Kansas Geological Survey, Bulletin 233, p. 163-176
by
Roger L. Kaesler
University of Kansas
In recent years the thinking of paleontologists has become increasingly oriented toward the biologic sciences and less toward sedimentology and stratigraphy. For this reason advances in paleontology in the past three decades have not found their way into the repertoire of some sedimentologists. This is perhaps especially true of those who are most heavily involved with sedimentologic modeling. Organisms respond to changes in the environment, but they also contribute to these changes; thus information about them needs to be incorporated into sedimentary models. The advances in paleontology that are important for modeling sedimentologists fall into three categories: the data of paleontology, interactions between fossils and paleoenvironments, and sources of permanent change through time. Interpreting the data of paleontology has been advanced by improvements in the understanding of taphonomy, time averaging, and the temporal resolution of the stratigraphic record. Interactions between fossils and their paleoenvironments can be expressed in terms of succession and diversity, both of which can be incorporated into sedimentary models. Some biologic communities contain highly interactive groups of species. The structure of such communities has typically been attributed to competition, but competition theory has recently been criticized by many ecologists, leaving community paleoecology somewhat up in the air. Studies of communities, however, remain applicable to paleoenvironmental analysis. Permanent changes through time are typically large-scale or long-term events that are rather easily incorporated into sedimentary models. They include organic evolution, community evolution, and extinction.
An Acrobat PDF file containing the complete paper is available (1.1 MB).
The revitalization of paleontology was a major development of the late 1960's and the 1970's. Since then and as a direct result of the confluence of a number of historically fascinating events, paleontologists' views have changed: views of themselves, of their science, of its role in the broader scheme of things, and, indeed, of the fossil record itself. Subsequent rapid advances have come as paleontologists have incorporated new knowledge from other fields. Moreover, to an extent most of us would have thought impossible 25 years ago, paleontologists have begun to contribute to the development of ecology and evolutionary theory rather than being mere consumers of progress in these disciplines [see Gould (1980)].
As paleontologists have become increasingly biologic in their outlook, paleontology has improved. The cost of this improvement--and nothing is done without cost--has been borne by geology. Today, paleontologists typically do not concern themselves with biostratigraphic problems to the extent that they did in the past. Many paleontologists are not academically grounded in the systematics of a specific group of fossil organisms, and some are content to study only data about fossils rather than the fossils themselves. As a result, much of the progress in paleontology has not been transferred into the literature of sedimentology and physical stratigraphy.
Cross and Harbaugh (1990, p. 9) emphasized that modelers of quantitative dynamic stratigraphy need to select carefully the ranges of variables that operate in their models. It is probably true that what sedimentary modelers need most from paleontologists is good biostratigraphy to provide better resolution of geologic time and good paleoecology to enable them to estimate water depth better. This is especially true in sequence stratigraphy, where progress in some directions awaits more refined biostratigraphy and paleobathymetry.
My goal here is less pragmatic: to summarize some of the advances in paleontology, especially community paleoecology (as broadly construed; table 1), that can enhance the work of sedimentary modelers. This goal is set in the belief that no discussion of sedimentology or stratigraphy and certainly no sedimentary models are complete unless they incorporate information from the fossil record. In the interest of brevity, I cite examples from the literature but do not discuss them in detail. Instead, I provide some of the conceptual background that has contributed to the growth of our understanding of fossil communities. Finally, although my approach is eclectic rather than exhaustive, I focus attention on ancient organisms as potential sedimentary particles and agents of sedimentation in hopes of making my remarks more useful to modelers.
Table 1--Classification of topics in community paleoecology that bear on sedimentary modeling.
Interpreting the data of paleontology
|
Biological community has been defined in many ways. Most definitions refer to a community as a group of species that live together and that interact with each other in various ways. Such ancillary terms as "assemblage" and "association" have been used to refer to parts of communities or to mixtures of communities or their remains. Moreover, some paleontologists have used the term "paleocommunity" to emphasize that fossils are unique and that paleontologists cannot study holistic communities (Kauffman and Scott, 1976). Ecologists studying modern environments, however, are nearly as limited as paleontologists in their approach to communities by the logistic difficulties of conducting thorough ecologic studies.
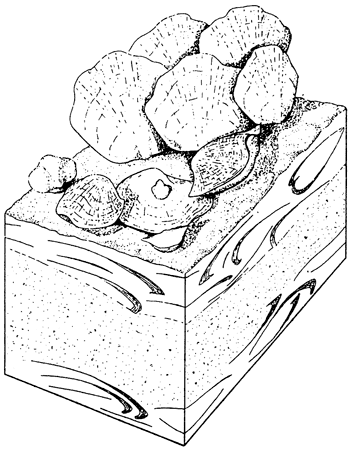
Studies of communities in the fossil record are of two kinds: reconstructional and quantitative. The reconstructional approach is widely used and is typified by Ziegler et al. (1968) and McKerrow (1978). Usually on the basis of extensive field experience, the investigators assess the relative importance of the kinds of organisms in the community and their contribution to the sedimentary rock record. The organisms and their fossilizable remains in the substrate are often depicted in block diagrams in which the abundances of species are more or less proportional to their importance in the community (fig. 1). Such an approach to communities in the fossil record allows the investigators to convey their impressions of the community. The reconstructional approach relies heavily on interpretation, however, and in the present context is of limited usefulness because it does not lend itself to the development of quantitative sedimentary models. Moreover, it is based on extensive paleoecologic experience of the investigators, experience that most sedimentary modelers do not have.
Figure 1--Block diagram showing reconstruction of the Lower Silurian Costistricklandia community. Costistricklandia, which is the largest brachiopod in the community, is also the most abundant--nearly 50% of the fauna. Its contribution to the sediment, both as whole and as disarticulated specimens, is largely confined to shell beds, as indicated in the cutaway view of the substrate below the brachiopod-encrusted surface [modified from Ziegler et al. (1968, fig. 8, p. 17)].

The quantitative approach to ancient communities grew out of the biofacies analysis of the 1950's (Phleger, 1951; Imbrie, 1955) and the subsequent quantitative recognition of communities by Fager (1957), Johnson (1960,1962), Kaesler (1966), and others. A method that lends itself to sedimentary modeling is Murray's (1973) straightforward quantitative discrimination of paleoenvironments by using ternary diagrams with the foraminiferal suborders Textulariina, Miliolina, and Rotaliina at the apices. A nonspecialist can learn to recognize suborders of foraminifers rather easily. By plotting the relative abundances of individuals belonging to each of the three suborders, one can assign a sample to any of several marine environments with a fair degree of accuracy. Murray's book is replete with examples. His method applies especially well to Cenozoic foraminifers but has not yet been applied extensively to the Mesozoic and Paleozoic.
Any environment inhabited by organisms will change as a result of the activities of those organisms. The activities can be as subtle as the binding of sediment by foraminifers or the instigation of early diagenesis by interstitial bacteria. They can be as brash as the construction of a barrier reef. Moreover, once organisms have established themselves in an environment, those with mineralized skeletons contribute sedimentary particles and enter the domain of biostratinomy. The result is that no model of stratigraphic succession is complete without paleontology. Models will be stronger if they are based on current concepts of paleontology, incorporating the results of our rapid progress in understanding communities of ancient organisms and their effects on sedimentary environments.
The most important points that one can make about the data of paleontology are that taxonomy is of utmost importance and that the success of research based on paleontology depends on both accurate and precise taxonomy. This is true whether one is interested in systematic paleontology, paleoecology, paleoenvironmental analysis, or sedimentary modeling. Ecologists and paleoecologists have long recognized sound taxonomy as the conditio sine qua non of their field. In large part the need for sound taxonomy stems from the key role that taxonomic uniformitarianism (Lawrence, 1971; Dodd and Stanton, 1990, pp. 5-12) plays in using fossils to decipher the past. Unfortunately, as a kind of substantive uniformitarianism, taxonomic uniformitarianism is flawed, ignores evolution, and, if applied strictly, can lead to errors (Gould, 1965).
Recognition of the importance of taxonomy has not carried over into sedimentology. A flagrant example comes from the use of data from point counts of thin sections, where the data may be taxonomically imprecise. That a thin section of limestone contains 5% ostracodes, for example, tells one only that the rock was deposited in an aquatic environment. Learning in addition that the rock's fauna also includes brachiopods, crinoids, cephalopods, or trilobites demonstrates only its marine origin. On the other hand, the knowledge that 90% of the foraminiferal assemblage belongs to the suborder Miliolina gives one confidence that the limestone was deposited in a shallow, marginal marine setting with a variable environment, such as a backreef lagoon. The more precise the identification, the more likely the fossils are to yield useful information; and one should identify species whenever possible. Most fossil species cannot be identified in thin section, of course, so geologists must bolster data from point counts by spending additional time describing the rock and its fauna in the field or in the laboratory. The paleontologic contribution to studies of well cuttings is likely to be limited to information from microfossils.
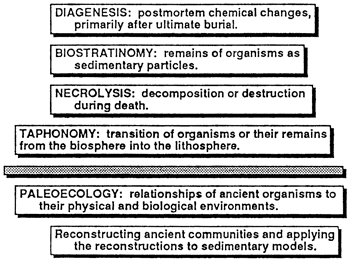
Taphonomy, from the Greek taphos (grave), is the system of knowledge that deals with the transition of organisms or their remains from the biosphere into the lithosphere, the postmortem changes of fossils. In the past taphonomy was widely regarded as an overprint or veil that hampers paleoecology by concealing from view the ecology of ancient organisms or by distorting our perception of it (Lawrence, 1968). More recently, paleoecologists interested in taphonomy have taken a more positive view, using taphonomy for the information it provides rather than bemoaning the loss of the information that it conceals [see Behrensmeyer and Kidwell (1988)]. This change of emphasis, especially Speyer and Brett's (1988) work on taphofacies, has made the subject more important to sedimentary modelers.
Taphonomy has three aspects (fig. 2): necrolysis, biostratinomy, and diagenesis. Necrolysis [from the Greek necros (death) and lysis (loosening)] is the disaggregation, decomposition, or destruction of organisms during death. The necrolysis of an organism can range from negligible to total. A soft-bodied organism ingested by a carnivorous predator, for example, may vanish without a trace. Weakly mineralized skeletons are unlikely to endure necrolysis without being dissolved, crushed, or broken; weakly articulated skeletons are unlikely to come through intact. On the other hand, robust clams drilled by predaceous gastropods may have only small drill holes that have little effect on the likelihood of the shells' resisting later stages of taphonomy. Finally, organisms that die from natural causes, especially those in burrows, are likely to show no trace of necrolysis whatsoever. Few studies have been aimed solely at the effects of necrolysis on the fossil record [but see Maddocks (1988)].
Figure 2--Relationship of taphonomy to community paleoecology. For our purposes the goal is reconstruction of ancient communities that can be used to help build sedimentary models. Beyond the taphonomic veil, shown by the shaded bar, are the three aspects of taphonomy--necrolysis, biostratinomy, and diagenesis--which obscure our view of the ancient world and render some parts of it beyond our comprehension.
Biostratinomy deals with the remains of organisms as sedimentary particles before their final burial. It has been the subject of a great deal of work in recent years and is the taphonomic topic that has received more attention from paleontologists than any other (Behrensmeyer and Kidwell, 1988; International Geological Congress, 1989). A great deal remains to be learned, however. Chave (1964), for example, pioneered quantitative biostratinomy, which enables sedimentary modelers to incorporate measurements of biostratinomic properties and processes into their models. This subject has only recently been the focus of renewed investigation (Kaesler and Kontrovitz, 1989; Kidwell and Baumiller, 1989).
Biostratinomy encompasses both the physical and the biologic effects of the environment on the remains of organisms. Almost exclusively, the physical effects result in destruction of the remains of fossils. Wave action, movement by currents, and abrasion disarticulate and disaggregate skeletal remains and reduce their size. Until protected by burial, the resulting skeletal fragments may be repeatedly rounded by abrasion and broken into angular fragments, often until little remains of them but carbonate mud. Early diagenesis that occurs before ultimate burial and while the remains of organisms are still in the sedimentary regime is legitimately regarded both as biostratinomy and as diagenesis. Physical biostratinomy can be incorporated into models of carbonate sedimentology because it determines the nature of the carbonate grains that form limestone.
The biologic effects of biostratinomy are pervasive, and study of this aspect of biostratinomy is an actively growing field. The destructive actions of organisms include the effects of bacteria on both soft remains and mineralized skeletons, some of which are best regarded as diagenetic effects, and destructive boring by algae and sponges. Bioturbating organisms reintroduce buried shells into the sedimentary environment, where both physical and biologic agents can renew their destructive attacks on them. The importance of repeatedly exhuming skeletal remains and their repeated exposure to the destructive environment is apparent to students of modern nearshore marine environments, and a number of sedimentary models incorporate this phenomenon [e.g., Foster (1985)].
Conversely, the activities of other organisms may protect both soft tissues and skeletal material from destruction. Bacteria produce anoxic environments in which soft tissues decay less rapidly, and such encrusting organisms as red algae and bryozoans commonly cover less robust skeletons, protecting them from destruction by waves and currents.
Finally, not all biostratinomic factors result in destruction of potential fossils or in their enhanced preservation. Remains of organisms in the sedimentary environment behave in much the same way as other sedimentary grains, with a few important additions. Of greatest importance are the initial sizes and shapes of the skeletal grains. Characteristics of skeletal grains are not determined by either the strength of currents, which help determine the size of terrigenous grains, or the provenance, which may determine the original shape of terrigenous grains; they are determined by the kinds and sizes of organisms that lived in the environment of deposition and their population dynamics and survivorship. Craig and Oertel's (1966) computer simulation of population dynamics includes a number of factors important to sedimentary modelers interested in the size distribution of organisms. Such simulation analysis requires modelers to specify the parameters to be used in the model, a requirement that is likely to drive them back into the laboratory or the field. Depending on the physical environment, skeletal remains are variously oriented, transported, and sorted in ways that can be readily quantified to add realism to sedimentary models (Nagle, 1967; Behrens and Watson, 1969; Futterer, 1978).
Diagenesis occupies much of the effort of sedimentary geologists and is important in deciphering both the processes of sedimentology and the provenances of sedimentary rocks. In a sense, diagenesis is to sedimentology and paleoenvironmental analysis what taphonomy as a whole is to paleontology and paleoecology--a glass through which we see darkly. Yet diagenesis provides evidence of post-depositional processes that have important implications for interpretation of the original structure of communities. In recent years paleontologists have begun to work closely with sedimentary geochemists to investigate diagenesis of fossils. Among the frontiers where significant progress is expected are studies of the importance of early diagenesis for subsequent biostratinomy of exhumed fossils and the importance of late deep-basin diagenesis in the destruction of the remains of fossils. Much remains to be learned, and three recent books on biomineralization (Lowenstam and Weiner, 1989; Simkiss and Wilbur, 1989; Carter, 1990) are as outstanding for their dearth of material on diagenesis as for their thorough coverage of the processes and patterns of biomineralization.
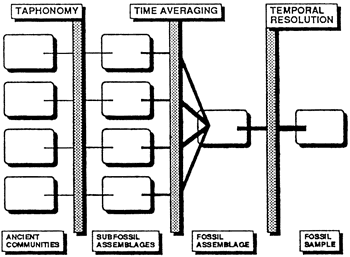
Time averaging is the product of taphonomic processes that obscure the temporal differences between diachronous events [see Staff and Powell (1988, p. 74)]. Common sense and careful fieldwork tell us that time averaging has been important in determining the quality of the fossil record and the nature of the information we can derive from its study. The result of time averaging is that even the fossils on a single bedding plane cannot be assumed to have lived at the same time and to have interacted with each other. The temporal disparity may confound the interpretation of the remains of organisms that were active at different times of the day, lived at different seasons of the year, occurred in the same environment at widely separated times, or occupied different environments of deposition. The effect of time averaging is to detract from our ability both to interpret the structure of ancient communities and to extend our knowledge of community structure to sedimentary modelers.
Three causes of time averaging are particularly important in the present context because they can be readily incorporated into sedimentary models: size of fossils, lag deposits, and bioturbation. [See Staff et al. (1986) for a more complete discussion of the types of time averaging.] Size of fossils contributes to time averaging of assemblages in a simple manner. The vertical dimension of a fossil in a stable position of rest may be greater than the thickness of the enclosing sediment that accumulates during the lifetime of the organism. If so, the sediment that encloses such a fossil will necessarily represent a longer time than the fossil's life span. Two such fossils in the same bed, therefore, need not have lived at the same time. This effect is enhanced by compaction of fine-grained sediment.
An extreme instance of the effect of size occurs when fossils form lag deposits in which finer-grained sediment was not deposited or from which it has been subsequently removed by penecontemporaneous erosion. The result can be a persistent shell bed on the seafloor. The presence of exposed shells, which offer good sites for attachment of other shell-bearing benthic organisms, enhances colonization of the seafloor and may accelerate the formation of the shell bed. Ultimately, if such a shell bed is to be preserved, it must be covered by sediment. The temporal relationships among the fossils will be obscure, however, and the deposit may have resulted from accumulation of diachronous fossils [see Walker and Diehl (1986)].
A more profound cause of time averaging is bioturbation, which is pervasive in most marine environments. As a result of the activities of bioturbating organisms, subfossil skeletal remains of organisms can be mixed through an appreciable thickness of sediment that may have been deposited in a variety of depositional environments and at widely different times. Drosser and Bottjer (1986, 1988) developed a classification of the effects of bioturbation on bedding. Because their model is simple and linear rather than complicated and hierarchical, their classification lends itself to incorporation into sedimentary models. Foster's (1985) computer model simulates the bioturbation of sediment on a cell-by-cell basis. It allows the user to consider an array of environmental parameters and to vary rate and depth of bioturbation and rate of sedimentation. It is worth noting that bioturbation enhances the effectiveness of taphonomic agents by reintroducing buried subfossil material into the biostratinomic regime.
The field geologist is a bioturbating organism whose importance is rarely considered in evaluating ancient sedimentary environments. Irrespective of the scale at which events are recorded, the stratigraphic record can be interpreted at a scale no finer than the scale at which the rocks are studied. Detailed sampling may enable one to make detailed interpretations. Coarse sampling--operator-induced time averaging--limits one to coarse interpretations. In this connection, the ideas presented in the next section on resolution in the stratigraphic record come into play. Moreover, because detailed sampling is always done at some cost, the results that it produces may not be worth the extra effort that it requires.
The effects of taphonomy and time averaging combine to limit the temporal resolution that is possible with the stratigraphic record, but these are not the only limiting factors. The nature of sedimentation itself is equally important. Although sedimentary modeling is no doubt made easier by assuming constant rates of sedimentation, a number of researchers have recently shown the weakness of the concept of continuous sedimentation, the need for more precise understanding of the idea of gaps in the stratigraphic record, and the limits on resolution imposed by the nature of the record. In this connection, Ager's (1973) book, The Nature of the Stratigraphic Record, is particularly enlightening, especially his chapter entitled "More Gaps than Record," in which he is concerned principally with the outmoded notion of continuous sedimentation.
Sadler and Dingus [1982, p. 461; see also Sadler (1981)] defined a complete stratigraphic section as one that contains "some sediment that is representative of each time unit at [the specified time] scale." They provided an empirical procedure for estimating completeness. Schindel (1980, 1982a,b) showed some of the kinds of paleontologic and sedimentologic events that can be detected with sampling plans of various precision. More recently, Allmon (1989) applied these ideas to the fossil record of lower Tertiary mollusks of the Gulf and Atlantic coastal plains. He concluded that the "molluskan record is probably not complete enough for meaningful investigations of evolutionary tempo and mode" because the required resolution of 1,000 to 100,000 years is not possible.
A useful guide to our thinking about temporal resolution is Kitts's (1977, pp. 134-147) discussion of the importance of geologic signals and their velocities in geologic correlation, especially the use of correlation to establish simultaneity.
Taphonomy, time averaging, and factors that diminish temporal resolution affect the fossil record of ancient communities, complicate their interpretation, and detract from their usefulness to sedimentary modelers (fig. 3). Although necrolysis and diagenesis have not yet been investigated thoroughly, we now know a great deal about the behavior of skeletal remains in the sedimentary environment (Nagle, 1967; Behrens and Watson, 1969; Futterer, 1978; Staff and Powell, 1988).
Figure 3--Importance of taphonomy, time averaging, and temporal resolution in the formation and interpretation of ancient communities, subfossil assemblages, fossil assemblages, and time-averaged samples of fossils. The weight of the horizontal or branching lines connecting the various boxes shows the relative confidence of paleoecologists in their interpretation after the material has been affected by taphonomy, time averaging, and other factors that diminish temporal resolution.
Several aspects of biostratinomy need more study. Experimental taphonomy is needed to determine how shells break or abrade under various conditions of environmental energy. We need to assess the importance of bioturbation in exhuming shells repeatedly and thereby subjecting them to the rigors of the sedimentary environment. We need to know more about the effects of such boring organisms as algae, sponges, and clams and of such encrusting organisms as red algae, bryozoans, and barnacles on long-term preservation of shells.
Study of early diagenesis of fossils may be particularly important for helping us to see through the taphonomic veil. What is the effect of early diagenesis of shells on their biostratinomic history if they are exhumed by bioturbating organisms? Will they break more readily? Will boring and encrusting organisms attack such shells less vigorously? How does early diagenesis affect the supply of carbonate sediment, and how can this information be incorporated into sedimentary models? These questions remain unanswered and hint at a fruitful field of diagenetic studies lying fallow before us.
The principal concepts to be discussed in this section are succession, diversity, and competition among the organisms that make up ancient communities as they apply to the work of sedimentary modelers. Before dealing with these concepts, however, I briefly cover some other topics that relate generally to fossils and paleoenvironments and especially to the scale at which communities can be studied. Here the matter of temporal resolution in the stratigraphic record becomes especially important.
Ichnology, including paleoichnology, lies at the interface across which paleontology and sedimentology impinge on each other most beneficially. Bioturbation is a nearly ubiquitous phenomenon in the sedimentary environment, and the study of trace fossils provides a great deal of information about the kinds of bioturbating organisms present, their behaviors, and the paleoenvironments of which they were a part (Frey et al., 1990). Moreover, trace fossils are extremely unlikely to be transported, except those that have been bored into hard substrates. Because of many readily available, useful summaries of paleoichnology in the literature, I do not cover the topic in detail here. The main point that needs to be made to sedimentary modelers is that much of the trace fossil literature that purports to interpret paleoenvironments is the result, instead, of overinterpreting or misinterpreting the data. Except in the broadest terms, individual genera of trace fossils can rarely be used as indexes of facies. In this respect they resemble body fossils, which also should be considered as they occur in assemblages rather than individually. Beware of those who do violence to Seilacher's (1970) classical model by seeking to oversimplify its application to the real world.
Similarly, one must be wary of attempts to use paleontologic data to interpret short-term changes of the biota or the environment, such as ecologic succession that takes place over a period of time ranging from a few hours or days to, say, a hundred years (Schindel, 1980). In this regard, two concepts are important to sedimentary modelers. The first is recognition that there are two relevant time scales: ecologic time and evolutionary time, the latter of which is sometimes referred to as geologic time. Most changes in communities of organisms that are of interest to paleoecologists, who are attempting to apply concepts of ecology to the fossil record, have occurred on a scale of ecologic time. These include such local environmental changes as tidal cycles, seasonal fluctuations, short-term climatic changes, and seral succession. Because of time averaging and the loss of temporal resolution in the stratigraphic record, however, most changes that are recorded by sedimentary rocks have happened on a scale of evolutionary time. These include permanent changes of substrate type and evolution of new species. In general, the stratigraphic record has not preserved details of events that happened on a scale of ecologic time, suggesting that sedimentary modelers can safely ignore a whole range of short-term ecologic phenomena.
The second concept, Markov memory, was first suggested to me by John C. Griffiths (personal communication, 1969). A Markov process is one "in which the probability of the process being in a given state at a particular time may be deduced from knowledge of the immediately preceding state" (Harbaugh and Bonham-Carter, 1970, p. 98). The concept of Markov memory comes into play when one asks, How previous? how immediately? To pick an extreme example, in retrospect, on the basis of conditions at the time, one would not have wanted in 1914 to try to predict today's political situation in central Europe. Similarly, one would not attempt to retrodict the Devonian from knowledge of the Silurian, even if one's knowledge of the Silurian were as complete as the geologic record will allow. The Markov memories of both twentieth-century European politics and the middle Paleozoic geologic systems are too short. Rollins et al. (1979, p. 91), discussing the effects of transgression and regression on biologic succession, generalized: "Transgressive-regressive cycles of environmental change result in an asymmetry of successional change and community replacement since the colonizing biota is under predominantly allogenic stress, whereas the regressive biota is under both allogenic and autogenic stress." [Note that Rollins et al. (1979, p. 89) have correctly pointed out that succession "should only be used in the study of marine level-bottom communities where organizational changes can be demonstrated to be caused by autogenic effects."] One expects changes in evolutionary time of communities that were tracking regressive environments to have been different from those tracking transgressive environments. One would not, however, expect communities to be aware of whether they were occupying a transgressive or a regressive environment. The successional changes taking place in an ecologic time frame have no Markov memory of such evolutionary time events.
Table 2--Johnson's (1972, pp. 152-153) seven theoretical propositions regarding disturbances in ancient communities
|
More than any other paleoecologist, Johnson (1972) has dealt with the effects of environmental perturbation of marine faunas. His views (table 2) pertain to succession and diversity in both the modern world and the fossil record. They are particularly applicable to sedimentary modelers trying to interject biotic factors into their models. Johnson's work (1972, p. 153) led him to view "the community [as] a collection of the relics of former disasters," a useful guide to the thinking of anyone who studies the ecology of ancient communities.
Paleontologists' search for succession in the fossil record has been replete with discovery--but in most instances not the discovery of succession. The concept of succession is important for sedimentary modelers for two reasons. First, although ecologists' understanding of succession is not as firmly grounded in theory as it once was, some communities may undergo orderly changes in ecologic time that might be incorporated into models (table 3). Second and perhaps of greater importance, most of what has been called succession in the stratigraphic record is something quite different.
Table 3--Models of species' response to environmental change and kinds of seral successional change
|
Pioneering or opportunistic species (weeds)
Equilibrium species
Facilitation model of succession: Following a disturbance, only early successional species colonize an area. They modify the habitat to facilitate the colonization of species that occur later in succession. Tolerance model of succession: Species that appear later in succession either arrive early and grow slowly or arrive late. Early and late colonizers are able to tolerate each other. The sequence of succession is a result of differences in species' life histories. Inhibition model of succession: Following a disturbance, species that arrive early inhibit other species from colonizing the habitat. Colonization by later arriving species occurs only when space is cleared of inhibiting species. Source: Connell and Slatyer (1977). |
"Succession refers to the changes observed in an ecological community following a perturbation that opens up a relatively large space" (Connell and Slatyer, 1977, p. 1,119). Ecologists typically regard succession as occurring through a "series of stages of community change in a particular area leading toward a stable state" (Ricklefs, 1983, p. 479). Such a series of stages, changing in ecologic time, is termed a "sere"; and the succession that results is termed "seral succession." In the ecology literature, to specify that succession is seral succession is redundant; ecologists rarely deal with any other kind of succession. In the paleoecology literature, however, succession has been applied to everything from seral succession to mere tracking of changing environments by communities of organisms. Because succession occurs in ecologic time, instances of true seral succession are not likely to be discernible in the stratigraphic record (Walker and Alberstadt, 1975; Walker and Diehl, 1986; Kaesler and Peterson, 1989). Walker and Alberstadt (1975) and Walker and Diehl (1986) have discussed succession in reefs, which in some instances is a long-term analogue of seral succession. The extent to which succession in reefs mimics seral succession seems in large part to depend on rates of subsidence and of growth of the reef.
Walker and Alberstadt (1975) introduced the terms allogenic succession" and "autogenic succession," of which only the latter occurs in a short time span and is analogous to the seral succession seen in modern environments (Walker and Diehl, 1986, p. 65). Bretsky and Bretsky (1975), for example, noted changing faunas through a thick sequence of Ordovician rocks, clearly an example of allogenic succession. They described the changes that they observed in the terms used for seral succession, which occurs in ecologic time rather than evolutionary time. Gould (1980, p. 103) regarded such extension of terminology from the phenomena of ecologic time to the phenomena of evolutionary time as an instance of "serious errors of scaling." The long-term tracking of environments of deposition by communities of ancient organisms is an interesting phenomenon that is vital to the success of sedimentary models, but it should not be described in terms that suggest analogy to the short-term events studied by ecologists.
The concept of species diversity is inseparable from succession, although it is now studied less by ecologists that in the recent past. In general, one expects newly perturbed environments to be occupied by low-diversity communities that include opportunistic species. Stable environments ought to be occupied by equilibrium species, typically in communities of high diversity. Exceptions to these heuristics are commonplace, enough so that many ecologists have abandoned the study of species diversity, judging the results of such study to be merely descriptive and without predictive value. Succession in reefs is a glaring example of an exception if one looks only at the reef core. As a reef matures and undergoes reef succession, it grows into the surf zone where few species can live. As a result, diversity is reduced, space-clearing events are commonplace, and the environment is often perturbed by events that downgrade the community.
For all its shortcomings, the concept of species diversity can be readily integrated into sedimentary models because it can be expressed either semiquantitatively or quantitatively. One approach to species diversity is simply to use species richness, an integer that expresses the number of species present in the environment or in samples from it. Species richness, however, conveys no information about the evenness of the distribution of the species. A species that makes up 95% of the fauna and one that makes up only 1% make the same contribution to species richness. Moreover, species richness does not consider other aspects of communities, such as the size of organisms and the trophic structure.
An alternative to species richness is the use of indexes of species diversity from information theory (Shannon and Weaver, 1949; Brillouin, 1962; Pielou 1975, 1977). Viewed in this way, species diversity is a number that expresses (or confounds) both the number of species and the evenness with which individual organisms are distributed among them. Of the three equations that have been used to compute species diversity from information theory (table 4) (Kaesler and Herricks, 1976; Kaesler et al., 1978), Brillouin's equation is especially appropriate for incorporation into sedimentary models.
Table 4--Equations from information theory used to compute species diversity and hierarchical diversity
|
Shannon-Weaver H'= -Σ pi ln(pi) Brillouin H = (I / N)[ ln(N!/N1!N2!N3! ... Ns!)] Approximate H"=-Σ(Ni/N)ln(Ni/N)
Modified from Kaesler et al. (1978). |
Another aspect of species diversity from information theory could be incorporated into sedimentary models, especially those that deal with various closely related taxa that produce carbonate grains or bioturbate sediment. Diversity can be structured hierarchically and computed so that components of diversity from various taxonomic categories are additive (Pielou, 1967; Mulvaney and Kaesler, 1976; Kaesler and Herricks, 1979).
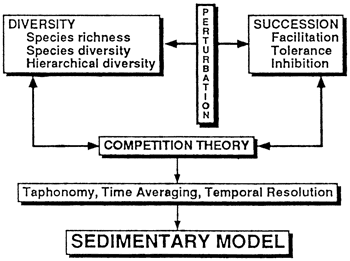
One of the linchpins of community ecology and paleoecology is competition theory, the idea that the structure of communities in nature is determined largely by the competition among populations of species for resources that are in limited supply, such as food, space, and trace nutrients. The strength of the body of theory that governs much of paleoecologists' approach to the study of communities, therefore, hinges on the strength of competition theory. Until quite recently, competition was widely viewed as the dominant factor in the assembly and structuring of communities. More recently, emphasis on null hypotheses and neutral models in ecology has brought competition theory into disrepute, at least among some ecologists who want to see more emphasis on the roles of predation, pathogens, and parasites (May, 1984; Slobodkin, 1987).
Nevertheless, much current community paleoecology should be of interest to sedimentary modelers (Yu et al., 1987). The continued interest of paleoecologists in ancient communities and the progress that such interest has engendered have probably come about in large part because of the close link between community paleoecology and paleoenvironmental analysis (Hoffman, 1979). Sedimentary modelers can largely ignore conceptual developments in community paleoecology sensu stricto and focus their attention on the applications of the studies of communities to paleoenvironmental analysis.
Figure 4--Relationships among diversity, succession (both affected by environmental perturbations), and competition. These three parameters, either as patterns or processes, affect ancient communities but are partially obscured from view by taphonomy and time averaging, which lead to loss of temporal resolution. Nevertheless, they bear strongly on sedimentary models and are relatively straightforward to model in a quantitative manner.
The processes and patterns in the fossil record of communities that are relevant to the work of sedimentary modelers are the sort that either detract from the usefulness of the fossil record or are associated with short-term, repeatable changes. Thus taphonomy and time averaging detract from the usefulness of the fossil record and lead to lack of temporal resolution. Succession and a change in diversity may result from environmental perturbations, either in the short term or in the long term; but neither implies a long-term change in the community. Similarly, competition, whatever its importance, occurs only when an environmental resource is in such short supply that it limits the growth of populations. In this section I deal with three long-term changes of communities that are not reversible: organic evolution, community evolution, and extinction theory.
Neo-Darwinian evolutionary synthesis has received a great deal of attention from paleontologists since Eldredge and Gould (1972) painted two contrasting pictures of organic evolution: phyletic gradualism and punctuated equilibria. Although few sedimentary modelers deal with evolution in their models, Eldredge and Gould's pictures of evolution pertain to sedimentary models because they bear on our idea of how rapidly new species and higher taxa replace old ones and on our view of the reliability of biostratigraphic data.
Eldredge and Gould (1972) listed the tenets of phyletic gradualism and punctuated equilibrium and the consequences of each for the interpretation of the fossil record. Phyletic gradualism is inconsistent with much of what is seen in the stratigraphic record: abrupt appearance of new species; thick stratigraphic intervals with no directional, morphologic change within species (i.e., morphologic stasis); and abrupt extinction of species that is often simultaneous with their replacement by other species (Sylvester-Bradley, 1956).
An important contribution of the model of punctuated equilibria to paleontology is a new view of the fossil record. Paleontologists have begun to take the fossil record more seriously than before, and they no longer regard gaps and morphologic stasis as lack of data. The importance for sedimentary modelers is that, in general, they can accept data from biostratigraphy at face value--data on abrupt appearances of fossils, ranges of species, and abrupt extinctions. Of course, the fossil record is not complete; it has gaps. Nevertheless, paleontologists and sedimentary modelers are now encouraged to seek trends and signals in what was previously regarded largely as noise. A major consequence of renewed interest of paleontologists in evolutionary theory has been new developments in macroevolutionary theory, the origin of species and higher taxa. Although this is a fascinating subject, it is unlikely to play much of a role in the work of sedimentary modelers in the near future.
Community evolution involves the irreversible changes of community composition and structure through time. The changes are irreversible because they consist of originations and extinctions of species and, probably to a much less important extent, changes of niches by species. Concepts of communities and of organic evolution color our view of community evolution. Near one end of the spectrum, Clements (1916, p. 3) regarded ecologic communities as superorganisms: "The developmental study of vegetation necessarily rests upon the assumption that the unit or climax formation is an organic entity." Talent (1983) followed this view but did not subscribe to it in his critique of community evolution. Near the other end of the spectrum, Hoffman (1979, p. 370) regarded communities as mere epiphenomena "of the overlap in distributional patterns of various organisms controlled primarily by the environmental framework." Opposed to this view is the basic assumption of community ecology and paleoecology (Hoffman, 1979, p. 364) that "ecological communities . . . represent a distinct level of biotic organization achieved through ecological integration and coevolution among the species." In any case, one must be wary of the notion that evolution of any sort, either organic evolution sensu stricto or community evolution, implies progress [see Nitecki (1989)].
The modeling sedimentologist can take a more pragmatic view of communities. Physical environments change on an ecologic time scale, and organisms adapt to such change on a slower evolutionary time scale. There can be no catching up, no optimal adaptation, and, given fluctuating environments, no progress. Except for immigration of new organisms into the community, changes in the biologic environment and adaptation to them are likely to be protracted, taking place on an evolutionary time scale. Nevertheless, as communities change through time, the raw materials of sedimentology change, especially the nature of carbonate grains. Sedimentary modelers can incorporate these changes into their models by considering the kinds of questions that address temporal changes of the physical environment and the biota (table 5).
Table 5--Kinds of questions from the study of community evolution that pertain to sedimentary models
|
Long-term changes in the physical environment
Long-term changes in the biologic environment
|
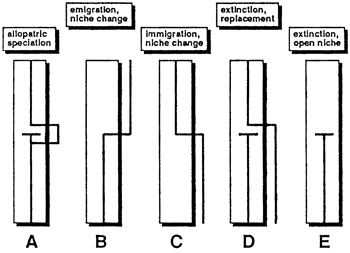
Melchert (1982, pp. 28-30) discussed five models of community evolution, regarding community evolution as the replacement of one species in a community by another (fig. 5). His work illustrates that community evolution is not limited to the evolution of new species but also involves immigration, emigration, and extinction. Determining what processes produced the patterns in the stratigraphic record is not always possible. Sedimentary modelers, however, are not so constrained and can draw on any of Melchert's five models to introduce temporal changes in community structure into their models.
Figure 5--Five models of species replacement important in the evolution of communities. The vertical dimension is time; the boxes represent communities; and the lines that enter and leave communities are species. (A) Allopatric speciation followed by immigration into the community and extinction of the ancestral form. (B) Emigration of species from the community resulting from an evolutionary change of niche. (C) Immigration of species into the community from outside because of an evolutionary change of niche. (D) Extinction of species with replacement by a species from outside the community that is not necessarily closely related. (E) Extinction of species with no replacement; niche remains empty. [Modified from Melchert (1982).]
The names of the geologic eras tell us that the kinds of fossils found in the rocks have changed through time. Among the marine invertebrates, three major, successive evolutionary faunas have been identified (Sepkoski, 1981): the Cambrian fauna, dominated by trilobites; the later Paleozoic fauna, dominated by brachiopods; and the Mesozoic-Cenozoic fauna, dominated by mollusks. Moreover, paleontologists have long been keenly aware of other instances in the geologic past when a large number of different kinds of organisms became extinct in a relatively short interval of time (Newell, 1967).
The history of paleontology is studded with the waxing and waning of interest in such big-picture events as mass extinctions, which have long-term consequences for the history of life. At present the interest in mass extinction has reached an almost feverish pitch. The episode of renewed interest was stimulated in part by the proposition that the extinctions at the Cretaceous-Tertiary boundary were triggered by the impact of a bolide (Alvarez et al., 1980), followed by the recognition that the probability of such impacts throughout geologic time is rather high (McLaren, 1982, 1983, 1986) and that events that have brought about mass extinctions may have occurred at intervals of approximately 26 m.y. (Raup and Sepkoski, 1984, 1986). The ultimate stimulus, however, was probably paleontologists' renewed confidence in the value of the fossil record, which has come about since the introduction by Eldredge and Gould (1972) of the idea of punctuated equilibria in evolution.
Several recent collections of papers deal with extinction and long-term changes in the diversity of the biosphere. Some are cited here as useful means of gaining insight into paleontologists' current thinking about mass extinction, including extinction events that may have resulted from impact of bolides: Berggren and Van Couvering (1984), Nitecki (1984), Valentine (1985), Elliott (1986), Raup and Jablonski (1986), Larwood (1988), and Donovan (1989).
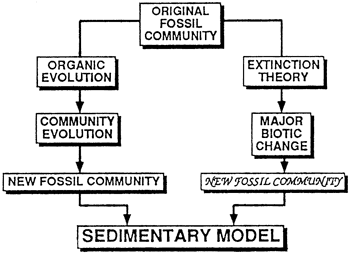
The processes that bring about permanent changes through time in communities of organisms--organic evolution, community evolution, and mass extinction--can be readily incorporated into sedimentary models (fig. 6). The punctuational view of evolution implies that the data of biostratigraphy can be taken at face value, especially in local stratigraphic sections, where abrupt appearance and termination of species and long intervals of morphologic stasis are expected. Community evolution can be considered in either the short term or the long term. In the short term local communities should be characterized by species that appear and disappear in the community suddenly. In the long term communities change fundamentally as new kinds of organisms evolve and become extinct and as the physical environment undergoes long-term changes.
Figure 6--Implications of long-term irreversible changes in biologic communities for sedimentary models. Organic evolution leads to community evolution, the change of species composition of a community. The result of this process may be a new fossil community that is similar in many respects to its antecedent. Mass extinction leads to major biotic change and new fossil communities that may be wholly unlike previous ones.
Incorporating ideas about mass extinctions into sedimentary models is largely a matter of realizing their importance for community evolution, notably that mass extinction is likely to occur irrespective of adaptations of organisms to their environments. Viewed in this light, mass extinction is seen as being of overriding importance in the history of life. If so, microevolution, the changes of gene frequency in a biologic population that lead to adaptations to the environment, may be responsible for little more than fine tuning.
My purpose here has not been to model paleocommunities but rather to show that community paleoecology can contribute to sedimentary modeling. The reason for this approach stems from my view of the purpose of modeling, especially simulation modeling, and the pitfalls placed in the path of its paleontologic practitioners. For this reason I present here my views of simulation modeling in paleontology and geology. Most of these comments pertain to deterministic and, where they exist, stochastic models. They do not apply so much to conceptual models, which usually do not involve simulation and in any event are typically just hypotheses. Some might inveigh against some uses of simulation modeling, but even the most curmudgeonly are not against hypotheses in science.
Simulation modelers outside geology design systems. They predict--that is, they declare in advance--what a system will be like on the basis of a set of parameters that they present to their simulator. Today the simulator is a digital computer, and the parameters are simply inputs. The simulation model is intended to give the appearance or the effect of the system being simulated or to have the characteristics of that system. The ultimate goal is to facilitate design. We sometimes see examples of such simulation models in advertisements for automobiles on television. The simulation model allows the design engineers to view their product from any perspective before production, thus evading Edselesque embarrassments.
Compare simulation modeling for design (i.e., for prediction) with what geologists typically do in the name of simulation. A good example from sedimentology is the pioneering, dynamic quantitative model of Briggs and Pollack (1967) with which the authors interpreted the distribution of Upper Silurian salt in the Michigan basin [see also Harbaugh and Bonham-Carter (1970, pp. 4-11)]. Among numerous paleontologic examples, I refer readers to work by Raup (1966), Chang et al. (1974), and Savazzi (1990). In the first place, none of these researchers was interested in design. Upper Silurian salt deposits and fossils, both from the geologic past, do not need to be designed. In effect, the geologists and paleontologists in our examples have sat as if before a black box covered with dials. They have twisted those dials until they achieved a result--termed "output"--that matches the real world. Thus they have sought to make their computers emulate the real world, that is, to imitate, equal, or approach equality with the real world (Gove, 1976, p. 744) rather than simulating in the strict sense of the word. Neither does one make predictions about the past. The geologic computer emulators are trying to retrodict, that is, "to infer (a past state of affairs) from present observational data" [Gove, 1976, p. 1940; see also Kitts (1977, pp. 8, 39-45)]. It seems that discussions of modeling in geology will be enhanced and the purposes of modeling made clearer if researchers, instead of referring to simulation (which has design as its goal) and to prediction of the past, refer to computer emulation and retrodiction.
I am grateful to E. K. Franseen, J. A. French, C. Kendall, C. G. Maples, W. L. Watney, and R. R. West, whose careful review of this manuscript contributed to its relevance to the problems faced by sedimentary modelers. I have incorporated most of their suggestions and take responsibility for omissions where our opinions differed.
Ager, D. V., 1973, The nature of the stratigraphic record: Macmillan, London, 114 p.
Allmon, W. D., 1989, Paleontological completeness of the record of lower Tertiary mollusks, US Gulf and Atlantic coastal plains--implications for phylogenetic studies: Historical Biology, v. 3, p. 141-158
Alvarez, L. W., Alvarez, W., Asaro, F., and Michel, H. V., 1980, Extraterrestrial cause for the Cretaceous-Tertiary extinction: Science, v. 208, p. 1,095-1,108
Behrens, E. W., and Watson, R. L., 1969, Differential sorting of pelecypod valves in the swash zone: Journal of Sedimentary Petrology, v. 39, p. 159-165
Behrensmeyer, A. K., and Kidwell, S. M., eds., 1988, Ecological and evolutionary implications of taphonomic processes: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 63, 291 p.
Berggren, W. A., and Van Couvering, J. A., eds., 1984, Catastrophes and earth history: Princeton University Press, Princeton, New Jersey, 464 p.
Bretsky, P. W., and Bretsky, S. S., 1975, Succession and repetition of Late Ordovician fossil assemblages from the Nicolet River Valley, Quebec: Paleobiology, v. 1, p. 225-237
Briggs, L. I., and Pollack, H. N., 1967, Digital model of evaporite sedimentation: Science, v. 155, p. 453-456
Brillouin, L., 1962, Science and information theory: 2d ed., Academic Press, New York, 347 p.
Carter, J. G., ed., 1990, Skeletal biomineralization: Van Nostrand Reinhold, New York, 640 p.
Chang, Y.-M., Kaesler, R. L., and Merrill, W. M., 1974, Simulation of growth of the Foraminiferida Ammonia beccarii by computer: Geological Society of America, Bulletin, v. 85, p. 745-748
Chave, K. E., 1964, Skeletal durability and preservation; in, Approaches to Paleoecology, J. Imbrie and N. D. Newell, eds.: John Wiley and Sons, Inc., New York, p. 377-387
Clements, F. E., 1916, Plant succession--an analysis of the development of vegetation: Carnegie Institution of Washington, Publication 242, Washington, DC, 512 p.
Connell, J. H., and Slatyer, R. O., 1977, Mechanisms of succession in natural communities and their role in community stability and organization: American Naturalist, v. 111, p. 1,119-1,144
Craig, G. Y., and Oertel, G., 1966, Deterministic models of living and fossil populations of animals: Quarterly Journal of the Geological Society of London, v. 122, p. 315-355
Cross, T. A., and Harbaugh, J. W., 1990, Quantitative dynamic stratigraphy--a workshop, a philosophy, a methodology; in, Quantitative Dynamic Stratigraphy, T. A. Cross, ed.: Prentice-Hall, Englewood Cliffs, New Jersey, p. 3-20
Dodd, J. R., and Stanton, R. J., Jr., 1990, Paleoecology--concepts and applications: 2d ed., Wiley-Interscience, New York, 502 p.
Donovan, S. K., ed., 1989, Mass extinctions--processes and evidence: Columbia University Press, New York, 266 p.
Drosser, M. L., and Bottjer, D. J., 1986, A semiquantitative field classification of ichnofabric: Journal of Sedimentary Petrology, v. 56, p. 558-559
Drosser, M. L., and Bottjer, D. J., 1988, Trends in depth and extent of bioturbation in Cambrian carbonate marine environments, western United States: Geology, v. 16, p. 223-236
Eldredge, N., and Gould, S. J., 1972, Punctuated equilibria--an alternative to phyletic gradualism; in, Models in Paleobiology, T. J. M. Schopf, ed.: Freeman, Cooper & Co., San Francisco, California, p. 82-115
Elliott, D. K., ed., 1986, Dynamics of extinction: Wiley-Interscience, New York, 294 p.
Fager, E. W., 1957, Determination and analysis of recurrent groups: Ecology, v. 38, p. 586-595
Foster, D. W., 1985, BIOTURB--A Fortran program to simulate the effects of bioturbation on the vertical distribution of sediment: Computers & Geosciences, v. 11, p. 39-45
Frey, R. W., Pemberton, S. G., and Thomas, D. A., 1990, Ichnofacies and bathymetry--a passive relationship: Journal of Paleontology, v. 64, p. 155-158
Futterer, E., 1978, Studien über die Einregelung, Anlagerung und Einbettung biogener Hartteile im Strömungskanal: Neues Jahrbuch Paläontologie, Abhandlungen, v. 156, p. 87-131
Gould, S. J., 1965, Is uniformitarianism necessary?: American Journal of Science, v. 263, p. 223-228
Gould, S. J., 1980, The promise of paleobiology as a nomothetic, evolutionary discipline: Paleobiology, v. 6, p. 96-118
Gould, S. J., 1989, Wonderful life: W.W. Norton & Co., New York, 347 p.
Gove, P. B., ed., 1976, Webster's third new international dictionary of the English language unabridged: G. & C. Merriam Company, Springfield, Massachusetts, 2,662 p.
Harbaugh, J. W., and Bonham-Carter, G., 1970, Computer simulation in geology: Wiley-Interscience, New York, 575 p.
Hoffman, A., 1979, Community paleoecology as an epiphenomenal science: Paleobiology, v. 5, p. 357-379
Imbrie, J., 1955, Biofacies analysis; in, Crust of the Earth, A. Poldervaart, ed.: Geological Society of America, Special Paper 62, p. 449-464
International Geological Congress, 1989, Abstracts, 28th International Geological Congress: Washington, DC, 3 v.
Johnson, R. G., 1960, Models and methods for analysis of the mode of formation of fossil assemblages: Geological Society of America, Bulletin, v. 71, p. 1,075-1,086
Johnson, R. G., 1962, Intraspecific associations in Pennsylvanian fossil assemblages: Journal of Geology, v. 70, p. 32-55
Johnson, R. G., 1972, Conceptual models of benthic marine communities; in, Models in Paleobiology, T. J. M. Schopf, ed.: Freeman, Cooper & Co., San Francisco, California, p. 148-159
Kaesler, R. L., 1966, Quantitative re-evaluation of ecology and distribution of recent Foraminifera and Ostracoda of Todos Santos Bay, Baja California, Mexico: University of Kansas Paleontological Contributions, Paper 10, 50 p. [available online]
Kaesler, R. L., and Herricks, E. E., 1976, Analysis of data from biological surveys of streams--diversity and sample size: Water Resources Bulletin, v. 12, p. 125-135
Kaesler, R. L., and Herricks, E. E., 1979, Hierarchical diversity of communities of aquatic insects and fishes: Water Resources Bulletin, v. 15, p. 1,117-1,125
Kaesler, R. L., and Kontrovitz, M., 1989, Taphonomy of subfossil, intertidal Ostracoda--experimental approach: Abstracts, 28th International Geological Congress, v. 2, p. 2-144
Kaesler, R. L., and Peterson, R. M., 1989, Succession and diversity in a rapidly changing environment--evidence from Carboniferous ostracode assemblages: Senckenbergiana Lethaia, v. 69, p. 523-534
Kaesler, R. L., Herricks, E. E., and Crossman, J. S., 1978, Use of indices of diversity and hierarchical diversity in stream surveys: American Society for Testing and Materials, Special Technical Publication 652, p. 92-112
Kauffman, E. G., and Scott, R. W., 1976, Basic concepts of community ecology and paleoecology; in, Structure and Classification of Paleocommunities, R. W. Scott and R. R. West, eds.: Dowden, Hutchinson & Ross, Inc., Stroudsburg, Pennsylvania, p. 1-28
Kidwell, S. M., and Baumiller, T. K., 1989, Postmortem disintegration of echinoids--effects of temperature, oxygenation, tumbling, and algal coats: Abstracts, 28th International Geological Congress, v. 2, p. 2-188-2-189
Kitts, D. B., 1977, The structure of geology: Southern Methodist University Press, Dallas, Texas, 180 p.
Larwood, G. P., ed., 1988, Extinction and survival in the fossil record: Oxford University Press, New York, 365 p.
Lawrence, D. R., 1968, Taphonomy and information losses in fossil communities: Geological Society of America, Bulletin, v. 79, p. 1,315-1,330
Lawrence, D. R., 1971, The nature and structure of paleoecology: Journal of Paleontology, v. 45, p. 593-607
Lowenstam, H. A., and Weiner, S., 1989, On biomineralization: Oxford University Press, New York, 324 p.
Maddocks, R. F., 1988, One hundred million years of predation on ostracodes--the fossil record in Texas; in, Evolutionary Biology of Ostracodes, T. Hanai, N. Ikeya, and K. Ishizaki, eds.: Elsevier, Amsterdam, p. 637-657
May, R. M., 1984, An overview-real and apparent patterns in community structure; in, Ecological Communities--Conceptual Issues and the Evidence, D. R. Strong, Jr., D. Simberloff, L. G. Abele, and A. B. Thistle, eds.: Princeton University Press, Princeton, New Jersey, p. 3-16
McKerrow, W. S., ed., 1978, The ecology of fossils: MIT Press, Cambridge, Massachusetts, 384 p.
McLaren, D. J., 1982, Frasnian-Famennian extinctions: Geological Society of America, Special Paper 190, p. 477-484
McLaren, D. J., 1983, Bolides and biostratigraphy: Geological Society of America, Bulletin, v. 94, p. 313-324
McLaren, D. J., 1986, Abrupt extinctions; in, Dynamics of Extinction, D. K. Elliott, ed.: Wiley-Interscience, New York, p. 37-46
McMenamin, M. A. S., and McMenamin, D. L. S., 1990, The emergence of animals: Columbia University Press, New York, 217 p.
Melchert, G. D., 1982, Evolution of the ostracode community associated with Myalina (Orthomyalina): M.S. thesis, University of Kansas, Lawrence, 63 p.
Mulvany, P. S., and Kaesler, R. L., 1976, Fortran IV program to compute hierarchical diversity: Computers & Geosciences, v. 2, p. 521-529
Murray, J. W., 1973, Distribution and ecology of living benthic foraminiferids: Heinemann Educational Books, London, 274 P.
Nagle, J. S., 1967, Wave and current orientation of shells: Journal of Sedimentary Petrology, v. 37, p. 1,124-1,138
Newell, N. D., 1967, Revolutions in the history of life: Geological Society of America, Special Paper 89, p. 63-91
Nitecki, M. H., ed., 1984, Extinctions: University of Chicago Press, Chicago, Illinois, 354 p.
Nitecki, M. H., ed., 1989, Evolutionary progress: University of Chicago Press, Chicago, Illinois, 354 p.
Phleger, F. B., 1951, Ecology of Foraminifera, northwest Gulf of Mexico--pt. I, Foraminifera distribution: Geological Society of America, Memoir 46, 88 p.
Pielou, E. C., 1967, The use of information theory in the study of the diversity of biological populations: Fifth Berkeley Symposium of Mathematical Statistics and Probability, Proceedings, v. 4, p. 163-177
Pielou, E. C., 1975, Ecological diversity: Wiley-Interscience, New York, 165 p.
Pielou, E. C., 1977, Mathematical ecology: John Wiley & Sons, New York, 385 p.
Price, P. W., Slobodchikoff, C. N., and Gaud, W. S., eds., 1984, A new ecology--novel approaches to interactive systems: John Wiley & Sons, New York, 515 p.
Raup, D. M., 1966, Geometric analysis of shell coiling-general problems: Journal of Paleontology, v. 40, p. 1,178-1,190
Raup, D. M., and Jablonski, D., eds., 1986, Patterns and processes in the history of life: Springer-Verlag, New York, 447 p.
Raup, D. M., and Sepkoski, J. J., Jr., 1984, Periodicity of extinctions in the geologic past: Proceedings of the National Academy of Science USA, v. 81, p. 801-805
Raup, D. M., and Sepkoski, J. J., Jr., 1986, Periodicity in marine extinction events; in, Dynamics of Extinction, D. K. Elliott, ed.: Wiley-Interscience, New York, p. 3-36
Ricklefs, R. E., 1983, The economy of nature: Chiron Press, New York, 5 10 p.
Rollins, H. B., Carothers, M., and Donahue, J., 1979, Transgression, regression and fossil community succession: Lethaia, v. 12, p. 89-104
Sadler, P. M., 1981, Sediment accumulation rates and the completeness of stratigraphic sections: Journal of Geology, v. 89, p. 569584
Sadler, P. M., and Dingus, L. W., 1982, Expected completeness of sedimentary sections--estimating a time-scale dependent, limiting factor in the resolution of the fossil record: Third North American Paleontological Convention, Proceedings, v. 2, p. 461-464
Sandberg, P. A., 1983, An oscillating trend in Phanerozoic nonskeletal carbonate mineralogy: Nature, v. 305, p. 19-22
Savazzi, E., 1990, Theoretical morphology of shells aided by microcomputers; in, Microcomputer Applications in Geology, J. T. Hanley and D. F. Merriam, eds.: Pergamon Press, New York, v. 2, p. 229-240
Schindel, D. E., 1980, Microstratigraphic sampling and the limits of paleontologic resolution: Paleobiology, v. 6, p. 408-426
Schindel, D. E., 1982a, Resolution analysis--a new approach to the gaps in the fossil record: Paleobiology, v. 8, p. 340-353
Schindel, D. E., 982b, Time resolution in cyclic Pennsylvanian strata--implications for evolutionary patterns in Glabrocingulum (Mollusca; Archaeogastropoda): Third North American Paleontological Convention, Proceedings, v. 2, p. 482a-482e
Seilacher, A., 1970, Arbeitskonzept zur Konstruktions-morphologie: Lethaia, v. 3, p. 393-396
Sepkoski, J. J., Jr., 1981, A factor analytic description of the Phanerozoic marine fossil record: Paleobiology, v. 7, p. 36-53
Shannon, C. E., and Weaver, W., 1949, The mathematical theory of communication: University of Illinois Press, Urbana, Illinois, 125 p.
Simkiss, K., and Wilbur, K. M., 1989, Biomineralization---cell biology and mineral deposition: Academic Press, San Diego, California, 337 p.
Slobodkin, L. B., 1987, How to be objective in community studies; in, Neutral Models in Biology, M. H. Nitecki and A. Hoffman, eds.: Oxford University Press, New York, p. 93-108
Speyer, S. E., and Brett, C. E., 1988, Taphofacies models for epeiric sea environments--middle Paleozoic examples: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 63, p. 225-262
Staff, G. M., and Powell, E. N., 1988, The paleoecological significance of diversity--the effect of time averaging and differential preservation on macroinvertebrate species richness in death assemblages: Palaeogeography, Palaeoclimatology, Palaeoecology, v. 63, p. 73-89
Staff, G. M., Stanton, R. J., Jr., Powell, E. N., and Cummings, H., 1986, Time-averaging, taphonomy and their impact on paleocommunity reconstruction--death assemblages in Texas bays: Geological Society of America, Bulletin, v. 97, p. 428-443
Sylvester-Bradley, P. C., ed., 1956, The species concept in palaeontology: Systematics Association, Publication 2, 144 p.
Talent, J. A., 1983, On the question of "community evolution"; in, Sreda i Zhizn' v Geologischeskom Proshlom--Paleobiogeografiya i Paleoekologiya, Betekhtina, 0. A., and Zhuravleva, 1. T., eds.: Akedemiya Nauk SSSR, Sibirskoye Otdeleniye, Trudy Instituta Geologii i Geofiziki, v. 569, p. 46-53
Valentine, J. W., ed., 1985, Phanerozoic diversity patterns--profiles in macroevolution: Princeton University Press, Princeton, New Jersey, 441 p.
Walker, K. R., and Alberstadt, L. P., 1975, Ecological succession as an aspect of structure in fossil communities: Paleobiology, v. 1, p. 238-257
Walker, K. R., and Diehl, W. W., 1986, The effect of synsedimentary substrate modification on the composition of paleocommunities--paleoecologic succession revisited: Palaios, v. 1, p. 65-74
Yu, W., Boucot, A. J., Rong, J.-Y., and Yang, X.-C., 1987, Community paleoecology as a geologic tool--the Chinese Ashgillian-Eifelian (latest Ordovician through early Middle Devonian) as an example: Geological Society of America, Special Paper 211, 100 p.
Ziegler, A. M., Cocks. L. R. M., and Bambach, R. K., 1968, The composition and structure of Lower Silurian marine communities: Lethaia, v. 1, p. 1-27
Kansas Geological Survey
Comments to webadmin@kgs.ku.edu
Web version April 2, 2010. Original publication date 1991.
URL=http://www.kgs.ku.edu/Publications/Bulletins/233/Kaesler/index.html