 | 0.083 | 60 | 0.52 | 0.159 | 0.24 |
| 25† | 3.5 | 11.5 | 22.2 | 19.6 | 56.8 | 0.283 | 39 | 1.34 | 0.211 | <0.06 |
| 26 | 6.5 | 12.6 | 15.3 | 4.6 | 38.9 | 0.222 | 58 | 0.72 | 0.308 | 0.61 |
| 27† | <0.1 | 3.3 | 7.6 | 7.4 | 18.3 | 0.086 | 30 | 0.47 | 0.183 | <0.06 |
| 28 | 4.5 | 5.3 | 3.8 | <0.1 | 13.5 | 0.087 | 74 | 0.55 | 0.159 | <0.06 |
| 29 | 3.1 | 4.1 | 4.3 | 1.4 | 12.9 | 0.078 | 64 | 0.49 | 0.158 | 0.06 |
| 30 | 4.3 | 18.4 | 33.1 | 21.6 | 77.5 | 0.393 | 42 | 1.54 | 0.255 | 1.83 |
| 31 | 3.6 | 13.4 | 19.7 | 10.4 | 47.1 | 0.248 | 47 | 1.00 | 0.248 | 1.16 |
| 32 | 35.7 | 27.5 | 19.4 | 3.4 | 86.0 | 0.573 | 77 | 2.43 | 0.236 | 2.32 |
| 33 | 1.1 | 4.3 | 8.5 | 5.9 | 19.8 | 0.100 | 41 | 0.60 | 0.166 | 0.12 |
| 34 | 3.4 | 5.2 | 5.4 | 1.9 | 15.8 | 0.093 | 62 | 0.50 | 0.186 | <0.06 |
| 35 | 11.0 | 10.7 | 7.9 | 1.0 | 30.6 | 0.199 | 74 | 0.88 | 0.226 | 1.22 |
| 36 | 3.6 | 3.1 | 1.6 | <0.1 | 8.3 | 0.057 | 80 | 0.27 | 0.210 | 0.85 |
| 37 | 1.5 | 2.1 | 1.7 | <0.1 | 5.3 | 0.033 | 71 | 0.21 | 0.158 | <0.06 |
| 38‡ | <0.1 | 0.4 | 1.4 | 3.9 | 5.8 | 0.025 | 16 | 0.29 | 0.086 | >24.40 |
| 39 | 0.3 | 1.3 | 3.6 | 4.0 | 9.2 | 0.044 | 31 | 0.31 | 0.141 | 7.63 |
| 40 | 4.2 | 10.7 | 12.7 | 5.7 | 33.4 | 0.184 | 54 | 0.98 | 0.188 | 0.24 |
| 41 | 9.6 | 17.9 | 22.3 | 9.4 | 59.2 | 0.334 | 57 | 1.27 | 0.263 | 0.61 |
| 42 | 7.4 | 12.4 | 15.6 | 6.4 | 41.8 | 0.238 | 58 | 1.10 | 0.216 | 3.48 |
| 43 | 5.9 | 15.0 | 29.3 | 23.2 | 73.5 | 0.374 | 42 | 1.02 | 0.366 | 7.93 |
| 44 | 0.4 | 6.5 | 7.2 | 3.1 | 17.3 | 0.090 | 46 | 0.50 | 0.180 | 0.06 |
| 45 | 3.4 | 6.8 | 8.1 | 3.4 | 21.8 | 0.123 | 57 | 0.80 | 0.153 | 0.55 |
| 46 | 1.3 | 3.7 | 4.6 | 1.9 | 11.4 | 0.063 | 53 | 0.36 | 0.174 | <0.06 |
| 47 | 1.3 | 11.6 | 58.2 | 107 | 178.0 | 0.784 | 19 | 2.14 | 0.367 | ND** |
| 48 | 7.2 | 4.3 | 2.0 | <0.1 | 13.5 | 0.096 | 84 | 0.37 | 0.257 | ND |
| 49* | 52.6 | 43.5 | 32.9 | 5.5 | 134.4 | 0.885 | 76 | 2.45 | 0.362 | 9.94 |
| 50 | 2.3 | 3.9 | 5.2 | 2.2 | 13.6 | 0.077 | 57 | 0.48 | 0.160 | 6.34 |
| Mean | 13.5 | 11.7 | 13.3 | 8.2 | 46.7 | 0.281 | 61 | 1.03 | 0.242 | 2.69 |
| SD | 20.9 | 9.5 | 11.5 | 16.7 | 39.5 | 0.250 | 18 | 0.76 | 0.070 | 3.09 |
| G. Mean | 5.4 | 8.5 | 9.2 | 2.2 | 32.5 | 0.192 | 58 | 0.81 | 0.232 | 0.95 |
| Median | 4.3 | 10.2 | 8.7 | 3.3 | 30.6 | 0.188 | 62 | 0.84 | 0.227 | 1.65 |
| *This sample was filtered through a glass-fiber filter (934 AH) to remove suspended solids. †Free chlorine was detected in the untreated sample (this was not checked in samples 1-23 or 36-50, except for sample 8). ‡No free chlorine residual was detected following chlorination, perhaps due to the high concentration of H2S. Therefore, the TFP and chlorine demand data for this sample were excluded from all statistical summaries and correlations. **Not determined |
||||||||||
Table 4 shows the terminal THM concentrations of the finished water samples collected from public-water supplies (i.e. those using chlorine to disinfect the water). A majority of the samples still contained free chlorine at the time the water was analyzed for THMs. Table 5 shows the instantaneous THM and TOC concentrations for the finished water samples.
Table 4--Terminal trihalomethane concentrations in finished water samples.
| Sample | CHCl3 µg/L |
CHCl2Br µg/L |
CHClBr2 µg/L |
CHBr3 µg/L |
Term. THM | Yield* µmoles/mg |
Free Chlorine Remaining |
||
|---|---|---|---|---|---|---|---|---|---|
| µg/L | µM | %Cl | |||||||
| 1 | 4.7 | 8.5 | 9.8 | 3.7 | 26.7 | 0.153 | 59 | 0.222 | yes |
| 3 | 53.9 | 18.6 | 9.5 | 1.4 | 83.5 | 0.617 | 88 | 0.241 | yes |
| 4 | 0.5 | 0.9 | 0.6 | <0.1 | 2.0 | 0.012 | 70 | 0.033 | no |
| 5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | <0.004 | yes† |
| 6 | <0.1 | 1.0 | 7.5 | 31.1 | 39.5 | 0.165 | 10 | 0.118 | no |
| 7 | 12.0 | 17.7 | 24.8 | 16.9 | 71.4 | 0.394 | 54 | 0.296 | yes |
| 8 | 20.5 | 19.0 | 23.1 | 6.2 | 68.8 | 0.423 | 68 | 0.338 | yes |
| 9 | <0.1 | 0.2 | 0.2 | <0.1 | 0.3 | 0.002 | 52 | <0.001 | no |
| 11 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | <0.002 | no |
| 12 | <0.1 | 1.5 | 2.0 | 0.9 | 4.3 | 0.022 | 42 | 0.032 | yes |
| 13 | <0.1 | 1.3 | 2.2 | 2.1 | 5.6 | 0.027 | 33 | 0.159 | yes |
| 14 | <0.1 | 2.1 | 1.9 | 0.7 | 4.7 | 0.025 | 47 | 0.147 | yes |
| 16 | 12.9 | 15.1 | 12.1 | 2.1 | 42.2 | 0.266 | 71 | 0.169 | yes |
| 17 | 14.4 | 14.8 | 10.5 | 1.4 | 41.2 | 0.267 | 74 | 0.267 | yes |
| 18 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | <0.005 | no |
| 20 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | <0.001 | no |
| 24 | 0.8 | 1.6 | 3.3 | 3.4 | 9.1 | 0.046 | 40 | 0.208 | yes |
| 25 | 1.5 | 5.8 | 17.5 | 19.9 | 44.7 | 0.211 | 30 | 0.155 | yes |
| 27 | <0.1 | 0.8 | 2.5 | 3.4 | 6.7 | 0.030 | 24 | 0.178 | yes |
| 28 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | <0.007 | no |
| 30 | 5.7 | 17.5 | 25.4 | 13.4 | 62.0 | 0.329 | 48 | 0.212 | yes |
| 32 | 10.3 | 15.0 | 13.9 | 3.3 | 42.5 | 0.257 | 66 | 0.105 | yes |
| 36 | 2.9 | 2.4 | 1.4 | <0.1 | 6.7 | 0.045 | 80 | 0.182 | yes |
| 37 | 0.7 | 1.3 | 1.4 | 0.4 | 3.8 | 0.022 | 61 | 0.136 | yes |
| 38 | 0.5 | 1.2 | 4.0 | 5.1 | 10.8 | 0.051 | 30 | 0.106 | yes |
| 39 | <0.1 | 0.7 | 4.0 | 10.7 | 15.4 | 0.066 | 14 | 0.095 | yes |
| 40 | 0.4 | 2.6 | 7.1 | 6.9 | 17.1 | 0.081 | 31 | 0.072 | yes |
| 42 | 0.3 | <0.1 | <0.1 | <0.1 | 0.3 | 0.003 | 100 | 0.002 | no |
| 45 | 0.2 | <0.1 | <0.1 | <0.1 | 0.2 | 0.002 | 100 | <0.001 | no |
| 49 | 91.9 | 42.7 | 17.8 | 1.9 | 154.2 | 1.123 | 87 | 0.459 | yes |
| 50 | 1.6 | 4.6 | 10.7 | 10.4 | 27.2 | 0.133 | 37 | 0.278 | yes |
| Mean | 7.6 | 6.4 | 6.9 | 4.7 | 25.5 | 0.154 | 54 | 0.136 | - |
| SD | 18.8 | 9.5 | 7.9 | 7.2 | 34.4 | 0.237 | 25 | 0.118 | - |
| G. Mean | 0.6 | 1.4 | 1.8 | 0.9 | 6.1 | 0.037 | 47 | 0.044 | - |
| Median | 0.5 | 1.5 | 3.3 | 1.9 | 9.1 | 0.046 | 52 | 0.136 | - |
| *Based on the TOC concentration of the sample analyzed for instantaneous THMs (except for samples 49 and 50, in which case the yield was based on the TOC value of the raw water sample). †The detection of free chlorine in this sample may have been an artifact. It is more likely that this sample contained combined chlorine and enough iodide ion to cause monochloramine to be mistaken for free chlorine (see table 5). |
|||||||||
Table 5--Instantaneous THM and TOC concentration in finished water samples.
| Sample | CHCl3 µg/L |
CHCl2Br µg/L |
CHClBr2 µg/L |
CHBr3 µg/L |
Inst. THM | TOC mg/L |
Yield* µmoles/mg |
Free Cl2 (Field) mg/L |
||
|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | µM | %Cl | ||||||||
| 1 | 0.7 | 2.4 | 3.7 | 1.8 | 8.5 | 0.045 | 48 | 0.69* | 0.065 | 2.5 |
| 3 | 19.3 | 10.4 | 5.6 | 0.6 | 35.9 | 0.254 | 84 | 2.56* | 0.099 | 2.5 |
| 4 | 0.5 | 0.6 | 0.5 | 0.0 | 1.6 | 0.010 | 73 | 0.36* | 0.028 | 0.2 |
| 5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 0.83 | <0.004 | 0.2 (1.3)† |
| 6 | <0.1 | <0.1 | 1.2 | 5.7 | 6.9 | 0.028 | 7 | 1.40 | 0.020 | 0.5 |
| 7 | 3.1 | 4.3 | 6.9 | 4.5 | 18.8 | 0.103 | 53 | 1.33 | 0.077 | 2.0 |
| 8 | 12.4 | 0.9 | 1.4 | 1.0 | 15.7 | 0.120 | 91 | 1.25 | 0.096 | 4.0 |
| 9 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 2.48 | <0.001 | 0.2 |
| 11 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 1.93 | <0.002 | 0.2 |
| 12 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 0.69 | <0.004 | 1.7 |
| 13 | <0.1 | 0.5 | 1.0 | 1.3 | 2.8 | 0.013 | 28 | 0.17 | 0.076 | 1.0 |
| 14 | <0.1 | 0.2 | 0.4 | 0.3 | 0.9 | 0.004 | 34 | 0.17 | 0.023 | 2.0 |
| 16 | 0.6 | 1.3 | 1.8 | 0.6 | 4.3 | 0.023 | 55 | 1.57 | 0.015 | 2.7 |
| 17 | 1.1 | 0.7 | 0.8 | 0.9 | 3.5 | 0.021 | 64 | 1.00 | 0.021 | 1.5 |
| 18 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 0.64 | <0.005 | 0.2 |
| 20 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 2.08 | <0.001 | 1.0 |
| 24 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 0.22 | <0.014 | 1.0 |
| 25 | <0.1 | 0.8 | 2.9 | 5.0 | 8.7 | 0.038 | 20 | 1.36 | 0.028 | 2.0 |
| 27 | <0.1 | <0.1 | 0.5 | 1.1 | 1.6 | 0.007 | 12 | 0.17 | 0.041 | 1.3 |
| 28 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 0.42 | <0.007 | 0.2 |
| 30 | 1.3 | 1.9 | 3.7 | 3.3 | 10.2 | 0.053 | 46 | 1.55 | 0.034 | 4.0 |
| 32 | 0.4 | 1.1 | 2.1 | 0.5 | 4.1 | 0.022 | 51 | 2.44 | 0.009 | 1.3 |
| 36 | <0.1 | 1.1 | 0.2 | <0.1 | 1.3 | 0.007 | 63 | 0.25 | 0.030 | 2.7 |
| 37 | 0.3 | <0.1 | <0.1 | <0.1 | 0.3 | 0.002 | 100 | 0.16 | 0.015 | 2.7 |
| 38 | <0.1 | 0.2 | 0.4 | <0.1 | 0.6 | 0.003 | 46 | 0.48 | 0.006 | 4.0 |
| 39 | <0.1 | 0.1 | 0.4 | <0.1 | 0.5 | 0.002 | 41 | 0.69 | 0.003 | 1.3 |
| 40 | 0.4 | 0.1 | <0.1 | <0.1 | 0.5 | 0.004 | 95 | 1.12 | 0.004 | 1.2 |
| 42 | <0.1 | <0.1 | <0.1 | <0.1 | <0.4 | <0.003 | - | 1.34 | <0.002 | 0.2 |
| 45 | 0.3 | <0.1 | <0.1 | <0.1 | 0.3 | 0.002 | 100 | 2.61 | <0.001 | 0.2 |
| 49 | 42.4 | 27.3 | 12.4 | 1.3 | 83.4 | 0.586 | 83 | 2.45 | 0.239 | 2.0 (2.5)t |
| 50 | 1.4 | 0.9 | 0.9 | <0.1 | 3.1 | 0.021 | 78 | 0.48* | 0.044 | 4.0 |
| Mean | 2.7 | 1.8 | 1.5 | 0.9 | 6.9 | 0.045 | 58 | 1.13 | 0.032 | 1.6 |
| SD | 8.4 | 5.1 | 2.6 | 1.6 | 16.0 | 0.112 | 28 | 0.81 | 0.048 | 1.3 |
| G. Mean | 0.2 | 0.3 | 0.4 | 0.2 | 1.4 | 0.009 | 49 | 0.81 | 0.011 | 1.0 |
| Median | <0.1 | 0.2 | 0.4 | <0.1 | 1.3 | 0.007 | 52 | 1.00 | 0.015 | 1.3 |
| * Raw water TOC (other TOC values were determined on the treated sample taken for analysis of instantaneous THMs). † The total residual chlorine concentration was greater than the free chlorine concentration (the total concentration is shown in parentheses). |
||||||||||
Table 6 gives the results of the geochemical analyses. The laboratory pH was generally higher than the field pH, as would be expected with the escape of CO2. A large difference between field and laboratory conductance might indicate precipitation of minerals from the water after sampling, but the values were generally close. Ionic balances were computed for these results, and the greatest deviation from electroneutrality (the difference between meq/L of anions and meq/L of cations divided by their sum) was 1.87%.
Table 6--Results of the geochemical analysis.1
| No. | Temp °C |
pH Fld |
pH Lab |
Cond Fld |
Cond Lab |
Ca mg/L |
Mg mg/L |
Na mg/L |
K mg/L |
Sr mg/L |
HCO3- mg/L |
SO4-2 mg/L |
Cl- mg/L |
NO3- mg/L |
NH4+ mg/L |
Ba µg/L |
Fe µg/L |
Mn µg/L |
H2S µg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 12 | 6.95 | 7.40 | 740 | 663 | 106 | 14 | 15 | 1.6 | 0.5 | 371 | 36 | 5.2 | 0.1 | 0.1 | 490 | 1530 | 422 | ND |
| 02 | 15 | 6.95 | 7.50 | 940 | 908 | 123 | 35 | 29 | 5.4 | 0.9 | 588 | 15 | 6.6 | <0.1 | 1.1 | 868 | 12900 | 713 | ND |
| 03 | 15 | 7.05 | 7.60 | 830 | 810 | 90 | 28 | 46 | 5.9 | 0.7 | 398 | 96 | 14 | <0.1 | 0.7 | 674 | 11100 | 706 | ND |
| 04 | 16 | 7.05 | 7.60 | 550 | 562 | 90 | 13 | 16 | 1.1 | 0.8 | 352 | 12 | 2.2 | <0.1 | 0.2 | 131 | 664 | 24 | ND |
| 05 | 19 | 7.70 | 8.00 | 1450 | 1300 | 20 | 8.2 | 251 | 2.7 | 1.0 | 333 | 19 | 248 | <0.1 | 1.0 | 31 | 4300 | 36 | 1.0 |
| 06 | 11 | 6.95 | 7.45 | 950 | 1020 | 142 | 26 | 33 | 5.3 | 2.3 | 407 | 94 | 56 | 46 | 0.1 | 193 | 152 | 9 | ND |
| 07 | 15 | 7.05 | 7.90 | 660 | 685 | 69 | 17 | 43 | 11.0 | 0.6 | 241 | 95 | 40 | 2.0 | 0.1 | 371 | 93 | 769 | ND |
| 08 | 16 | 6.95 | 7.60 | 1450 | 1430 | 207 | 42 | 52 | 11.0 | 2.7 | 420 | 382 | 45 | 24 | 0.1 | 55 | BQ | 411 | ND |
| 09 | 14 | 7.15 | 7.60 | 1030 | 1098 | 138 | 17 | 68 | 12.0 | 0.7 | 381 | 154 | 74 | 17.0 | 0.1 | 207 | 311 | 545 | ND |
| 10 | 14 | 6.85 | 7.45 | 720 | 750 | 103 | 16 | 34 | 4.1 | 0.4 | 395 | 64 | 16 | 0.1 | 0.4 | 168 | 2630 | 837 | ND |
| 11 | 14 | 7.10 | 7.70 | 960 | 1000 | 140 | 28 | 34 | 6.0 | 1.6 | 457 | 119 | 43 | 0.1 | 0.7 | 108 | 7520 | 937 | ND |
| 12 | 15 | 6.35 | 6.80 | 310 | 333 | 30 | 6.9 | 24 | 2.1 | 0.2 | 86 | 46 | 19 | 20 | <0.1 | 158 | BQ | 16 | ND |
| 13 | 14 | 7.10 | 7.65 | 530 | 570 | 89 | 9.0 | 17 | 2.5 | 0.4 | 292 | 21 | 26 | 4.2 | <0.1 | 188 | BQ | BQ | ND |
| 14 | 14 | 7.10 | 7.60 | 380 | 412 | 58 | 7.5 | 18 | 1.2 | 0.3 | 208 | 20 | 6.1 | 21 | <0.1 | 122 | BQ | BQ | ND |
| 15 | 14 | 7.15 | 7.60 | 850 | 909 | 94 | 14 | 84 | 1.0 | 0.4 | 391 | 46 | 73 | 19 | <0.1 | 130 | BQ | BQ | ND |
| 16 | 16 | 7.05 | 7.50 | 780 | 830 | 124 | 26 | 18 | 1.3 | 2.4 | 485 | 54 | 13 | 6.7 | 0.1 | 218 | BQ | BQ | ND |
| 17 | 15 | 7.05 | 7.85 | 2250 | 2350 | 167 | 37 | 263 | 7.1 | 1.3 | 322 | 188 | 487 | 22 | <0.1 | 65 | 947 | BQ | ND |
| 18 | 14 | 7.25 | 7.85 | 1130 | 1180 | 29 | 7.7 | 229 | 6.1 | 0.4 | 418 | 116 | 102 | 0.4 | 0.8 | 24 | 127 | 25 | ND |
| 19 | 15 | 7.15 | 7.80 | 620 | 602 | 82 | 14 | 29 | 2.5 | 0.5 | 389 | 13 | 4.9 | 0.2 | 0.1 | 231 | 175 | 395 | ND |
| 20 | 14 | 7.25 | 7.95 | 1010 | 1042 | 140 | 45 | 34 | 16.0 | 1.2 | 704 | 7.6 | 18 | 0.2 | 1.5 | 38402 | 140002 | 124002 | ND |
| 21 | 8 | 6.35 | 6.90 | 1100 | 1160 | 44 | 14 | 168 | 2.9 | 1.2 | 155 | 61 | 226 | 40 | 0.3 | 45 | 27 | 211 | ND |
| 22 | 8 | 6.90 | 7.60 | 800 | 838 | 103 | 28 | 42 | 1.9 | 1.0 | 397 | 93 | 29 | 4.0 | 0.1 | 71 | 377 | 8 | ND |
| 23 | 9 | 7.10 | 7.90 | 710 | 650 | 76 | 23 | 29 | 3.8 | 1.1 | 401 | 18 | 12 | 0.1 | 0.5 | 190 | 811 | 126 | ND |
| 24 | 15 | 7.50 | 8.00 | 410 | 415 | 40 | 15 | 24 | 5.7 | 0.6 | 213 | 23 | 6.4 | 14 | <0.1 | 82 | BQ | BQ | ND |
| 25 | 14 | 7.10 | 7.85 | 625 | 610 | 62 | 24 | 21 | 4.6 | 1.2 | 175 | 74 | 49 | 25 | <0.1 | 86 | 70 | BQ | ND |
| 26 | 16 | 7.15 | 7.90 | 640 | 608 | 60 | 27 | 29 | 3.8 | 1.2 | 265 | 84 | 13 | 9.3 | 0.1 | 118 | 41 | 19 | ND |
| 27 | 16 | 7.05 | 7.85 | 710 | 730 | 65 | 27 | 50 | 4.5 | 1.5 | 224 | 162 | 16 | 14 | <0.1 | 20 | 16 | BQ | ND |
| 28 | 15 | 7.35 | 8.10 | 385 | 400 | 53 | 5.7 | 22 | 3.2 | 0.3 | 193 | 18 | 11 | 20 | <0.1 | 141 | BQ | BQ | ND |
| 29 | 15 | 7.30 | 8.00 | 500 | 492 | 75 | 9.0 | 14 | 3.0 | 0.5 | 261 | 14 | 14 | 16 | 0.1 | 206 | 145 | BQ | ND |
| 30 | 14 | 6.85 | 7.70 | 1000 | 1050 | 176 | 14 | 29 | 4.6 | 0.7 | 347 | 180 | 71 | 0.3 | 0.1 | 197 | 904 | 52 | ND |
| 31 | 14 | 6.95 | 7.60 | 850 | 885 | 124 | 19 | 28 | 6.6 | 1.1 | 371 | 75 | 38 | 49 | <0.1 | 213 | 22 | BQ | ND |
| 32 | 13 | 7.15 | 7.55 | 785 | 820 | 126 | 10 | 35 | 4.8 | 0.7 | 333 | 95 | 46 | 3.3 | <0.1 | 204 | 45 | 140 | ND |
| 33 | 16 | 7.35 | 7.85 | 3700 | 3500 | 93 | 30 | 622 | 9.1 | 1.3 | 251 | 286 | 853 | 5.8 | <0.1 | 38 | BQ | BQ | ND |
| 34 | 12 | 7.50 | 8.00 | 935 | 827 | 41 | 11 | 120 | 2.9 | 0.5 | 257 | 82 | 79 | 7.0 | 0.1 | 37 | 32 | BQ | ND |
| 35 | 15 | 7.45 | 7.85 | 600 | 580 | 57 | 28 | 29 | 1.7 | 0.7 | 324 | 23 | 14 | 13 | 0.1 | 136 | BQ | BQ | ND |
| 36 | 18 | 7.10 | 8.00 | 480 | 451 | 47 | 22 | 18 | 3.9 | 0.7 | 234 | 50 | 4.8 | <0.1 | 0.2 | 72 | 166 | BQ | ND |
| 37 | 19 | 7.20 | 8.05 | 450 | 423 | 49 | 18 | 14 | 1.8 | 0.1 | 176 | 64 | 14 | 0.1 | 0.1 | 82 | 44 | 7 | ND |
| 38 | 20 | 7.35 | 7.60 | 780 | 790 | 51 | 24 | 87 | 5.3 | 0.8 | 320 | 34 | 86 | <0.1 | 0.3 | 357 | BQ | BQ | 7.5 |
| 39 | 23 | 7.25 | 7.85 | 1200 | 1140 | 70 | 34 | 125 | 7.6 | 1.3 | 323 | 96 | 169 | <0.1 | 0.4 | 52 | 122 | BQ | 3.5 |
| 40 | 14 | 6.80 | 7.65 | 820 | 820 | 118 | 14 | 38 | 6.9 | 0.7 | 352 | 71 | 45 | 27 | 0.1 | 178 | BQ | BQ | ND |
| 41 | 14 | 6.95 | 7.60 | 1040 | 1030 | 137 | 20 | 65 | 4.9 | 0.6 | 475 | 107 | 46 | 15 | 0.1 | 115 | 846 | 117 | ND |
| 42 | 14 | 6.95 | 7.90 | 950 | 923 | 142 | 15 | 42 | 5.0 | 0.8 | 476 | 96 | 22 | 0.6 | 0.3 | 122 | 3470 | 669 | ND |
| 43 | 13 | 6.80 | 7.60 | 1620 | 1481 | 184 | 43 | 88 | 7.4 | 2.1 | 392 | 379 | 100 | 0.2 | 1.0 | 39 | 616 | 2620 | ND |
| 44 | 13 | 7.05 | 7.80 | 840 | 778 | 95 | 32 | 18 | 0.9 | 0.3 | 386 | 21 | 29 | 36 | 0.2 | 556 | BQ | BQ | ND |
| 45 | 13 | 6.95 | 7.60 | 1900 | 1670 | 303 | 54 | 32 | 2.3 | 5.3 | 401 | 687 | 31 | 3.1 | 0.1 | 26 | 924 | 14 | ND |
| 46 | 15 | 6.95 | 8.00 | 2200 | 1960 | 78 | 12 | 319 | 6.8 | 0.5 | 250 | 59 | 474 | 6.0 | <0.1 | 203 | 651 | 37 | ND |
| 47 | 13 | 6.60 | 6.85 | 1590 | 1520 | 227 | 30 | 47 | 1.4 | 0.9 | 352 | 140 | 134 | 188 | 0.1 | 192 | BQ | BQ | ND |
| 48 | 16 | 7.15 | 7.45 | 590 | 560 | 86 | 12 | 22 | 1.1 | 0.4 | 335 | 24 | 2.2 | 0.1 | 0.2 | 12 | 396 | 48 | ND |
| 49 | 21 | 7.25 | 7.85 | 685 | 725 | 106 | 16 | 22 | 2.7 | 1.0 | 385 | 30 | 17 | 0.1 | 1.1 | 805 | 9270 | 1790 | ND |
| 50 | 22 | 7.25 | 7.45 | 785 | 830 | 69 | 29 | 60 | 6.2 | 0.8 | 326 | 83 | 54 | <0.1 | 0.3 | 108 | 40 | BQ | 2.0 |
| Mean | 14.8 | 7.08 | 7.69 | 956 | 942 | 100 | 21 | 72 | 4.7 | 1.0 | 340 | 97 | 80 | 13.6 | 0.3 | 186 | 1258 | 240 | - |
| SD | 2.9 | 0.25 | 0.28 | 584 | 546 | 55 | 11 | 105 | 3.2 | 0.8 | 110 | 118 | 150 | 28.3 | 0.3 | 191 | 2887 | 495 | - |
| GM | 14.5 | 7.07 | 7.68 | 842 | 837 | 87 | 19 | 43 | 3.7 | 0.8 | 321 | 59 | 33 | 1.8 | 0.2 | 122 | 139 | 21 | - |
| Med. | 14.5 | 7.10 | 7.68 | 810 | 824 | 90 | 19 | 34 | 4.4 | 0.8 | 350 | 68 | 30 | 4.1 | 0.1 | 131 | 122 | 14 | - |
| 1 Notation: Fld = Field value; Lab = Lab value Cond = Specific conductance in µmhos/cm BQ = Below quantifiable limit (26 µg/L for Fe and 4 µg/L for Mn) SD = Standard Deviation GM = Geometric Mean Med. = Median 2 Sample 20 contained a substantial amount of sediment, and the acidified sample had to be filtered prior to analysis for Ba, Fe, and Mn. The high values for these constituents probably reflect dissolution of particulate matter and not the presence of dissolved minerals. These values were discarded in all statistical summaries and correlations. |
|||||||||||||||||||
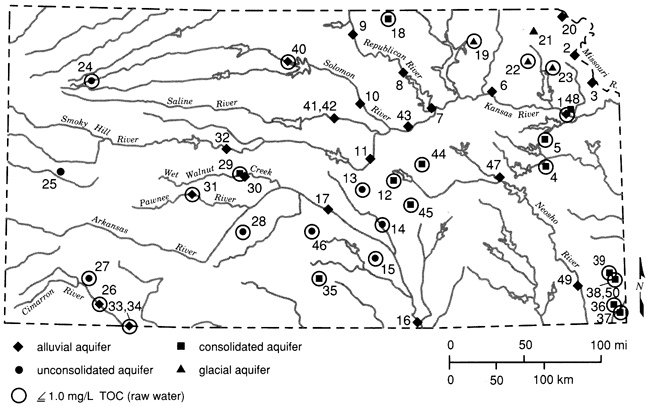
The raw-water TOC concentrations (table 3) ranged from 0.21 to 3.31 mg/L with a median value of 0.84 and a mean of 1.03 mg/L. Fig. 3 shows the statewide distribution of TOC concentrations greater than and less than 1.0 mg/L. Values greater than 1 mg/L were found throughout the state but primarily in alluvial aquifers. All of the consolidated aquifers sampled had TOC concentrations below 1 mg/L. The highest values (i.e. those > 2.0 mg/L) were found exclusively in the eastern third of the state.
Figure 3--Statewide distribution of TOC concentrations (base map from Steeples and Buchanan, 1983).

The TFPs (table 3) ranged from 5.3 to 178 mg/L, with a median value of 30.6 µg/L, a mean of 46.7 µg/L, and a geometric mean of 32.5 µg/L. On the average, only about 61% of the THM-halogen atoms were chlorine, illustrating the importance of bromide in THM formation in Kansas. For 29 of the 50 samples, either CHClBR2 or CHCl2BR was the most abundant THM species, with another five samples dominated by CHBR3. Sample 47 had anomalously high concentrations of brominated THMs, presumably due to an unusually high concentration of bromide in the raw water.
Of the 50 samples analyzed, only four (8%, all in Missouri or Neosho River alluvium) had TFPs exceeding the current MCL for THMs of 100 µg/L. However, 28 (56%) had TFPs exceeding 25 µg/L, and 45 (90%) had TFPs exceeding 10 µg/L. Hence, if the MCL were set at a substantially lower level, a significant number of water utilities relying on ground water as a source of supply might have difficulty complying with the new MCL.
TOC and TFP (µM) were very strongly correlated (r = 0.953), as shown in fig. 4 and table 7. This was expected based on the results of many previous investigations of THM formation in surface waters; this relationship is reflected in the relatively low standard deviation in TFP yield (± 25%, as shown in table 3). This demonstrates that TOC would be an excellent surrogate measure for THM formation, which might prove particularly useful in monitoring and regulatory efforts.
Figure 4--TFP as a function of TOC and aquifer type.
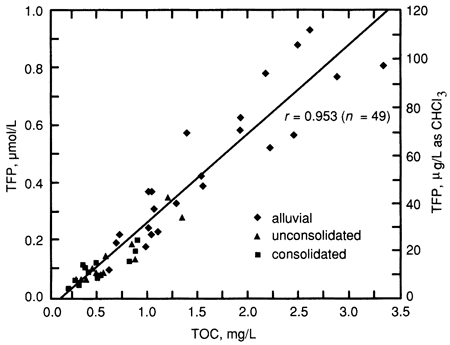
Fig. 4 and table 7 also show the TOC and TFP concentrations for each of the three major aquifer types. The highest concentrations (TOC values > 1.5 mg/L and TFP values > 50 µg/L) were found exclusively in alluvial aquifers, including those of the Missouri, Neosho, Smoky Hill, and Republican rivers. Only two nonalluvial aquifer samples had TOC concentrations greater than 1 mg/L; one was from a glacial buried-valley aquifer and one was from the Ogallala Formation. The remaining nonalluvial aquifer samples had TOC concentrations ranging from 0.2 to 0.9 mg/L, a range which includes only three alluvial aquifer samples.
As shown in table 7, the mean TOC and TFP concentrations were substantially higher for the alluvial aquifer samples than for the samples from consolidated and unconsolidated aquifers. River waters often carry large organic loads, and other investigations (see Introduction) have shown that river waters generally have higher TOC concentrations than ground waters. The recharge and discharge relationship of a river and its adjoining alluvium is probably a major factor in the amount of TOC in samples from alluvial sources. Alluvial aquifers which are at least partially recharged by river waters would be expected to have higher TOC levels. Organic materials deposited along with the alluvial sediments might also impart a significant amount of TOC to alluvial waters.
Table 7--Statistical summary of TOC and TFP data by aquifer type.1
| Parameter | All Samples |
Alluvial Aquifers (23) |
Consolidated Aquifers (14) |
Unconsolidated Aquifers (12) |
|---|---|---|---|---|
| TOC, mg/L | 1.03 ± 0.76 | 1.59 ± 0.76 | 0.48 ± 0.22 | 0.65 ± 0.34 |
| TFP, µg/L | 46.7 ± 39.5 | 76.2 ± 37.6 | 16.3 ± 7.4 | 25.6 ± 21.1 |
| TFP, µM | 0.28 ± 0.25 | 0.47 ± 0.25 | 0.10 ± 0.05 | 0.14 ± 0.09 |
| TFP Yield, µmoles/mgC | 0.24 ± 0.07 | 0.29 ± 0.07 | 0.20 ± 0.05 | 0.20 ± 0.04 |
| Percent Cl | 61 ± 18 | 62 ± 19 | 63 ± 19 | 56 ± 18 |
| Cl2 demand | 2.69 ± 3.09 | 3.70 ± 3.42 | 2.76 ± 3.19 | 0.99 ± 1.29 |
| Correlation coefficients: | ||||
| TFP, µg/L vs TOC | 0.887 | 0.766 | 0.875 | 0.902 |
| TFP, µM vs TOC | 0.953 | 0.911 | 0.887 | 0.934 |
| TFP yield vs TOC | 0.531 | 0.187 | -0.072 | 0.360 |
| Cl2 demand vs TOC | 0.597 | 0.737 | 0.095 | 0.358 |
| Cl2 demand vs TFP, µg/L | 0.565 | 0.708 | 0.201 | 0.476 |
| Calculated demand vs. actual demand |
0.853 | 0.965 | 0.848 | 0.906 |
| 1 Excluding THM and Cl2 data for sample 38, excluding sample 34 from the aquifer types, and excluding Cl2 demand for samples 47 and 48. | ||||
Table 7 also presents some statistical information regarding TFP yields, which were, on the average, about 50% higher in the alluvial aquifers in comparison to the nonalluvial aquifers. The TFP yields did not vary much, suggesting that the organic matter in Kansas ground waters has somewhat similar characteristics from place to place. When grouped by aquifer type, TFP yields were not correlated with TOC concentration; the weak correlation (r = 0.531) for the entire sample set is an artifact resulting from the combining of different sample populations.
Chlorine demand, determined simultaneously with TFP, averaged 2.69 mg/L and ranged from < 0.1 mg/L to 10.6 mg/L, excluding sample 38 (table 3). As shown in table 7, the average chlorine demand was significantly higher for the alluvial aquifer samples than for the samples from consolidated and unconsolidated aquifers. TOC and TFP appear to be weakly correlated with chlorine demand for the grouping of all samples and for samples from alluvial aquifers (table 7), but this in an artifact caused by a cluster of alluvial aquifer samples which contained both high amounts of TOC (and TFP) and high concentrations of ammonium, iron, and manganese.
To determine how well the measured chlorine demand values would compare to those expected on the basis of the chemical constituents present in the samples, the chlorine demand for each sample was calculated using the formula: 5.91(NH4+) + 0.63(Fe) + 1.29(Mn) + 8.34(H2S) + TOC, where all concentrations are expressed as mg/L. (The first four terms are based on stoichiometry, assuming all of the Fe and Mn present in divalent form.) The average calculated chlorine demand was 6.1 mg/L, substantially higher than the average measured chlorine demand. There are several reasons why this should be so: 1) a few of the raw-water samples, including samples 8, 25, and 27, already had some chlorine in them at the time they were collected; 2) Fe and Mn were not necessarily present in a reduced state, because oxygen introduced into the samples during pumping and handling could have oxidized the Fe and Mn prior to chlorination; and 3) some of the H2S may have escaped by volatilization. Nevertheless, the measured and calculated chlorine demands were strongly correlated for all samples (r = 0.853) and for each aquifer type (table 7).
Samples from the 31 public water-supply wells were analyzed for terminal and instantaneous THM concentrations (THM and ITHM, respectively), and the results are shown in tables 4 and 5. Additional statistical information is presented in table 8, which includes a separate category for the 21 samples that had a free chlorine residual remaining at the end of the TTHM incubation period (the TTHM results for the other samples were questionable).
The finished-water TOC concentrations were determined on 26 of the 31 ITHM samples. As shown in table 8, the average finished-water and raw-water TOC concentrations were quite similar and raw-water TOC was strongly correlated with finished-water TOC, as would be expected. The raw-water TOC concentration for these samples averaged 1.04 ± 0.74 mg/L, in very close agreement with the raw-water TOC concentration of 1.03 ± 0.76 mg/L for all 50 samples. Hence these samples are a very representative subset.
Table 8--Statistical summary of ITHM and TTHM data.
| Parameter | All (31) Samples |
Selected Samples1 |
|---|---|---|
| Raw water TOC, mg/L | 1.04 ± 0.742 | 0.93 ± 0.692 |
| Finished water TOC, mg/L | 1.09 ± 0.772 | 0.86 ± 0.672 |
| ITHM, µg/L | 6.95 ± 16 | 9.78 ± 18.9 |
| TTHM, µg/L | 35.6 ± 37.0 | |
| ITHM/RTHM | 0.19 ± 0.15 | |
| ITHM/RFP | 0.11 ± 0.133 | 0.15 ± 0.143 |
| TTHM/TFP | 0.78 ± 0.413 | |
| Correlation coefficients: | ||
| ITHM vs TOC4 | 0.433 | 0.658 |
| TTHM vs TOC4 | 0.819 | |
| TTHM vs TFP | 0.9263 | |
| TOC, Finished vs Raw | 0.8222 | 0.9442 |
| 1Those 21 samples, excluding sample 5, for which the TTHM sample had a free chlorine residual at the end of the incubation period. 2 Excluding samples 1, 3, 4, 49, and 50, for which finished water TOC was not determined. 3 Excluding sample 38 4 Finished water TOC |
||
The average ITHM concentration for all 31 samples was only 6.95 µg/L, and the average ratio of ITHM to TFP was only 11%. Similarly, for the 21 TTHM samples having a free chlorine residual, the average ratio of ITHM to TTHM was only 19%. Hence, the concentration of THMs in the finished water generally represented only a small fraction of the THM concentration to which the consumers have been exposed. ITHM concentrations were only weakly correlated with TOC (r = 0.433), reflecting the strong influence of other factors, such as temperature, reaction time, pH, and chlorine dosage, on the initial rate of THM formation.
The mean TTHM concentration was 35.6 ± 37.0 µg/L. Since the TTHM incubation conditions (4 days at 25°C [77°F]) were probably, in most cases, a bit more severe than those actually present in the distribution system, the TTHM values represent a conservative estimate of the THM concentrations actually present in the distribution system.
The TTHM concentrations were strongly correlated with TOC (r = 0.819) and very strongly correlated with TFP (r = 0.926), as would be expected when the THM formation reaction is allowed to go to completion in the presence of excess free chlorine. Hence, TOC and TFP appear to be useful as predictors of distribution system THM concentrations. However, the average ratio of TTHM to TFP (based on 21 samples) was only 78%. The difference is readily attributable to the more extreme conditions (pH and chlorine dosage) of the TFP analysis. A pH of 8.2 was used for the TFP analysis, whereas the average pH of the raw water was 7.1 (table 5), and the average free chlorine residual in the TFP samples was undoubtedly a bit higher than in the TTHM samples. Clearly, it would be better to simulate conditions in the distribution system if the goal is to predict the distribution system THM concentrations; however, the TFP analysis provides a superior basis for comparisons among water sources, which was a major goal of this study.
TOC is generally expected to decrease with depth due to adsorption and biodegradation of organic matter as the water percolates downward through the sediments. Also, wells screened closer to the ground level are generally more susceptible to contamination by high-TOC surface water percolating into the shallow ground water through cracks, fissures, permeable deposits, or poorly constructed wells. Therefore, the data were examined to see if there might be a relationship between TOC concentration and the depth of the top of the well screen, the mid-depth of the well screen, or the depth of the top of the well screen below the water table. A statistical summary of this examination is presented in table 9, and fig. 5 shows TOC, as a function of aquifer type, versus the depth of the top of the well screen.
Figure 5--TOC as a function of depth to the top of the well screen.
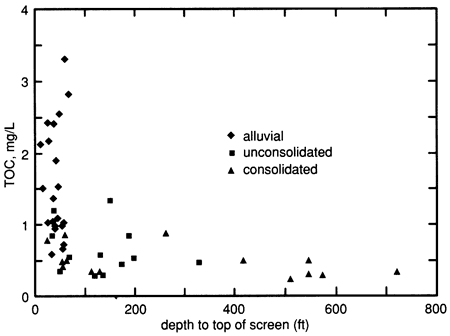
Table 9--Correlation of TOC and well depth.
| Linear Regression Correlation Coefficient1 | ||||
|---|---|---|---|---|
| All Samples |
Alluvial Aquifers |
Consolidated Aquifers |
Unconsolidated Aquifers |
|
| TOC vs depth to top of screen |
-0.455(48) | -0.008(22) | -0.540(14) | -0.215(12) |
| TOC vs mid depth of well screen |
-0.443(48) | -0.060(22) | -0.582(14) | -0.249(12) |
| TOC vs depth from water table to top of screen |
-0.377(46) | +0.014(21) | -0.550(13) | -0.414(12) |
| 1 The number of samples included in the correlation is shown in parentheses (the necessary data were unavailable for several wells). |
||||
As shown in table 9, TOC was weakly correlated with depth when considering all of the samples or only those from consolidated aquifers, but there was no correlation between TOC and depth for the alluvial aquifers. This was true whether the depth was measured to the top of the screen, to mid-depth, or from the water table to the top of the screen. A close examination of fig. 5 reveals that the weak correlations for the consolidated aquifers are really artifacts due to a data cluster associated with the very deep open-hole wells (the six deepest wells). Similarly, the weak correlations for all of the samples are attributable to the combining of two different populations; the alluvial aquifers had, on the average, much higher TOC values than the other types and their top-of-screen depths were all less than 80 ft (24 m) The curvilinear relationship shown in fig. 5 suggests that an exponential curve might better fit the data, and indeed the correlation coefficient for a semilogarithmic plot was higher (-0.593), but this too is attributable to the combining of two different populations of aquifers. Thus, it can be concluded that there is a general trend of decreasing TOC with depth, but only because alluvial aquifers tend to be shallow and high in TOC.
Fig. 6 is a modified Piper diagram summarizing the geochemical composition of the samples. Water-type assignments were made according to dominant contributions (>50%) of particular ions to the total milliequivalents per liter of cations or anions in solution. Samples not dominated by a particular cation or anion were designated as "Mix" types. A majority (27) of the samples were CaHCO3 type waters, 15 of these being from alluvial aquifers (see table 10). All of the Equus Beds aquifer samples, three glacial buried-valley samples, and two Pennsylvanian aquifer samples also were CA-HCO3 type waters.
Table 10--Classification of aquifers by water type.
| Sample | Aquifer* | TOC(mg/L) |
|---|---|---|
| Ca-HCO3 Type Waters | ||
| 1 | Kansas(A) | 0.69 |
| 2 | Missouri(A) | 3.31 |
| 3 | Missouri(A) | 2.56 |
| 4 | Pennsylvanian(C) | 0.36 |
| 6 | Kansas(A) | 1.04 |
| 7 | Smoky Hill(A) | 1.90 |
| 9 | Republican(A) | 2.19 |
| 10 | Solomon(A) | 1.03 |
| 11 | Smoky Hill(A) | 1.90 |
| 13 | Equus Beds(U) | 0.31 |
| 14 | Equus Beds(U) | 0.30 |
| 15 | Equus Beds(U) | 0.85 |
| 16 | Arkansas(A) | 1.52 |
| 19 | Glacial(U) | 0.45 |
| 20 | Missouri(A) | 2.84 |
| 22 | Glacial(U) | 0.58 |
| 23 | Glacial(U) | 0.83 |
| 28 | Big Bend(U) | 0.55 |
| 29 | Dakota(C) | 0.49 |
| 31 | Pawnee(A) | 1.00 |
| 32 | Smoky Hill(A) | 2.43 |
| 37 | Arbuckle(C) | 0.21 |
| 40 | Solomon(A) | 0.98 |
| 42 | Saline(A) | 1.10 |
| 44 | Permian(C) | 0.50 |
| 48 | Pennsylvanian(C) | 0.37 |
| 49 | Neosho(A) | 2.45 |
| Ca-SO4 Type Waters | ||
| 45 | Permian(C) | 0.80 |
| Ca-Mix Type Waters | ||
| 8 | Republican(A) | 1.37 |
| 25 | Ogallala(U) | 1.34 |
| 30 | Walnut(A) | 1.54 |
| 43 | Solomon(A) | 1.02 |
| 47** | Neosho(A) | 2.14 |
| Mix-Mix Type Waters | ||
| 12 | Dakota(C) | 0.41 |
| 27 | Ogallala(U) | 0.47 |
| 39 | Arbuckle(C) | 0.31 |
| Mix-HCO3 Type Waters | ||
| 24 | Ogallala(U) | 0.52 |
| 26 | Cimarron(A) | 0.72 |
| 35 | Permian(C) | 0.88 |
| 36 | Arbuckle(C) | 0.27 |
| 38 | Arbuckle(C) | 0.29 |
| 50 | Arbuckle(C) | 0.48 |
| Na-HCO3 Type Waters | ||
| 18 | Dakota(C) | 0.87 |
| 34 | Pleistocene(U) | 0.50 |
| Na-Cl Type Waters | ||
| 5 | Pennsylvanian(C) | 0.48 |
| 17** | Arkansas(A) | 1.06 |
| 21 | Glacial(U) | 1.20 |
| 33 | Cimarron(A) | 0.60 |
| 46 | Big Bend(U) | 0.36 |
| *A = alluvial, C = consolidated, U = unconsolidated **The ratio of Na to Cl in mg/L is less than 0.65. |
||
Figure 6--Modified Piper diagram showing study samples.
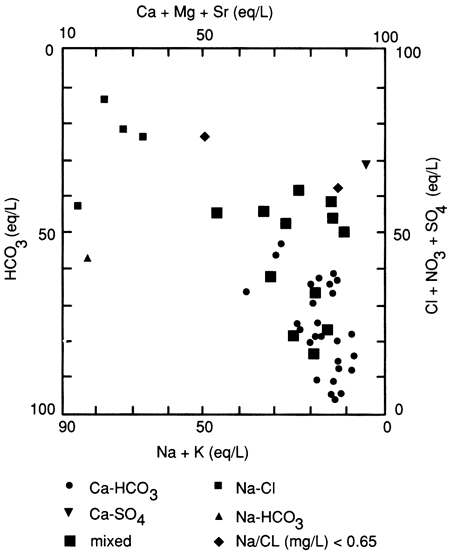
There does not appear to be any significant relationship between TOC and water type. The five Ca-Mix waters had TOC levels greater than 1.0 mg/L, ranging from 1.02 to 2.14 mg/L, but four of these samples were from alluvial aquifers. The Mix-Mix, Mix-HCO3, and CaSO4 waters all had TOC concentrations close to or less than the median concentration (0.8 mg/L), but only one of these samples came from an alluvial aquifer. Na-Cl type waters had TOC concentrations ranging from 0.36 to 1.20 mg/L.
A simple statistical analysis of the geochemical data according to aquifer type is presented in table 11. Inspection of the means and medians reveals that the mean and median concentration of every constituent was higher in the alluvial aquifers than in the consolidated and unconsolidated aquifers, with only three minor exceptions: 1) the mean and median pH values were slightly lower for the alluvial aquifers (meaning that the hydrogen ion concentration was actually higher); 2) the median NO3- concentration was highest for the unconsolidated samples; and 3) the median ammonium concentration was highest for the consolidated aquifers. Hydrogen sulfide was excluded from the data summary, because only four samples contained a detectable amount, but all four came from deep open-hole wells in consolidated aquifers.
Specific conductance, calcium, bicarbonate, sulfate, chloride, barium, iron, and manganese were, on the average, present in substantially higher concentrations in the alluvial aquifers. Hence, these parameters would be expected to be correlated (associated) with TOC even where no causal relationship exists. For this reason, it was necessary to examine the relationship between TOC and the inorganic constituents for each individual type of aquifer, as described in the following section. The consolidated-aquifer samples were, on the average, quite similar to the unconsolidated samples; however, the consolidated aquifer samples had slightly higher concentrations of the majority of constituents, especially bicarbonate, sulfate, and iron.
Table 11--Summary of geochemical data by aquifer type.
| Constituent, units | All Aquifers (50) | Alluvial (23) | Consolidated (14) | Unconsolidated (12) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | |
| Field pH, pH units | 7.08 ± 0.25 | 7.10 | 7.01 ± 0.17 | 6.95 | 7.17 ± 0.30 | 7.23 | 7.07 ± 0.28 | 7.10 |
| Field Spec. Cond., µmhos/cm |
956 ± 584 | 810 | 1130 ± 677 | 950 | 826 ± 447 | 690 | 777 ± 495 | 668 |
| Ca, mg/L | 100 ± 55 | 90 | 132 ± 40 | 126 | 77 ± 69 | 63 | 70 ± 20 | 71 |
| Mg, mg/L | 21 ± 11 | 19 | 25 ± 10 | 26 | 21 ± 13 | 20 | 16 ± 8 | 14 |
| Na, mg/L | 72 ± 105 | 34 | 75 ± 129 | 35 | 67 ± 80 | 27 | 69 ± 90 | 29 |
| K, mg/L | 4.7 ± 3.2 | 4.4 | 6.3 ± 3.6 | 5.4 | 3.3 ± 2.2 | 2.5 | 3.4 ± 1.8 | 3.1 |
| Sr, mg/L | 1.0 ± 0.8 | 0.8 | 1.1 ± 0.7 | 0.9 | 1.0 ± 1.3 | 0.7 | 0.8 ± 0.4 | 0.6 |
| HCO3-, mg/L | 340 ± 110 | 350 | 399 ± 104 | 385 | 305 ± 90 | 325 | 274 ± 95 | 237 |
| SO4-2, mg/L | 97 ± 118 | 68 | 124 ± 102 | 95 | 92 ± 174 | 40 | 51 ± 44 | 35 |
| Cl-, mg/L | 80 ± 150 | 30 | 96 ± 192 | 43 | 56 ± 73 | 24 | 78 ± 139 | 21 |
| NO3-, mg/L | 13.6 ± 28.3 | 4.1 | 18 ± 40 | 3.3 | 6.4 ± 11.0 | 0.1 | 14 ± 12 | 14 |
| NH4+, mg/L | 0.3 ± 0.3 | 0.1 | 0.4 0.4 | 0.1 | 0.3 ± 0.3 | 0.2 | 0.1 ± 0.1 | 0.05 |
| Ba, µg/L* | 186 ± 191 | 131 | 256 239 | 193 | 139 ± 151 | 145 | 126 ± 67 | 126 |
| Fe, µg/L* | 1258 ±2887 | 122 | 2384 ±3972 | 464 | 499 ±1128 | 125 | 183 ± 280 | 22 |
| Mn, µg/L* | 240 ± 495 | 14 | 489 ± 658 | 276 | 13 ± 15 | 5 | 66 ±123 | 2 |
| * Excluding sample #20 | ||||||||
The data were statistically analyzed to reveal any significant geochemical relationships that might exist between TOC and various inorganic constituents. As shown in table 12, only a few of the correlations were statistically significant, and most of the statistically significant correlations were artifacts due to the combining of different populations (e.g., TOC versus Ca, Mg, hardness, K, and HCO3- for all 50 samples) or to the presence of outliers (e.g., TOC versus HCO3- or Ba for the alluvial aquifers and TOC versus NO3- for the unconsolidated aquifers). Upon closer inspection, the only potentially significant relationships were those involving NH4+, Fe, and Mn.
Table 12--Correlation of TOC and inorganic constituents.
| Constituent | Linear Regression Correlation Coefficient | |||
|---|---|---|---|---|
| All Aquifers (n=50) |
Alluvial Aquifers (n=23) |
Consolidated Aquifers (n=14) |
Unconsolidated Aquifers (n=12) |
|
| pH | -0.175 | 0.178 | 0.121 | -0.409 |
| Specific Conductance | 0.063 | -0.294 | 0.457 | 0.008 |
| Ca, mg/L | 0.420** | 0.061 | 0.337 | -0.244 |
| Mg, mg/L | 0.290* | 0.149 | 0.236 | 0.370 |
| Hardness, mg/L as CaCO3 | 0.419** | 0.101 | 0.327 | 0.007 |
| Na, mg/L | -0.149 | -0.328 | 0.263 | 0.012 |
| K, mg/L | 0.428** | 0.203 | -0.026 | 0.006 |
| Sr, mg/L | 0.131 | -0.179 | 0.390 | 0.546* |
| HCO3-, mg/L | 0.565** | 0.507* | 0.520* | -0.196 |
| SO4-2 mg/L | 0.057 | -0.353 | 0.436 | 0.151 |
| Cl-, mg/L | -0.116 | -0.334 | 0.021 | 0.042 |
| NO3-, mg/L | 0.150 | 0.013 | 0.184 | 0.594* |
| NH4+, mg/L | 0.500** | 0.600** | 0.183 | 0.395 |
| NH4+, mg/L1 | 0.736** | 0.676* | 0.594 | - |
| Ba, µg/L2 | 0.580** | 0.681** | -0.132 | -0.381 |
| Fe, µg/L2 | 0.648** | 0.669** | 0.049 | -0.044 |
| Mn, µg/L2 | 0.449** | 0.216 | 0.065 | 0.110 |
| Fe + Mn, µg/L3 | 0.966** | 0.991** | - | - |
| * Statistically significant at the 5% level of significance ** Statistically significant at the 1% level of significance 1 With values ≤ 0.1 mg/L excluded (n = 19, 8, 9, and 2, respectively) 2 Excluding sample #20 3 Excluding sample #20 and values < 1000 mg/L (n = 9, 8, 1, and 0, respectively) |
||||
Figs. 7 and 8 show NH4+ and Fe + Mn, respectively, as a function of TOC concentration for the alluvial aquifers. In each case, there is a subset of samples having an elevated concentration of NH4+ or Fe + Mn in which the concentration is linearly related to TOC. The correlation of Fe + Mn with TOC was especially strong (r = 0.991) for alluvial aquifer samples having a concentration of Fe + Mn greater than 1,000 µg/L (table 12 and fig. 8). Interestingly, the subsets of samples high in NH4+ and high in Fe + Mn are virtually identical, i.e. each of the alluvial aquifer samples having an NH4+ concentration greater than 0.1 mg/L also had an Fe + Mn concentration greater than 1,000 µg/L. Also, for the alluvial aquifer samples having > 0.1 mg/L of NH4+, NH4+ is linearly correlated to both Fe + Mn (r = 0.893) and to TOC (r = 0.845) when sample 43 is excluded as an outlier. Sample 43 had a high NH4+ concentration (1.0 mg/L) relative to its TOC concentration of 1.02 mg/L and, unlike the other high ammonium samples, it contained much more Mn than Fe. Sample 20 was not plotted in fig. 8, due to its excessive concentrations of Fe and Mn, but this sample had the highest concentration of NH4+ (1.5 mg/L) among all the samples.
Figure 7--Ammonium as a function of TOC for alluvial aquifer samples.
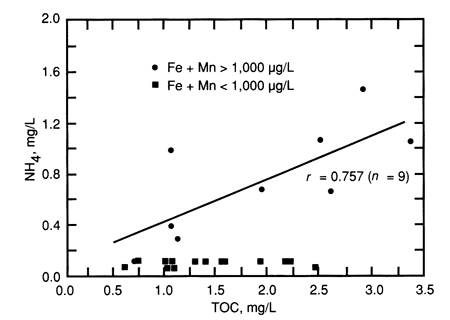
Figure 8--Fe + Mn as a function of TOC for alluvial aquifer samples.
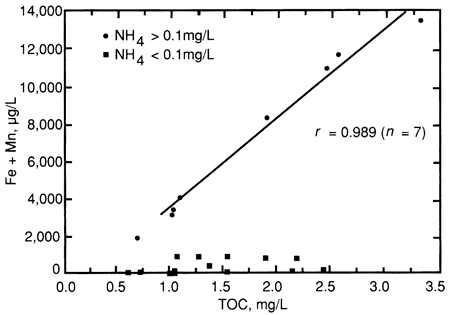
These results reveal two distinct populations of alluvial aquifers: one having elevated concentrations of NH4+, Fe, and Mn, and the other having low concentrations of these constituents. Presumably, the former population is associated with reducing (anoxic or anaerobic) conditions, under which iron and manganese were solubilized and the NH4+ released by biological activity could not be oxidized to nitrate. The TOC values of both populations vary over about the same range, but those for the population having high concentrations of NH4+, Fe, and Mn are linearly related to those constituents. There are several possible explanations for this relationship:
In any event, these relationships are strong enough and interesting enough to merit further investigation, especially in view of the fact that all of the constituents involved pose significant problems in regard to treatment of potable water supplies.
The current federal MCL for THMs for utilities serving more than 10,000 persons is 100 µg/L. The Kansas Department of Health and Environment (KDHE) also has applied this requirement to all new supplies and to small systems (serving less than 10,000 people) undergoing plant modifications. The federal standard is expected to be lowered, perhaps substantially, when the new standards for disinfection byproducts are released in the near future. Approximately 8% of the study samples had TFPs greater than 100 µg/L (the present MCL for THMs), but 56% had TFPs greater than 25 µg/L and 90% had TFPs greater than 10 µg/L. Hence, many water-supply systems using ground waters in Kansas might have difficulty in meeting a substantially lower THM limit.
The highest TFP concentrations were found in samples from alluvial aquifers, so it is clear that utilities using waters from alluvial sources would be the most greatly affected by a lower THM limit. Since many communities in Kansas, especially eastern Kansas, are largely dependent on alluvial aquifers as sources for public water supplies, special attention should be given to monitoring and control of THM concentrations in drinking-water supplies derived from these aquifers.
The four major alternatives to controlling THMs include 1) precursor removal, 2) use of an alternative disinfectant (eliminating the use of chlorine), 3) removal of THMs after they are formed, and 4) modification of the chlorination process to hinder the progress of the reaction. The simplest and most effective means of controlling THM formation for most water-treatment plants in Kansas is to modify the chlorination process and replace free chlorine with combined chlorine, since the latter does not form THMs. In Kansas, a free chlorine residual of 0.2 mg/L or a combined residual of 1.0 mg/L is required throughout the finished-water distribution system for disinfection purposes. Higher combined residuals are needed because combined chlorine is not as strong a disinfectant as free chlorine. There are several other advantages associated with combined chlorine: 1) it is more stable in the distribution system; 2) it can be used in higher concentrations than free chlorine, since it contributes less to taste and odor; and 3) it requires lower dosages of chlorine for waters already containing substantial concentrations of ammonium.
The use of combined chlorine in water supplies should only be implemented by those having adequate knowledge of the chemistry of chlorine and ammonium and the reactions between them, so that maximum disinfection can be achieved with minimum THM formation and a minimum of taste and odor problems. It also is important that any change in disinfection practice be carefully monitored to ensure that the microbial quality of the drinking water is not compromised.
The data also bear significant implications with regard to monitoring of water supplies for compliance with the THM regulations. Since TFP and TOC are very strongly correlated, TOC could be used as a surrogate measure of THM formation potential, and ground-water supplies having low concentrations of TOC could be exempted from monitoring for THMs. Also, because there was a strong correlation between the TFP and TTHM concentrations (with the former being generally higher), TFP analyses conducted in a centralized laboratory could be used as a substitute for TTHM analyses. The relationship between TOC and NH4+, Fe, and Mn suggests that alluvial aquifers having high concentrations of Fe, Mn, and NH4+ should receive the most immediate attention and closer monitoring.
Next Page--Report Start || Next Page--Summary, References
Kansas Geological Survey
Placed on web Nov. 6, 2012; originally published in 1991.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS12/02_results.html