Prev Page--Physiography || Next Page--Origin
Petrology
Thin sections of indurated sandstones from the Dakota formation and Kiowa shale were examined from samples from 31 localities in 10 counties in Kansas--Clay, Ellsworth, Kearny, Lincoln, McPherson, Ottawa, Rice, Russell, Saline, and Washington. Some mechanical analyses were made of the sand freed of cement (Table 1), chemical analyses were studied (Tables 2 and 3), and a few staining tests were made. The cementing materials show a wide range in composition and petrographic character, and in some instances the sand grains themselves show alteration related to the cement.
The indurated sandstones of the Dakota formation and Kiowa shale are grouped, on the basis of their cement, into four categories: iron oxide-cemented sandstone, calcite-cemented sandstone, dolomitic calcite-cemented sandstone (here designated simply as dolomite-cemented sandstone), and silica-cemented sandstone. A few miscellaneous types are also discussed, In all the samples herein described, unless otherwise noted, the clastic grains are predominantly quartz, with minor quantities of feldspar (plagioclase, microcline, orthoclase), chert, and quartzite.
Table 1--Size distribution of sand in carbonate-cemeitted sandstones. (Analyses by Carrie B. Thurber.)
| County | Location | Size disttribution in mm (percent by weight) | Type of cement |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| .71-.5 | .5-.35 | .35-.25 | .25-.177 | 177-.125 | .125-.088 | .088-.062 | .062 | |||
| Lincoln | 7-12-7W | 1.9 | 40.1 | 41.7 | 13.6 | 1.1 | 1.6 | Calcite | ||
| Lincoln | 7-12-10W | 0.0 | 73.9 | 20.0 | 2.8 | 2.3 | 0.4 | 0.5 | Dolomite | |
| McPherson | 19-17-2W | 0.0 | 0.1 | 0.8 | 33.8 | 55.9 | 5.5 | 3.8 | Dolomite | |
| Ellsworth | 19-16-6W | 0.0 | 0.0 | 88.3 | 9.5 | 0.5 | 0.8 | 0.2 | 0.7 | Calcite |
Table 2--Chemical composition of eight samples of indurated sandstones from the Dakota and Kiowa formations in Kansas. (Analyses by Russell Runnels.)
| County | Location | Chemical analysis (percent) | Type of cement |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loss on ignition | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | BaSO4 | SO3 | Sol. in HCl |
|||
| Lincoln | 13-12-8W | 16.60 | 58.02 | 1.49 | 0.08 | 0.59 | 21.05 | 0.44 | 0.00 | 0.03 | 39.74 | Calcite |
| Washington | 11-3-4E | 14.17 | 62.56 | 2.12* | 1.49 | 16.71 | 0.72 | 0.00 | 0.00 | 34.28 | Calcite | |
| Rice | 26-20-10W | 13.99 | 62.57 | 1.06 | 0.90 | 0.21 | 20.39 | 0.20 | 0.00 | 0.00 | 34.58 | Calcite |
| Lincoln | 7-12-10W | 16.73 | 60.95 | 0.76 | 0.25 | 0.82 | 13.74 | 6.31 | 0.00 | 0.00 | 37.13 | Dolomite |
| McPherson | 19-17-2W | 16.48 | 61.61 | 1.31 | 0.52 | 0.18 | 16.07 | 5.75 | 0.00 | 0.00 | 36.08 | Dolomite |
| McPherson | 1-18-2W | 18.35 | 55.88 | 2.37* | 0.50 | 13.51 | 6.92 | 0.00 | 0.07 | 41.34 | Dolomite | |
| McPherson | 25-17-1W | 13.61 | 60.29 | 2.07* | 0.19 | 17.82 | 1.42 | 2.95 | 0.36 | 35.45 | Calcite-barite | |
| Ellsworth | 14-15-6W | 2.04 | 83.76 | 4.06 | 8.69 | 0.48 | 0.00 | 0.94 | 0.00 | 0.00 | 2.69 | Ferric oxide |
| *Includes TiO2 | ||||||||||||
Iron Oxide-cemented Sandstone
Iron oxide is the most abundant cementing material of the sandstones in the Dakota formation; it is also common in the Kiowa shale. On outcrops it occurs chiefly in a case-hardened crust, which makes the sandstone extremely resistant to abrasion and further weathering. However, a few inches or feet in from the surface, most of the sandstones are probably soft and friable. Limonite is the predominant form of iron oxide in the case-hardened sandstone, but there are also small quantities of hematite. Hematite is much more prevalent in the subsurface Dakota than in outcropping sandstones, but it is well developed in a branch of Horsethief Canyon, in the Cen. sec. 9, T. 16 S., R. 6 W., Ellsworth County, and at Rocktown, sec. 4, T. 13 S., R. 11 W., Russell County.
Sands cemented with iron oxide are fine- to coarse-grained, and well- to poorly sorted. Many contain pellets of impure iron oxide slightly larger than the quartz grains, and small hollow concretionary nodules of limonite. Two thin sections of ferruginous sandstone were examined. One is case-hardened dark-brown sandstone from a channel sand of the Terra Cotta clay, NE sec. 2, T. 3 S., R. 4 E., Washington County (Pl. 5D). The cement is chiefly limonite, which fills more than half the space between the sand grains; this matrix is further characterized by pore spaces of about the diameter of coarse silt particles. Some of these pore spaces have a roughly rhombohedral shape, and the cement is judged to have been derived from siderite, because there are a few remnants of siderite rhombs in various stages of alteration. The sand grains themselves are well sorted but poorly packed. Many of the grains have been etched so that they exhibit extremely irregular boundaries. There are also small micro-clay-balls, or pellets, about 2 mm in diameter, which consist of impure limonite and hematite and contain much silt. As silt particles are not present elsewhere in the thin section, these clay balls must have been deposited as units.
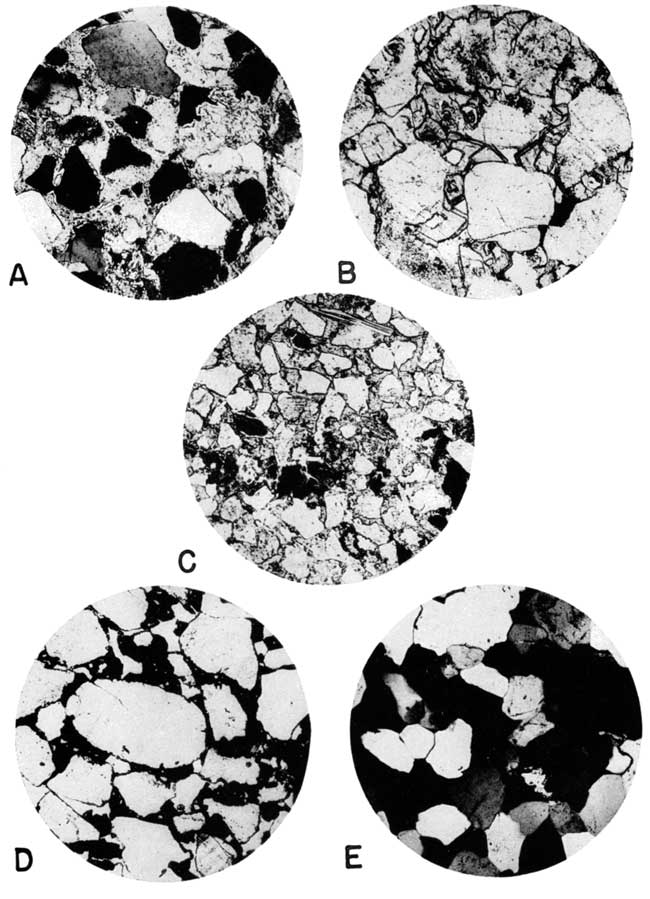
Plate 5--Thin sections from sandstone in the Kiowa and Dakota formations of Kansas. [Web image enlarged to show additional detail.]
A. Etched grains of quartz and feldspar, partly replaced by crystalline calcite cement. Dakota formation, Lincoln quarry, sec. 7, T. 12 S., R. 7 W., Lincoln County. Crossed nicols, X 53.
B. Sandstone cemented with dolomite rhombs. Kiowa shale, sec. 19, T. 17 S., R. 2 W., McPherson County. Plane polarized light, X 53.
C. Calcite-cemented micaceous sandstone with glauconite grains. Kiowa shale, sec. 27, T. 15 S., R. 2 W., Saline County. Plane polarized light, X 53.
D. Limonite-cemented sandstone, Dakota formation, NE sec. 2, T. 3 S., R. 4 E., Washington County. Plane polarized light, X 53.
E. Quartzite, Dakota formation, sec. 17, T. 25 S., R. 37 W., Kearny County. Note original outlines of grains, and ragged staurolite. Crossed nicols, X 53.
The second thin section of ferruginous sandstone is from the Terra Cotta clay near the type locality in Ellsworth County (SW sec. 14, T. 15 S., R. 6 W.), and was cut from somewhat friable sandstone below the case-hardened surface. It differs from the dark case-hardened sandstone described above, chiefly in the smaller quantity of iron oxide cement. The cement is limonite, but occurs as scattered patches or grains, and as coatings on the quartz particles. Many of the grains are probably pseudomorphs after pyrite, but their uniformly rhombohedral shape in thin section suggests that they may be pseudomorphs after siderite or ankerite. The sand grains are poorly sorted, but little or no silt is present. Heavy accessory minerals (tourmaline and zircon) are not coated with iron oxide, and are not etched, as are most of the quartz grains.
Calcite-cemented Sandstone
The calcite-cemented sandstone is hard, dense, quartzite-like rock which is gray to creamy gray on fresh surfaces and buff-colored or brown when weathered. Liesegang banding is developed on some of the weathered surfaces, but is not visible in thin sections or on freshly broken rock. The sand is fine- to coarse-grained, and in nearly every case extremely well sorted (Table 1). Thin sections show that the calcite cement occurs as large interlocking crystals, each enclosing several quartz grains. The diameter of the crystals may be observed in the hand specimen by reflection of sunlight from cleavage planes. If the crystals did not interlock, much of this sandstone would be similar in general appearance to the famed calcite "sand-crystals" of the White River Oligocene in South Dakota and Wyoming (Penfield and Ford, 1900; Ziegler, 1914, pp. 126-128; Wanless, 1922) and to the Fountainebleau sandstones of France. There are a few localities in the Dakota of Kansas where cement crystals are not entirely in contact with each other, notably in the SE sec. 13, T. 12 S., R. 8 W., Lincoln County; and also in the S2 sec. 20, T. 9 S., R. 2 W., Ottawa County, where small sand-crystals occur in a partly cemented zone between two spheroidal concretions.
The optical orientation of the calcite crystals seems to be random in most samples examined, but in others the orientation of each crystal differs only slightly from that of the adjacent crystal, giving the appearance of curved faces in the hand specimen. The grains of quartz and feldspar are characteristically etched (Pl. 5A). Glauconite is commonly present as irregular grains. The fact that some of the glauconite grains exhibit shrinkage cracks (Pl. 5C) suggests that they were transported only a short distance before deposition. Uncorroded fragments of shells also occur commonly. In some samples uncorroded oyster shells are associated with Turritella casts.
Pyrite
Much of the calcite-cemented sandstone contains small cubes of pyrite disseminated throughout the cement, and also nodules of pyrite cement up to 4 cm or more in diameter. Some pyrite nodules surround fragments of wood. Upon weathering, the pyrite alters to limonite which gives a yellowish-brown color to the rock. Hemispherical pits are formed in the exposed surface of the rock by the action of sulfuric acid, developed by the weathering of the pyrite nodules, on the calcite.
Sand-siderite pellets
At a few localities calcite-cemented sandstone peppered with small siderite or ankerite pellets occurs. Sand grains are included within the pellets. This feature is well developed in sandstone concretions in the W2 NW sec. 3, T. 14 S,. R. 6 W., Ellsworth County, and sec. 22, T. 9 S., R. 1 E., Clay County; and it seems to be restricted to the Dakota formation. As the pellets weather readily to limonite, they are not found in surface exposures unless protected as in the calcite-sandstone concretions.
Asphalt
Bituminous or asphaltic material is another impurity observed in some of the calcite-cemented sandstones. It is well developed in spherical concretions in the SE sec. 16, T. 18 S., R. 1 W., McPherson County, where it gives the rock a dark-gray or dark grayish-brown color. The asphalt occurs as small areas of cementing material admixed with calcite. Plate 6B shows this type of sandstone from the Kiowa shale (?) in the SW sec. 24, T. 18 S., R. 2 W., McPherson County.
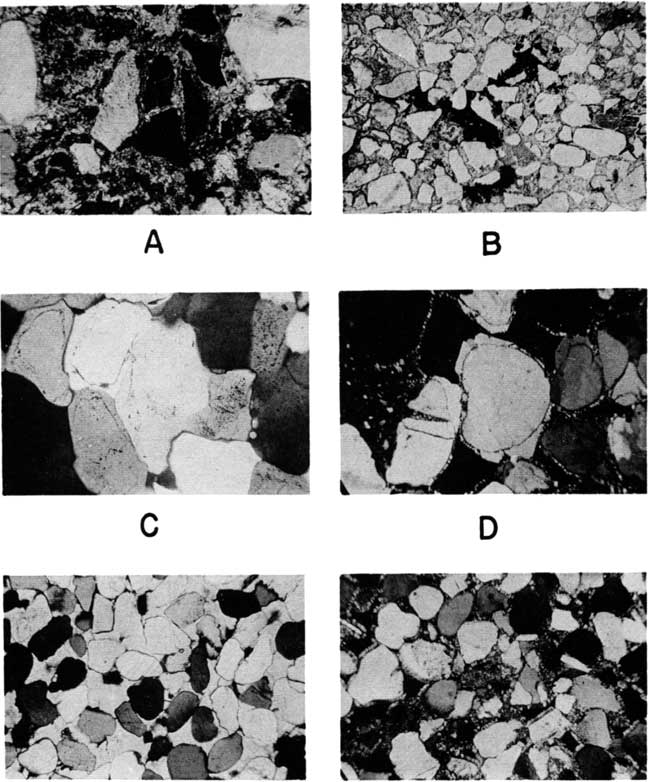
Plate 6--Thin sections from sandstone in the Kiowa and Dakota formations of Kansas. [Web image enlarged to show additional detail.]
A. Etched, displaced quartz grain in calcite-ankerite-cemented sandstone, Dakota formation. NE NW sec. 9, T. 1 S., R. 3 E., Washington County. Crossed nicols, X 120.
B. Asphalt in calcite-cemented sandstone, Kiowa shale. SW sec. 24, T. 18 S., R. 2 W., McPherson County. Plane polarized light, X 34.
C. Quartzite, Dakota formation, sec. 17, T. 25 S., R. 37 W., Kearny County, showing original grain outlines. Crossed nicols, X 120.
D. Silica-cemented sandstone, Kiowa shale (?), Twin Mounds. Sec. 1, T. 18 S., R. 2 W., McPherson County. Note chert in fractured grain, and secondary quartz projecting into pore spaces. Crossed nicols, X 120.
E. Celestite-cemented sandstone at base of Cretaceous in SW cor. sec. 2, T. 17 S., R. 5 W., McPherson County. Crossed nicols, X 29.
F. Silica-cemented sandstone from NW SW sec. 15, T. 18 S., R. 1 W., McPherson County. Crossed nicols, X 29.
Sand-barite
Sand barite crystals and rosettes characterize many outcrops of calcite-cemented sandstone in the Kiowa shale, but they have not been noted in the Dakota formation in Kansas. Barite crystals, however, have been described by Schramm (1943) in septaria-like concretions in the Dakota formation near Lincoln, Nebraska. The barite crystals are tabular and range in long diameter from 5 mm to more than 20 mm. The rosettes range from about 12 mm to the size of large walnuts, which many of them closely resemble in external appearance. Crystals and rosettes are not scattered uniformly throughout a bed, but are arranged in clusters or groups. Their relative insolubility causes them to protrude above the weathered surfaces of the sandstone outcrops. Localities where the barite sand crystals are well developed include: NE sec. 25, T. 17 S., R. 1 W., McPherson County; NW sec. 36, T. 14 S., R 4 W., Saline County; and the SW NW sec. 15, T. 20 S., R. 6 W., Rice County. Two types of barite rosettes are observed at the Saline County locality. Both types consist of aggregates of tabular barite crystals, but in one type all the crystals radiate about one of their axes (probably b), which they have in common; the other is the more usual rosette type, with two tabular crystals at right angles crossing a central depression. Similar forms from Permian rocks are described and illustrated by Tarr (1933), and others are illustrated by Walther (1924, p. 75). Tarr notes that these forms serve as horizon markers in the Garber formation and to a less extent in the Wellington formation in central Oklahoma. He believes that the barium was derived from barium silicates, from barium adsorbed in colloids, or from detrital grains of barite. He concludes that it was leached out by chloride waters (original brines, carried a short distance, and deposited as a cementing material through reaction with a soluble sulfate; and that the time of deposition was probably not long after the formation of the sandstone. The barite in the Kiowa shale probably had its source in the underlying Permian rocks, and, in accordance with the hypothesis of Tarr, may have been dissolved from the Permian deposits by the marine chloride waters of the Kiowa sea. The absence of barite in the Dakota formation may be ascribed in part to the quality of water associated with Dakota deposition and in part to the previous burial of the Permian rocks by Kiowa sediments.
In thin section the barite is seen to have slightly irregular contacts with the calcite cement in some areas and to be bounded by plane faces in others. The orientation of the tabular crystals is random. The sand grains surrounded by calcite are etched to a greater extent than those within the barite crystals. Some individual grains which penetrate a barite-calcite contact are etched on the calcite side and relatively unaffected on the barite side.
Dolomite-cemented Sandstone
Dolmite-cemented sandstone is known to occur in western Lincoln County (sec. 7, T. 12 S., R. 10 W.), at or near the top of the Dakota formation. It is also observed in the Kiowa shale in McPherson County in sec. 19, T. 17 S., R. 2 W., where it occurs as isolated concretionary masses on a hill side, and in large flattened spheroidal concretions a few feet above the base of Twin Mounds, near the NW cor. sec. 1, T. 18 S., R. 2 W. The appearance of this sandstone in the field is similar to that of much of the calcitecemented sandstone, and in some localities it may easily have been mistaken for the latter. The clastic grains are well sorted, as in the calcite-cemented sandstone (Table 1).
The dolomite cement occurs as small crystals about the size of the sand grains; the individual crystals seldom enclose quartz grains, and no sample was observed in which one crystal enclosed more than 3 or 4 sand grains. The dolomite may generally be distinguished from calcite in thin section by its occurrence as small rhombohedra (Pl. 5B). Staining tests (silver nitrate-potassium chromate) show that calcite in this sandstone occurs as isolated patches and as small veins less than 1 mm in diameter. The occurrence of pyrite is similar to that in the calcite-cemented sandstones. Chemical analyses (Tables 2 and 4) show that the sandstone contains from 5 to 7 percent magnesium oxide and from 11 to 16 percent calcium oxide. Dolomite-cemented sandstone and typical calcite-cemented sandstone have not been found together or in the same section in any area.
Silica-cemented Sandstone
Silica cement is observed in sandstones of the Kiowa shale in McPherson and Rice counties and in the Dakota formation in Kearny County. Hand specimens range in color from white to pink and buff, and several colors occur in one exposure. The sandstone is fine-to coarse-grained and dense to porous. Some of the white sandstone in Kearny County (sec. 17, T. 25 S., R. 37 W.) has a sugary texture. The silica cement is of several types, some of which occur together in the same exposure and even in the same thin section. Quartz cement optically continuous with the grains is noted in two thin sections from the Kearny County locality (Pls. 5E and 6C). The original outlines of many of the grains are made visible by an iron oxide coating, and the grains do not show the etching which is characteristic of the calcite-cemented sandstones. The space occupied by cement is also less than in the carbonate-cemented samples. A thin layer of quartzite containing similar cement and overlying calcite-cemented sandstone occurs in sec. 13, T. 19 S., R. 6 W., Rice County. A thin section from this locality shows relatively unetched sand grains surrounded by optically continuous quartz, although some pore spaces are left. In some areas of the thin section the cement is a coarse-grained chert.
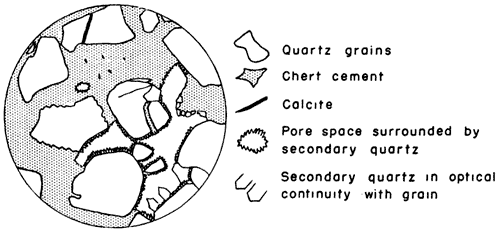
A mixture of chert and quartz cement, with a trace of calcite, is well shown in a thin section of partly cemented sandstone from Twin Mounds, McPherson County (Pl. 6D, Fig. 2). Calcite is observed in a small crack traversing a quartz grain which is surrounded by ebert, and also as minute specks in part of the chert cement. Most of the quartz cement occurs outside of a thin layer of chert cement which surrounds the grains, and projects as euhedral crystals into the pore spaces of the sandstone. The chert not only surrounds the grains, but also projects into wedge-shaped cracks and fractures, and cuts entirely across grains which have been fractured along planes of weakness and rotated, or displaced.
Fig. 2--Drawing from thin section of silica-cemented sandstone from Twin Mounds, McPherson County, showing evidence of replacement of calcite.
Uniformly medium-grained, granular chalcedonic chert is the cementing material in a thin section of pinkish-brown quartzite from the Kearny County locality. The quartz grains are closely packed, and the color results from large quantities of iron oxide coating the grains. A few of the grains show some indication of etching.
Chalcedonic chert cements the quartz grains in a thin section of sandstone from the NW SW sec. 15, T. 18 S., R. 1 W., McPherson County. It consists of thin bands of fibrous silica coating the grains, with the long dimensions of the fibers normal to the surfaces of the grains (Pl. 6F).
Most of the thin sections of silica-cemented sandstone are characterized by the extreme scarcity or absence of feldspars and by the absence or relative unimportance of etched surfaces of quartz grains. One thin section from Kearny County shows delicately etched staurolite, but other minerals are unaffected (Pl. 5E). The preservation of quartz grains in secondary silica is noted by Hatch, Rastall, and Black, (1938, p. 90).
Relation to other cements
In a description of cementing materials in sandstones of Mississippian to Cretaceous age in the Rocky Mountain region, Waldschmidt (1941, p. 1,858) indicates the following sequence of deposition of cements: quartz, dolomite, calcite, anhydrite. This type of sequence was not recognized in any of the sandstones examined by me. No beds or concretionary bodies in which both calcite and secondary silica were observable were noted in the field, and only one thin section (Twin Mounds, McPherson County) displayed this combination. The only other cementing material commonly found in association with silica is ferric oxide, some of which may be in the form of hematite.
The type of silica cement observed in a sandstone seemingly is related to the presence or absence of impurities in the sand. Chert cement or finely divided silica is associated with sandstone containing clay (as in the upper siltstone of the Dakota formation in Ford and Hodgeman counties) or iron oxide (as in some samples from McPherson and Kearny counties). Quartz cement occurs in the pure, well-sorted sandstones. This association is observed in other sands (Hadding, 1929, pp. 19-20).
Ankerite- and Celestite-cemented Sandstones
Ankerite cement occurs in a few concretionary sandstones in northern Washington County (Pl. 6A). Celestite cement occurs as very large crystals in a small lens of sandstone 6 inches thick and a lew feet in lateral extent at the base of the Kiowa in northwestern McPherson County (SW sec. 2, T. 17 S., R. 5 W.), where it is associated with the basal Cretaceous pebble zone (Plummer and Romary, 1942, p. 320). A thin section of this sandstone is shown in Plate 6E, which illustrates the close packing of the quartz grains and the absence of etching.
Prev Page--Physiography || Next Page--Origin
Kansas Geological Survey, Geology
Placed on web June 14, 2007; originally published Nov. 1947.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/70_4/05_petro.html