Kansas Geological Survey, Bulletin 96, Part 6, originally published in 1952
Originally published in 1952 as Kansas Geological Survey Bulletin 96, Part 6.
Six river and flood-plain sand samples from the vicinity of Kansas City, Wichita, Concordia, and Salina were treated by flotation methods to determine the feasibility of producing a feldspar concentrate and a silica concentrate for use in glass, glass fiber, and ceramic and allied industries. The following average values are from five of these tests: a feldspar concentrate yield of 27.1 percent with an average alkali content (K2O+Na2O) of 13.2 percent and a molecular ratio of K2O to Na2O of 3.6 to 1. The silica sand residue remaining after flotation of the feldspar is capable of being concentrated to an Fe2O3 content of less than 0.03 percent. These tests show that both a silica sand and feldspar product of commercial grade can be produced from the sand sampled at Concordia, Kansas City, and Wichita.
The ceramic, glass fiber, glass, and allied industries have been expanding into Kansas and adjacent areas during the past decade. The raw materials used in these industries are acquiring a greater market potential yearly. At the present time feldspar and silica sand are shipped into the Kansas region at considerable expense for freight and handling. The Geological Survey has published the results of one investigation (Nixon, Runnels, and Kulstad, 1950) on a source of silica sand. The present study was undertaken to determine if common river sands which are available, in very large tonnages could be utilized as a source for feldspar and silica sand. Preliminary examination has indicated that many river sands in Kansas contain almost 30 percent by weight of feldspar, orthoclase (potash) predominating. It was thought that suitable flotation methods could be worked out to produce a feldspar concentrate that would meet industrial specifications and that the silica sand fraction would meet, or could be further treated to meet, the glass and glass-fiber specifications.
The results of this study are presented in the following pages and show that feldspar concentrates and silica sand concentrates can be produced from common river sands. The analyses show that these products are comparable, with the possible exception of iron content, to the products now used and that they can be produced by standard commercial techniques. Due to the limited nature of this investigation, no attempt was made to prepare cost estimates although it is judged that the process described is economically feasible.
Samples of sand for this investigation were obtained from six widely separated sand plants which are located where there is a possible market for feldspar and silica products. The method of sand production in each of these plants is the same. A mixture of sand and water is pumped from a dredge in the river or pond to the sand preparation plant where it passes through a coarse screen to remove trash, mud balls, and gravel. The sand is then graded by means of screens or classifiers to yield one or more usable sand products and fine sand that is discarded unless there is a market for it. Samples of the various sand grades, including the fine sand reject, were taken at each plant. The operator's estimate of the relative amounts of the various grades was used in making up composite samples of sand from each plant. Table 1 gives data on the sampling and compositing of the sand from various locations.
Table 1--Location, company name, and proportions of sand samples used in this study.
| Name of sand company | Location of plant | Type of operation | Grades of sand produced | Proportions used in making up composite |
Date Sampled |
|---|---|---|---|---|---|
| Stewart Sand and Materials Co. | Turner, Kansas (near Kansas City) | Sand pumped from Kansas River | Concrete sand | 47.22 | June 1, 1951 |
| Mason's sand | 47.22 | ||||
| Fine sand | 5.56 | ||||
| Dolese Brothers | 21st Street and Big River, Wichita, Kansas | Sand pumped from Arkansas River | Concrete sand | 60.00 | June 6, 1951 |
| Plaster sand | 25.00 | ||||
| Fine sand | 15.00 | ||||
| Walt Keeler Co., Inc. | Southwest of Wichita on highway K-42 | Sand pumped from pond | Concrete sand | 80.00 | June 7, 1951 |
| Fine sand | 20.00 | ||||
| Black Cat Sand Co. | 47th Street and Little River, Wichita, Kansas | Sand pumped from Little Arkansas River | Coarse and medium mixed | 70.00 | June 7, 1951 |
| Fine sand | 30.00 | ||||
| Ross Sand Co. | East of Concordia | Sand pumped from pond beside Republican River | Road gravel | 26.70 | August 23, 1951 |
| Concrete aggregate | 33.30 | ||||
| Plaster sand | 26.70 | ||||
| Fine sand | 13.30 | ||||
| Salina Sand Co. | South of Salina | Sand pumped from pond beside Smoky Hill River | Concrete sand | 70.00 | August 24, 1951 |
| Fine sand | 30.00 |
Table 2 presents data on complete chemical analyses of head samples of the composite and the fine sand from four of the plants. In preparing these samples the sand was crushed in a disk pulverizer, resulting in the addition of abraded iron to the sample. Part or all of this iron was removed with a hand magnet, and it is probable that some magnetite originally in the sand was also removed. Thus the iron analyses represent the less magnetic iron in the sand. The analyses of the fine sand were included because at most plants the fines are either a waste product or are produced in excess of market requirements, thus constituting a potential source of raw material.
Table 2--Chemical analyses of head samples of the composite sand and the fine sand from four locations.
| Company | Product | SiO2* | Al2O3** | Fe2O3 | CaO | MgO | K2O | Na2O | Loss on ignition |
|---|---|---|---|---|---|---|---|---|---|
| Stewart Sand and Materials Co. | Composite | 87.37 | 7.08 | 0.65 | 0.72 | 0.10 | 2.46 | 1.23 | 0.39 |
| Fine sand | 85.24 | 7.55 | 0.71 | 1.31 | 0.15 | 2.81 | 1.46 | 0.77 | |
| Walt Keeler Co., Inc. | Composite | 87.54 | 6.87 | 0.72 | 0.35 | 0.07 | 3.06 | 1.11 | 0.28 |
| Fine sand | 85.75 | 7.58 | 1.11 | 0.61 | 0.15 | 3.16 | 1.33 | 0.32 | |
| Dolese Brothers | Composite | 87.38 | 7.09 | 0.88 | 0.49 | 0.10 | 2.62 | 1.21 | 0.23 |
| Fine sand | 86.48 | 7.70 | 1.37 | 0.63 | 0.16 | 2.09 | 1.29 | 0.28 | |
| Black Cat Sand Co. | Composite | 88.39 | 6.38 | 0.72 | 0.36 | 0.08 | 2.90 | 0.93 | 0.24 |
| Fine sand | 87.18 | 6.95 | 0.84 | 0.53 | 0.13 | 2.76 | 1.34 | 0.27 | |
| *By difference from 100. **Contains also MnO, TiO2, and P2O5 if present. |
|||||||||
Screen analyses of four of the composite samples are presented in Table 3.
Table 3--Screen analyses of four composite samples.
| Size, mm or mesh | Weight distribution, percent, of samples from | |||
|---|---|---|---|---|
| Stewart Sand and Materials Co. |
Walt Keeler Co., Inc. |
Dolese Brothers |
Black Cat Sand Co. |
|
| Plus 9.4 mm | Trace | 1.0 | 1.7 | |
| 9.4 mm-3 mesh | 1.1 | 1.7 | 2.8 | |
| 3-4 mesh | Trace | 1.6 | 1.6 | 3.0 |
| 4-6 | 1.2 | 3.2 | 3.2 | 5.5 |
| 6-8 | 2.5 | 5.3 | 4.6 | 7.7 |
| 8-10 | 4.1 | 8.0 | 6.3 | 11.8 |
| 10-14 | 5.4 | 9.3 | 6.9 | 12.2 |
| 14-20 | 6.5 | 10.7 | 7.4 | 11.8 |
| 20-28 | 11.5 | 15.8 | 12.6 | 9.5 |
| 28-35 | 13.6 | 15.2 | 15.9 | 10.8 |
| 35-48 | 23.7 | 15.4 | 21.4 | 11.2 |
| 48-65 | 16.7 | 8.5 | 11.6 | 7.4 |
| 65-100 | 10.9 | 4.2 | 4.6 | 3.4 |
| 100-150 | 2.7 | 1.0 | 0.8 | 0.6 |
| 150-200 | 0.9 | 0.4 | 0.2 | 0.2 |
| 200-270 | 0.2 | 0.1 | 0.1 | 0.1 |
| Minus 270 | 0.1 | 0.2 | 0.1 | 0.3 |
A number of preliminary tests were made to establish a procedure for separating the composite samples from the various plants into their constituent minerals. Several kilograms of the composite sand from each company was crushed to pass a 20-mesh screen by means of laboratory rolls in closed circuit with the screen. This was then weighed into 1 kilogram samples for use in the individual tests.
The degree of grinding to be used was selected by placing 1 kg charges of sand and 500 cc water in a 12 by 5 inch Denver laboratory steel ball mill with about 9 kilograms of steel balls, grinding for various lengths of time, and sizing the resulting pulp. A 15-minute grind was selected. Table 4 gives sizing data on sand ground for 15 and 20 minute periods.
Table 4--Sizing data on ground composite sand from Stewart Sand and Materials Company
| Size | Sand ground 20 minutes |
Sand ground 15 minutes |
|---|---|---|
| +48 mesh | 0.1 | 0.1 |
| 48-65 | 0.1 | 0.7 |
| 65-100 | 2.6 | 13.7 |
| 100-150 | 10.1 | 17.4 |
| 150-200 | 20.4 | 18.9 |
| -200 | 66.7 | 49.2 |
| Total | 100.0 | 100.0 |
Considerable iron-bearing magnetic mineral is present in the sands and also there was some iron abraded from the mill and balls. Because iron is most undesirable in feldspar and quartz products, most of it was removed magnetically. The ground sand for all tests was passed through a Frantz Ferrofilter. This device is a short cylinder filled with a network of soft iron grids that are magnetized by a direct current coil surrounding the cylinder. This forms a trap for magnetic particles, but lets nonmagnetic particles pass through with the water. After the current has been stopped the magnetic material may be washed out into a separate pan. In some of the tests the magnetic material was further separated by means of a hand magnet into products of different iron contents.
The extremely fine particles in a ground pulp, or slime as the finest material is called, often cause difficulty in nonsulfide flotation. For this reason the pulp in all the tests was deslimed before flotation. The analyses presented in Tables 5 to 10 show that in all cases the slime contained more iron than any of the flotation products so that its removal was helpful in lowering the iron content of the finished products. Only about 8 percent of the weight of the sand was removed as slime. The method of desliming the sand was to agitate the nonmagnetic pulp from the Ferrofilter in the 3-liter glass cell of a laboratory Fagergren flotation machine. A reagent was added to disperse the slime and then the sand was permitted to settle for 8 minutes before the slime-laden water was removed by a siphon. The sand was repulped with water and again permitted to settle for 8 minutes before the unsettled slime was removed. This was repeated (total of three deslimings which should remove practically all the slime if it were completely dispersed). A sufficient quantity of any of the three dispersing agents tried gave satisfactory results. When a 5 percent solution of Daxad 23 was used, 2 cc of solution did not give complete dispersion, but 10 cc of solution did. In one test a total of 50 cc of 5 percent sodium metasilicate was used to effect complete dispersion of the slime. With a 5 percent Calgon solution the addition of 10 cc did not seem to disperse the slime completely, while the addition of 20 cc gave excellent dispersion. The pulps that had been deslimed with Calgon as dispersing agent seemed to float better than those that had had Daxad treatment. Hence 20 cc of 5 percent Calgon per kilogram of sand (2 pounds per ton) was selected as the dispersing agent for subsequent tests.
The usual method of separating quartz and feldspar by flotation is to depress the quartz by means of hydrofluoric acid and float the feldspar with amine. Previous experience with the flotation of feldspar from quartz had indicated that good results could be obtained if 4 cc of 48 percent hydrofluoric acid was used in tests of the size being made. During the preliminary work the quantity of acid used in two tests was reduced to 0.8 cc and 2.0 cc respectively. The flotation results in each of these tests were definitely inferior to the results of similar tests in which 4 cc of acid was used. Therefore the larger quantity amounting to 4.4 pounds of HF per ton of sand was used in subsequent tests.
The primary amine acetates used as collector's in the flotation of the feldspar form a homologous series with a variable length hydrocarbon chain. Samples of distilled amine acetates with hydrocarbon chains ranging in length from 10 carbon atoms to 18 carbon atoms were supplied by Armour Chemical Division of Armour and Company. Different amines were used in a number of tests, the best results being obtained with the one having 12 carbon atoms. Thus in subsequent tests the collector was Armac 12-D, distilled dodecylamine acetate. Tests in which the quantity and method of addition of amine were varied showed that satisfactory results were obtained when it was added by stages of 0.1 pound per ton followed by conditioning for 1 minute and removal of the floatable material after each amine addition. The use of a little frother B-23 improved the flotation.
Previous experience with the flotation of Kansas River sand had shown that after the removal of the feldspar it was possible to float the quartz by raising the pH of the pulp to about 7 either by adding a caustic or by washing the acid out with fresh water. Very little additional amine is required to float a quartz product that is substantially more pure than the final nonfloated tailing.
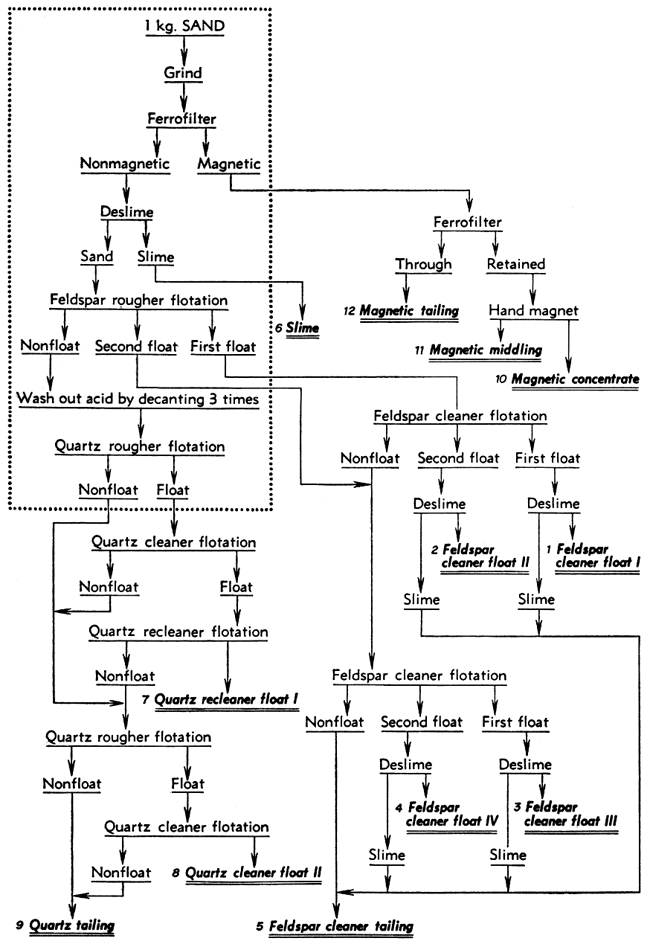
After a satisfactory procedure had been established for separating the composited sand from the Kansas River at Kansas City into mineral concentrates, the same procedure was applied with minor variations to each of the composited sand samples. The best possible quality was desired in the feldspar and quartz concentrates that were to be analyzed. To achieve this, the final tests were made using 3 kilograms of sand instead of 1 kilogram and the products were cleaned or re-cleaned by floating them a second or third time to give what seemed to be optimum results. The procedures used in these tests are shown as flowsheets (Figs. 1, 2, 3). The operations enclosed within the dotted rectangle were carried out with three separate 1-kilogram samples, the three charges being treated in parallel for that part of the test. Those operations shown outside the rectangle were carried out on the corresponding combined products from the parallel parts of the test.
Figure 1--Flow sheet for separation procedure for sand from Turner, Kansas (near Kansas City). Operations inside the rectangle were performed on three charges in parallel while those outside were performed on the combined products.

Figure 2--Flow sheet for separation procedure for sand from the areas of Concordia and Wichita, Kansas. Operations inside the rectangle were performed on three charges in parallel while those outside were performed on the combined products.
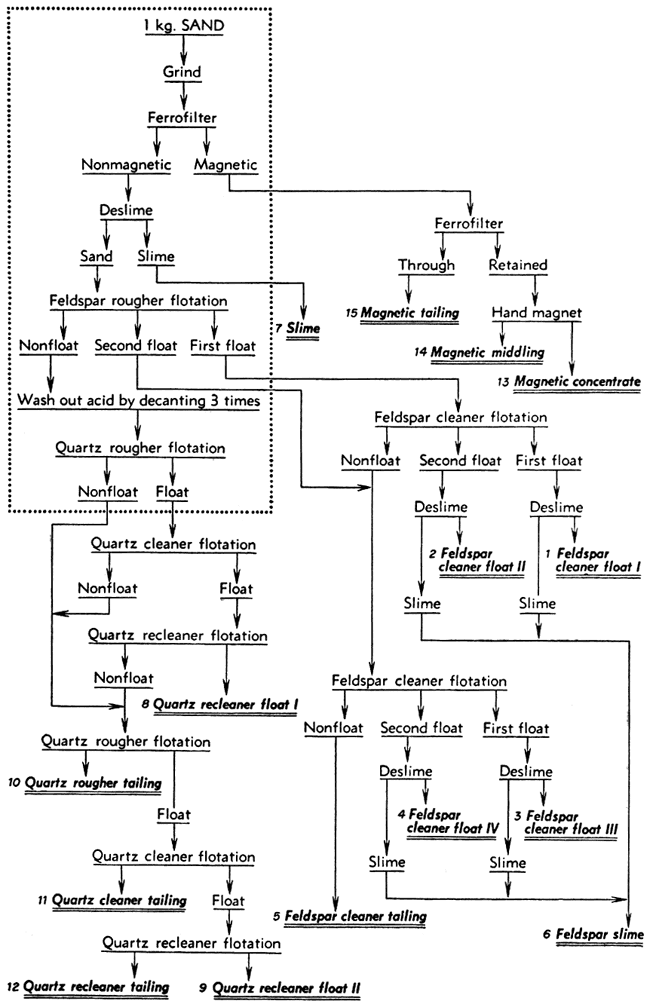
Figure 3--Flow sheet for separation procedure for sand from Salina, Kansas. Operations inside the rectangle were performed on three charges in parallel while those outside were performed on the combined products.
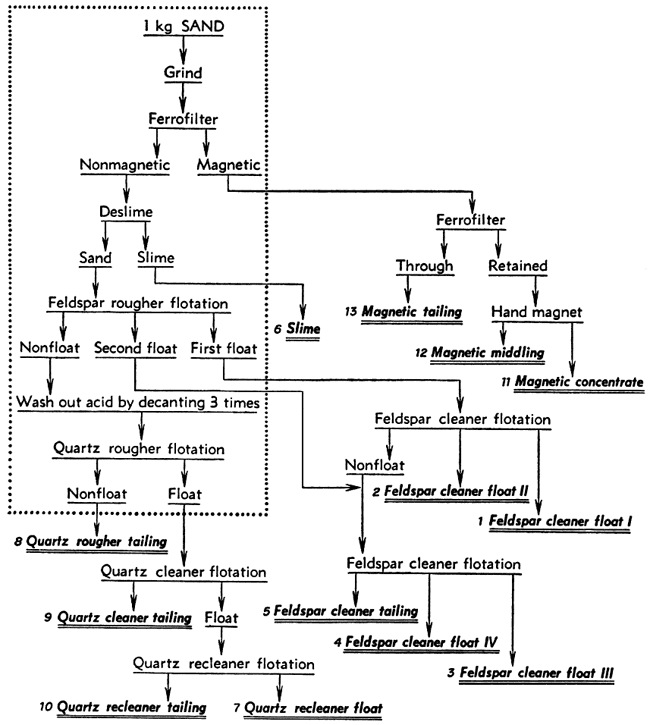
The separation of each sand composite was started in the same way and the parallel parts of the tests were all alike except for variations in the quantity of amine required. Each 1-kilogram charge of sand was ground in the steel mill for 15 minutes and then passed through the Ferrofilter to remove magnetic material. The magnetics removed from the three charges in each test were combined and again passed through the Ferrofilter to remove the nonmagnetic sand that had been caught mechanically. This was saved separately (called "magnetic tailing"). A hand magnet was used to produce a "magnetic middling" and "magnetic concentrate" from the material caught a second time in the Ferrofilter. The nonmagnetic sand from the Ferrofilter was deslimed by dispersing it with 2 pounds of Calgon per ton and then decanting it three times after 8-minute settling periods. The slimes from all three charges were combined.
The deslimed sand from each charge was prepared for feldspar flotation by agitating it with about 4.4 pounds of hydrofluoric acid per ton (4 cc of 48 percent HF per kilogram of sand) for 5 minutes. This gave a pH of about 3 with all the sands except that from the Salina Sand Company. With this sand the pH rose somewhat due to carbonate in the pulp so that 5 cc of acid instead of 4 cc was used. The feldspar was floated in stages using 0.1 pound of Armac 12-D per ton and about 0.05 pound of frother B-23. The sands from near Wichita and Concordia required only three stages to remove most of the feldspar. On these sands the first stage froth was considered the first float; the other two stages were combined as the second float. The sands from near Kansas City and Salina required four stages to float the feldspar. In these tests the first two, stages constituted the first float and the last two the second float.
The acid was removed from the tailings after feldspar flotation by letting the sand settle, pouring off the solution, and repulping with water until the sand had been decanted three times. Then the sand was repulped, a drop of frother B-23 added, and much of the silica floated without any additional amine. After this flotation no further separations were made on the 1-kilogram charges, but only upon combined products from the three parallel parts of the test.
The first feldspar floats were combined and cleaned by reflotation using only a little frother B-23, This first float became "feldspar cleaner float I." A little amine was then added to produce a second float, "feldspar cleaner float II." The tailings from this cleaner flotation, usually low in weight but containing much feldspar, and the second feldspar floats from the parallel parts of the test were combined for cleaning by the same method as for the first floats. "Feldspar cleaner float III" was produced without additional amine and "feldspar cleaner float IV" by the addition of amine. The froth in each of these feldspar concentrates was sprayed to break it down and the feldspar was stirred up in a pan of water. After the feldspar had settled, the water carrying some dark slime was decanted. These slimes were combined and saved separately or added to the "feldspar cleaner tailings" from the final flotation. In one test the feldspar products were not deslimed.
The quartz floats and quartz tailings from the 1-kilogram charges were re-treated to produce clean products. The combined quartz floats were refloated twice with only a little additional frother to give "quartz recleaner float I." The cleaner and recleaner tailings and the three quartz tailings from the 1-kilogram charges were combined for further flotation. A very small amount of amine was added and a rougher float made of the quartz. The froth was cleaned twice by flotation to give "quartz recleaner float II." In most of the tests the "quartz rougher tailings," "quartz cleaner tailing," and "quartz recleaner tailing" were saved separately. The procedures for cleaning of the quartz from the Kansas City sand and the Salina sand were modified somewhat as shown in Figures 1 and 3.
Representative samples of the various products were taken for chemical analysis, and analyzed without further grinding. In some instances special procedures were required to obtain reliable analyses. The modifications of the ordinary analytical methods are discussed briefly in the following paragraphs.
Two-gram samples were fused with a mixture of CaO and Na2CO3 at 1200° C. as described by Shell (1949). The fused cake was dissolved in HCl and water and then dehydrated to precipitate the silica. The dehydrated mass was then treated with HC1 and filtered to remove crude silica. The resulting solution was again dehydrated and the procedure repeated to recover the remaining crude silica. The combined precipitates of crude SiO2 were ignited to 1200° C., weighed, treated with HF and H2SO4 to evolve the silica, reignited, and weighed. The loss of weight was recorded as SiO2. It was found, however, that retention of calcium in the crude silica and the subsequent formation of calcium sulfate caused the final weighing to be high, thus giving a low value for the silica. This condition was corrected by determining the amount of retained sulfate with BaCl2 and adding this percentage to the observed percentage of silica. The necessity of this step was demonstrated to our satisfaction by repeated determinations of silica on the Bureau of Standard's standard feldspar No. 99.
When analyses had already been completed before the necessity of this silica correction was discovered, adjustment of the silica percentage, by difference from 100 percent (100.00 minus analyzed constituents equals SiO2) was considered safe and adequate.
The determination of R2O3 (Al2O3, Fe2O3, TiO2, ZrO2, V2O5, P2O5, and MnO) was done by accepted methods except for a gravimetric determination of TiO2 with cupferon reagent (Runnels, Utter, and Reed, 1950). Thus the reported results were Al2O3 (including P2O5 and MnO if present), Fe2O3, and TiO2 (including ZrO2 and V2O5 if present).
The alkalis were determined with a model 52-C Perkin-Elmer flame photometer after taking the sample into solution with HF and H2SO4. This procedure eliminates the tedious J. Lawrence Smith method of sintering the sample. Where the potassium content is higher than the sodium content, as in this study, the flame photometer gives reliable results for both elements (Broderick and Zack, 1951).
Qualitative spectrographic analyses of the feldspar products were made with an Applied Research Laboratory 1.5-meter grating spectrograph. The following elements were observed: manganese, silver, strontium, chromium, zirconium, nickel, copper, vanadium, and lead. All occurred in amounts estimated to be from 0.1 percent (strontium and manganese) to spectrographic traces. Lithium, rubidium, cesium, and barium were not detected.
Tables 5, 6, 7, 8, 9, and 10 present data on the weight distribution and the chemical analyses of the products from the large-scale tests discussed earlier.
Table 11 summarizes the composition and proportion of feldspar in the various composite sand samples as calculated from the analyses of the feldspar concentrates presented in Tables 5 through 10.
Table 5--Chemical analyses and weight distribution on products from composite sand sample from Stewart Sand and Materials Co., Turner, Kansas.
| No. on flowsheet (Fig. 1) |
Product | Weight distribution, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Feldspar cleaner float I | 8.5 | 63.82 | 19.21 | 0.49 | 0.18 | 0.83 | 1.16 | 10.20 | 3.73 | 0.38 | 100.00 |
| 2 | Feldspar cleaner float II | 8.2 | 65.36 | 18.61 | 0.44 | 0.26 | 1.32 | 0.87 | 8.72 | 4.10 | 0.32 | 100.00 |
| 3 | Feldspar cleaner float III | 8.8 | 64.57 | 19.65 | 1.01 | 0.26 | 0.74 | 1.11 | 7.97 | 4.12 | 0.57 | 100.00 |
| 4 | Feldspar cleaner float IV | 1.6 | 65.48 | 17.30 | 1.21 | 0.38 | 3.15 | 0.40 | 6.61 | 4.01 | 1.46 | 100.00 |
| 5 | Feldspar cleaner tailing | 3.2 | 79.24 | 8.37 | 1.04 | 1.53 | 2.25 | 0.49 | 3.38 | 2.06 | 1.68 | 100.00 |
| 6 | Slime | 6.7 | 76.94 | 11.26 | 2.93 | 0.13 | 0.43 | 0.36 | 3.78 | 1.86 | 2.29 | 100.00 |
| 7 | Quartz recleaner float I | 22.6 | 0.030 | |||||||||
| 8 | Quartz cleaner float II | 18.8 | 0.056 | |||||||||
| 9 | Quartz tailing | 17.5 | 0.34 | |||||||||
| 10 | Magnetic concentrate | 0.5 | 33.09 | 8.39 | 51.29 | 4.31 | ||||||
| 11 | Magnetic middling | 0.8 | 13.74 | |||||||||
| 12 | Magnetic tailing | 2.8 | 1.98 | |||||||||
| 100.0 | ||||||||||||
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. |
||||||||||||
Table 6--Chemical analyses and weight distribution data on products from composite sand sample from Walt Keeler Co., Inc., Wichita, Kansas.
| No. on flowsheet (Fig. 2) |
Product | Weight distribution, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Feldspar cleaner float I | 10.2 | 64.20 | 19.56 | 0.43 | 0.24 | 0.46 | 0.12 | 10.61 | 3.77 | 0.25 | 99.64 |
| 2 | Feldspar cleaner float II | 6.0 | 64.66 | 19.53 | 0.46 | 0.22 | 0.87 | 0.07 | 9.94 | 3.43 | 0.29 | 99.42 |
| 3 | Feldspar cleaner float III | 9.2 | 66.05 | 18.41 | 0.86 | 0.42 | 0.86 | 0.24 | 8.76 | 3.92 | 0.48 | 100.00 |
| 4 | Feldspar cleaner float IV | 1.5 | 70.52 | 15.75 | 1.33 | 0.64 | 1.26 | 0.28 | 5.48 | 3.95 | 0.79 | 100.00 |
| 5 | Feldspar cleaner tailing | 2.1 | 92.75 | 3.37 | 0.52 | 0.27 | 0.58 | 0.21 | 1.05 | 0.91 | 0.42 | 100.08 |
| 6 | Feldspar slimes | 0.6 | 66.23 | 14.29 | 3.20 | 0.67 | 2.60 | 0.79 | 5.25 | 3.21 | 3.76 | 100.00 |
| 7 | Slime | 7.6 | 77.76 | 10.77 | 2.81 | 0.55 | 0.88 | 0.42 | 3.01 | 1.80 | 2.00 | 100.00 |
| 8 | Quartz recleaner float I | 16.6 | 0.023 | 0.04 | 0.05 | |||||||
| 9 | Quartz recleaner float II | 13.2 | 0.033 | 0.05 | 0.05 | |||||||
| 10 | Quartz rougher tailing | 6.0 | 0.28 | 0.33 | 0.16 | |||||||
| 11 | Quartz cleaner tailing | 9.9 | 0.077 | 0.12 | 0.07 | |||||||
| 12 | Quartz recleaner tailing | 13.0 | 0.055 | 0.06 | 0.06 | |||||||
| 13 | Magnetic concentrate | 0.5 | 58.31 | |||||||||
| 14 | Magnetic middling | 1.1 | 11.20 | |||||||||
| 15 | Magnetic tailing | 2.5 | 1.22 | |||||||||
| 100.0 | ||||||||||||
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. |
||||||||||||
Table 7--Chemical analyses and weight distribution data on products from composite sand sample from Dolese Brothers, Wichita, Kansas
| No. on flowsheet (Fig. 2) |
Product | Weight distribution, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Feldspar cleaner float I | 12.4 | 65.40 | 19.21 | 0.46 | 0.09 | 0.83 | 0,.08 | 10.50 | 3.79 | 0.41 | 100.77 |
| 2 | Feldspar cleaner float II | 5.5 | 65.07 | 19.60 | 0.48 | 0.13 | 1.07 | 0.07 | 9.29 | 4.41 | 0.63 | 100.48 |
| 3 | Feldspar cleaner float III | 9.0 | 65.50 | 18.51 | 1.18 | 0.51 | 1.41 | 0.26 | 8.01 | 4.27 | 0.60 | 100.25 |
| 4 | Feldspar cleaner float IV | 1.1 | 70.12 | 15.40 | 1.55 | 0.63 | 1.99 | 0.68 | 5.19 | 3.59 | 0.85 | 100.00 |
| 5 | Feldspar cleaner tailing | 1.9 | 92.09 | 3.58 | 0.85 | 0.19 | 0.54 | 0.32 | 0.88 | 0.89 | 0.29 | 99.66 |
| 6 | Feldspar slimes | 1.2 | 67.28 | 16.12 | 2.72 | 0.42 | 1.64 | 0.49 | 6.67 | 3.03 | 1.63 | 100.00 |
| 7 | Slime | 7.3 | 76.62 | 10.32 | 2.38 | 0.33 | 0.71 | 0.36 | 3.96 | 1.99 | 3.07 | 99.74 |
| 8 | Quartz recleaner float I | 18.4 | 0.017 | 0.10 | 0.05 | |||||||
| 9 | Quartz recleaner float II | 14.4 | 0.044 | 0.05 | 0.04 | |||||||
| 10 | Quartz rougher tailing | 7.2 | 0.49 | 0.24 | 0.16 | |||||||
| 11 | Quartz cleaner tailing | 10.6 | 0.13 | 0.08 | 0.08 | |||||||
| 12 | Quartz recleaner tailing | 6.7 | 0.065 | 0.09 | 0.06 | |||||||
| 13 | Magnetic concentrate | 0.8 | 53.53 | |||||||||
| 14 | Magnetic middling | 0.9 | 13.19 | |||||||||
| 15 | Magnetic tailing | 2.6 | 1.56 | |||||||||
| 100.0 | ||||||||||||
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. |
||||||||||||
Table 8--Chemical analyses and weight distribution data on products from composite sand sample from Black Cat Sand Co., Wichita, Kansas
| No. on flowsheet (Fig. 2) |
Product | Weight distribution, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Feldspar cleaner float I | 11.6 | 64.47 | 19.39 | 0.43 | 0.14 | 0.72 | 0.18 | 10.47 | 3.81 | 0.31 | 99.92 |
| 2 | Feldspar cleaner float II | 5.6 | 65.90 | 19.33 | 0.40 | 0.22 | 0.74 | 0.15 | 10.20 | 3.39 | 0.30 | 100.66 |
| 3 | Feldspar cleaner float III | 8.6 | 66.27 | 18.45 | 0.76 | 0.48 | 1.03 | 0.22 | 7.77 | 3.91 | 0.54 | 99.43 |
| 4 | Feldspar cleaner float IV | 0.8 | 75.44 | 13.21 | 1.44 | 0.55 | 1.14 | 0.34 | 4.04 | 3.26 | 0.70 | 100.12 |
| 5 | Feldspar cleaner tailing | 1.9 | 95.05 | 2.19 | 0.61 | 0.30 | 0.37 | 0.11 | 0.71 | 0.42 | 0.41 | 99.80 |
| 6 | Feldspar slimes | 0.6 | 67.23 | 15.22 | 4.21 | 0.56 | 1.17 | 0.50 | 6.31 | 2.57 | 2.51 | 100.28 |
| 7 | Slime | 8.6 | 74.79 | 10.57 | 2.49 | 0.16 | 0.43 | 0.31 | 5.59 | 3.47 | 2.19 | 100.00 |
| 8 | Quartz recleaner float I | 17.2 | 0.028 | 0.06 | 0.04 | |||||||
| 9 | Quartz recleaner float II | 17.3 | 0.024 | 0.29 | 0.17 | |||||||
| 10 | Quartz rougher tailing | 6.8 | 0.37 | 0.22 | 0.11 | |||||||
| 11 | Quartz cleaner tailing | 11.2 | 0.075 | 0.10 | 0.06 | |||||||
| 12 | Quartz recleaner tailing | 5.7 | 0.045 | 0.08 | 0.04 | |||||||
| 13 | Magnetic concentrate | 0.6 | 56.61 | |||||||||
| 14 | Magnetic middling | 1.6 | 10.86 | |||||||||
| 15 | Magnetic tailing | 2.9 | 1.60 | |||||||||
| 100.0 | ||||||||||||
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. |
||||||||||||
Table 9--Chemical analyses and weight distribution data on products from composite sand sample from Ross Sand Co., Concordia, Kansas
| No. on flowsheet (Fig. 2) |
Product | Weight distribution, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Feldspar cleaner float I | 7.4 | 64.60 | 19.49 | 0.25 | 0.16 | 0.43 | 0.32 | 10.54 | 3.77 | 0.44 | 100.00 |
| 2 | Feldspar cleaner float II | 4.2 | 64.90 | 19.48 | 0.28 | 0.09 | 0.98 | 0.13 | 9.93 | 3.80 | 0.41 | 100.00 |
| 3 | Feldspar cleaner float III | 10.0 | 65.59 | 19.19 | 0.46 | 0.34 | 0.66 | 0.12 | 9.22 | 4.00 | 0.42 | 100.00 |
| 4 | Feldspar cleaner float IV | 5.2 | 64.82 | 19.15 | 0.81 | 0.26 | 1.44 | 0.11 | 7.89 | 4.68 | 0.49 | 99.65 |
| 5 | Feldspar cleaner tailing | 5.6 | 79.99 | 13.78 | 0.54 | 0.17 | 1.18 | 0.07 | 3.94 | 1.84 | 0.49 | 100.00 |
| 6 | Feldspar slimes | 0.6 | 64.35 | 15.26 | 3.99 | 0.76 | 3.39 | 0.67 | 5.50 | 2.60 | 3.48 | 100.00 |
| 7 | Slime | 9.3 | 75.51 | 10.89 | 2.70 | 0.17 | 1.55 | 0.36 | 4.28 | 2.11 | 2.32 | 99.89 |
| 8 | Quartz recleaner float I | 16.8 | 0.069 | 0.07 | 0.07 | |||||||
| 9 | Quartz recleaner float II | 18.4 | 0.025 | 0.02 | 0.04 | |||||||
| 10 | Quartz rougher tailing | 2.8 | 1.12 | 0.90 | 0.80 | |||||||
| 11 | Quartz cleaner tailing | 5.8 | 0.39 | 0.42 | 0.39 | |||||||
| 12 | Quartz recleaner tailing | 9.7 | 0.16 | 0.23 | 0.18 | |||||||
| 13 | Magnetic concentrate | 0.9 | 36.08 | |||||||||
| 14 | Magnetic middling | 0.6 | 10.80 | |||||||||
| 15 | Magnetic tailing | 2.7 | 1.56 | |||||||||
| 100.0 | ||||||||||||
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. |
||||||||||||
Table 10--Chemical analyses and weight distribution data on products from composite sand sample from Salina Sand Co., Salina, Kansas.
| No. on flowsheet (Fig. 3) |
Product | Weight distribution, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Feldspar cleaner float I | 8.6 | 64.62 | 18.24 | 1.87 | 0.15 | 1.16 | 0.18 | 9.91 | 3.22 | 0.92 | 100.27 |
| 2 | Feldspar cleaner float II | 3.3 | 64.21 | 17.07 | 2.49 | 0.59 | 2.85 | 0.41 | 7.96 | 2.81 | 1.51 | 100.35 |
| 3 | Feldspar cleaner float III | 6.6 | 70.53 | 10.05 | 5.29 | 0.74 | 4.54 | 0.22 | 4.61 | 1.71 | 2.43 | 100.00 |
| 4 | Feldspar cleaner float IV | 1.2 | 71.34 | 4.12 | 5.45 | ‡ | 11.50 | 0.33 | 1.06 | 0.89 | 5.31 | 100.00 |
| 5 | Feldspar cleaner tailing | 1.3 | 77.22 | 1.09 | 1.55 | 0.01 | 11.69 | 0.10 | 0.19 | 0.16 | 7.99 | 100.00 |
| 6 | Slime | 9.8 | 75.39 | 6.72 | 5.84 | 0.50 | 3.84 | 0.57 | 2.16 | 0.97 | 4.01 | 100.00 |
| 7 | Quartz recleaner float | 33.2 | 0.073 | 0.03 | 0.04 | |||||||
| 8 | Quartz rougher tailing | 13.0 | 3.56 | 0.46 | 0.21 | |||||||
| 9 | Quartz cleaner tailing | 5.5 | 0.67 | 0.23 | 0.13 | |||||||
| 10 | Quartz recleaner tailing | 12.3 | 0.20 | 0.11 | 0.07 | |||||||
| 11 | Magnetic concentrate | 0.6 | 59.01 | |||||||||
| 12 | Magnetic middling | 1.4 | 7.98 | |||||||||
| 13 | Magnetic tailing | 3.2 | ||||||||||
| 100.0 | ||||||||||||
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. ‡Reported with Al2O3 |
||||||||||||
Table 11--Calculated yield and chemical composition of combined feldspar products from composite sand samples. (Data from Tables 5 through 10.)
| Sand company | Yield weight, percent |
SiO2 | Al2O3* | Fe2O3 | TiO2** | CaO | MgO | K2O | Na2O | Loss on ignition† |
|---|---|---|---|---|---|---|---|---|---|---|
| Stewart Sand and Materials Co. | 27.11 | 64.63 | 19.06 | 0.69 | 0.24 | 1.08 | 1.01 | 8.82 | 3.99 | 0.49 |
| Walt Keeler Co., Inc. | 26.92 | 65.30 | 18.94 | 0.63 | 0.32 | 0.73 | 0.16 | 9.57 | 3.76 | 0.37 |
| Dolese Brothers | 27.87 | 65.55 | 18.91 | 0.74 | 0.25 | 1.11 | 0.16 | 9.25 | 4.06 | 0.53 |
| Black Cat Sand Co. | 26.58 | 65.67 | 18.89 | 0.56 | 0.28 | 0.84 | 0.19 | 9.35 | 3.74 | 0.39 |
| Ross Sand Co. | 26.79 | 65.06 | 19.31 | 0.44 | 0.24 | 0.80 | 0.18 | 9.44 | 4.04 | 0.44 |
| Salina Sand Co. | 19.66 | 66.94 | 14.45 | 3.41 | 0.44 | 3.20 | 0.24 | 7.27 | 2.50 | 1.79 |
| *Contains MnO and P2O5 when present. **Contains ZrO2 and V2O5 when present. †105° to 1000° C. |
||||||||||
Sands from the two major rivers in Kansas contain about 30 percent feldspar, about 65 percent quartz, and small amounts of many other minerals including magnetite and ilmenite. It was shown that the sands may be separated into a number of products having different mineralogical compositions. The object of such separations was to obtain a product containing the relatively valuable and abundant feldspar with a minimum of iron-bearing minerals. To accomplish this as much iron as possible was removed by magnetic separation and by desliming before flotation of the feldspar to separate it from the quartz. As a result of this treatment the quartz residues were easily treated by further flotation to make high-grade silica products.
Chemical analyses of the products showed that the feldspar concentrates contained nearly pure feldspar with an iron content somewhat higher than the best commercial grades. The silica products were of high quality and would meet the chemical requirements of most users.
Broderick, E. J., and Zack, P. G. (1951) Flame spectrophotometry for determination of sodium, potassium, and lithium in glass: Anal. Chemistry, vol. 23, no. 10, pp. 1455-1458, figs. 1-2.
Nixon, E. K., Runnels, R. T., and Kulstad, R. O. (1950) The Cheyenne sandstone of Barber, Comanche, and Kiowa Counties, Kansas, as raw material for glass manufacture: Kansas Geol. Survey, Bull. 86, pt. 3, pp. 41-84, figs. 1-4, pls. 1-2. [available online]
Runnels, R. T., Utter, M. G., and Reed, A. C. (1950) Determination of ferric oxide and titania in presence of alumina: Jour. Am. Ceramic Soc., vol. 33, no. 2, pp. 51-53.
Shell, H. R. (1949) Chemical analysis of clay: U. S. Bur. Mines, Rept. of Investi. 4420, pp. 1-36, fig. 1.
Kansas Geological Survey, Geology
Placed on web Jan. 3, 2008; originally published June 1, 1952.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/96_6/index.html